
Abstract
Aims: Only a few studies have examined the respective impact of low flow (LF), low gradient (LG) and low ejection fraction (LEF) on outcomes following transcatheter aortic valve replacement (TAVR). The purpose of this study was to assess the impact of preprocedural stroke volume index, aortic valve gradient, left ventricular ejection fraction (LVEF) and different flow/gradient/LVEF patterns on the clinical outcomes of patients with severe aortic stenosis (AS) who undergo TAVR.
Methods and results: We analysed the clinical, echocardiographic, and outcome data collected in 770 patients with AS who underwent TAVR. Overall, 357 patients had normal flow (NF) AS and 413 had LF AS. Patients with NF had similar one-year mortality (12.0% vs. 15.0%, p=0.23) compared with those in the LF group. Overall, patients with NF and/or HG had lower one-year mortality rates (11.7 to 13%) compared to those with paradoxical LF-LG with NEF (19%) and those with classical LF-LG with LEF (27.3%). Low mean gradient was an independent predictor of all-cause mortality (hazard ratio: 1.14, per 10 mmHg decrease, p=0.02). Despite significant association in univariable analyses, LF and LEF were not found to be predictors of outcomes in multivariable analyses.
Conclusions: Patients with HG and those with NF-LG have low one-year mortality rates following TAVR, whereas those with classical LF-LG and LEF and those with paradoxical LF-LG and NEF have high and intermediate risk of mortality, respectively. In contradiction to previous reports, LG but not LF or LEF is an independent predictor of late mortality in high-risk patients with severe AS undergoing TAVR.
Abbreviations
AS: aortic stenosis
AVA: aortic valve area
HF: high flow
HG: high gradient
LEF: low ejection fraction
LF: low flow
LG: low gradient
LVEF: left ventricular ejection fraction
LVOT: left ventricular outflow tract
NEF: normal ejection fraction
TAVR: transcatheter aortic valve replacement
SVi: stroke volume index
VTI: velocity time integral
Introduction
Patients with severe aortic stenosis (AS) are often categorised based on left ventricular stroke volume index (SVi), aortic valve gradient and left ventricular ejection fraction (LVEF)1. They are further subdivided into subcategories such as low flow (LF) versus normal flow (NF), high gradient (HG) versus low gradient (LG) and normal ejection fraction (NEF) versus low ejection fraction (LEF). LF, LG and LEF were all shown to be predictors of mortality following surgical aortic valve replacement (SAVR)1-4. On the basis of these three haemodynamic parameters, several patterns of flow/gradient/LVEF AS have been described: i) NF-HG (D1 Stage in the ACC/AHA guidelines); ii) NF-LG (no specific stage given in the guidelines); iii) LF-HG (D1 Stage); iv) paradoxical LF-LG with NEF (D3 Stage); and v) classical LF-LG with LEF (D2 Stage)5. A recent meta-analysis reported that, among patients with NEF, those with paradoxical LF-LG have worse outcomes compared to those with moderate AS and HG AS (D1) but their outcome was significantly improved by aortic valve replacement6. Furthermore, in this study, patients with NF-LG AS had similar survival to those with HG AS and their outcomes were also improved by surgical valve replacement.
Transcatheter aortic valve replacement (TAVR) has emerged as a treatment option for inoperable or high-risk surgical patients with severe AS7,8. Recent studies and meta-analyses have reported that the presence of low LVEF and low transvalvular aortic gradient prior to the procedure are associated with increased mortality following TAVR9-11. Nonetheless, only a few studies have examined the impact of LF or flow/gradient patterns on outcomes following TAVR12,13 and assessed the impact of the different flow/gradient categories on post-procedural outcomes. These studies represent the early experience with TAVR with significantly increased short-term mortality compared to recent years.
The objectives of the present study were to characterise patients with severe AS and LF who undergo TAVR and to assess the impact of preprocedural SVi, aortic valve gradient and LVEF on the clinical outcomes following this procedure.
Methods
We retrospectively examined the clinical and Doppler echocardiographic data prospectively collected in 825 consecutive patients who underwent TAVR between 2012 and 2014 at our institution. We excluded patients who had TAVR for indications other than severe AS or who had incomplete preprocedural Doppler echocardiographic data. Finally, 770 patients (93.3%) were included in the study. The study was approved by the institutional review board (IRB) and informed consent was obtained from all subjects.
All patients had congestive heart failure with New York Heart Association (NYHA) Class II-IV symptoms. Aortic valve disease was assessed with transthoracic echocardiography performed by experienced echocardiographers. Evaluation of AS severity was performed based on peak velocity, mean gradient and calculation of the aortic valve area (AVA) using the continuity equation as recommended by current guidelines5. All patients had an indexed AVA (AVA/body surface area) of <0.6 cm2/m2. Patients were considered to have HG severe AS if the mean transvalvular gradient was ≥40 mmHg and LG if it was <40 mmHg. Patients were also differentiated based on SVi (stroke volume/body surface area) into LF <35 ml/m2 and NF ≥35 ml/m2. Stroke volume was calculated using the following formula: left ventricular outflow tract (LVOT) area×LVOT velocity time integral (VTI)=π(LVOT diameter/2)2×LVOT VTI. Patients with LF-LG AS were further divided by their LVEF into LEF (LVEF <50%) and NEF (LVEF ≥50%) (Figure 1).
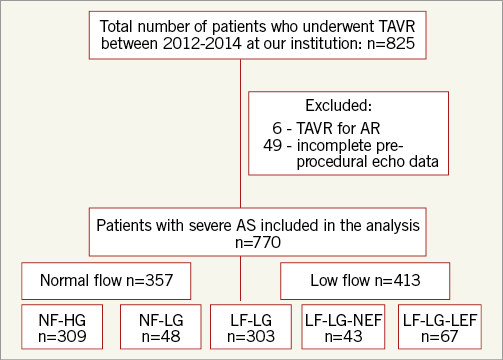
Figure 1. Flow chart for inclusion in the study. Patients were differentiated based on SVi (stroke volume/body surface area) into LF <35 ml/m2 and NF ≥35 ml/m2. Patients were considered to have HG severe AS if the mean transvalvular gradient was ≥40 mmHg and LG if it was <40 mmHg. LEF was defined as LVEF <50% and NEF as LVEF ≥50%. AS: aortic stenosis; LF-HG: low flow and high gradient; LF-LG-LEF: low flow, low gradient, and low ejection fraction; LF-LG-NEF: low flow, low gradient, and normal ejection fraction; NF-HG: normal flow and high gradient; NF-LG: normal flow and low gradient
Patients were also evaluated by an ECG-gated, multislice CT angiography study with a SOMATOM® Sensation Cardiac 64 scanner or a SOMATOM® Definition Flash CT scanner (Siemens Medical Solutions USA, Inc., Malvern, PA, USA). Aortic valve calcium was quantified by a standard Agatston methodology for all available non-contrast CT scans14.
All patients were considered high risk for valve surgery by our institutional Heart Team. Prosthetic valve size selection was based on CT or immediate preprocedural three-dimensional transoesophageal echocardiography. The vascular access approach was chosen on the basis of the individual patient’s risk profile. Baseline clinical, echocardiographic and procedural details for TAVR were recorded for all patients including one-month clinical and echocardiographic assessments during a follow-up visit. TAVR endpoints, device success and adverse events were recorded using the Valve Academic Research Consortium (VARC)-2 definitions15. The primary endpoint of this study was all-cause mortality. Secondary endpoints were cardiovascular mortality, 30-day mortality and complication rates.
STATISTICAL ANALYSIS
Continuous variables were tested for distribution normality with the Shapiro-Wilk test and expressed as mean±SD or median and interquartile range. Because the Society of Thoracic Surgeons score was not normally distributed, a natural log transformation was used for this variable. Differences between groups were assessed using analysis of variance with subsequent Tukey HSD pairwise comparisons, two-sided Student’s t-test or Wilcoxon rank-sum test, as appropriate. Categorical variables are expressed as number (percentage) and were compared using the Pearson χ² test or the Fisher’s exact test, as appropriate. Kaplan-Meier survival plot significance was estimated using the log-rank test. The effect of the clinical and Doppler echocardiographic variables on survival was assessed with Cox proportional hazards regression models for cumulative all-cause and cardiovascular mortality. Clinically relevant variables with a p-value ≤0.1 on individual analysis were included in the multivariable models. All of the analyses were considered significant at a two-tailed p-value of less than 0.05. The SPSS statistical package, Version 20.0, was used to perform all statistical evaluation (IBM Corp., Armonk, NY, USA).
Results
BASELINE AND PROCEDURAL CHARACTERISTICS
BASELINE AND PROCEDURAL CHARACTERISTICS ACCORDING TO FLOW STATUS
Overall, 357 (46.4%) patients had NF AS and 413 (53.6%) had LF AS. The baseline clinical patient characteristics and pre-TAVR imaging details of the study population are shown in Table 1. Compared to the NF group, patients in the LF group were predominantly male, had a higher body mass index, and a higher prevalence of diabetes mellitus, coronary artery disease and permanent atrial fibrillation/flutter. The LF group had significantly lower baseline aortic valve area, and lower aortic valve mean/peak echo gradients (0.58 cm2 and 42.6/78.4 mmHg vs. 0.70 cm2 and 48.8/81.6 mmHg, respectively; p<0.001 for all). They also had higher rates of moderate-severe mitral regurgitation (28.1% vs. 13.4%, respectively, p<0.001).
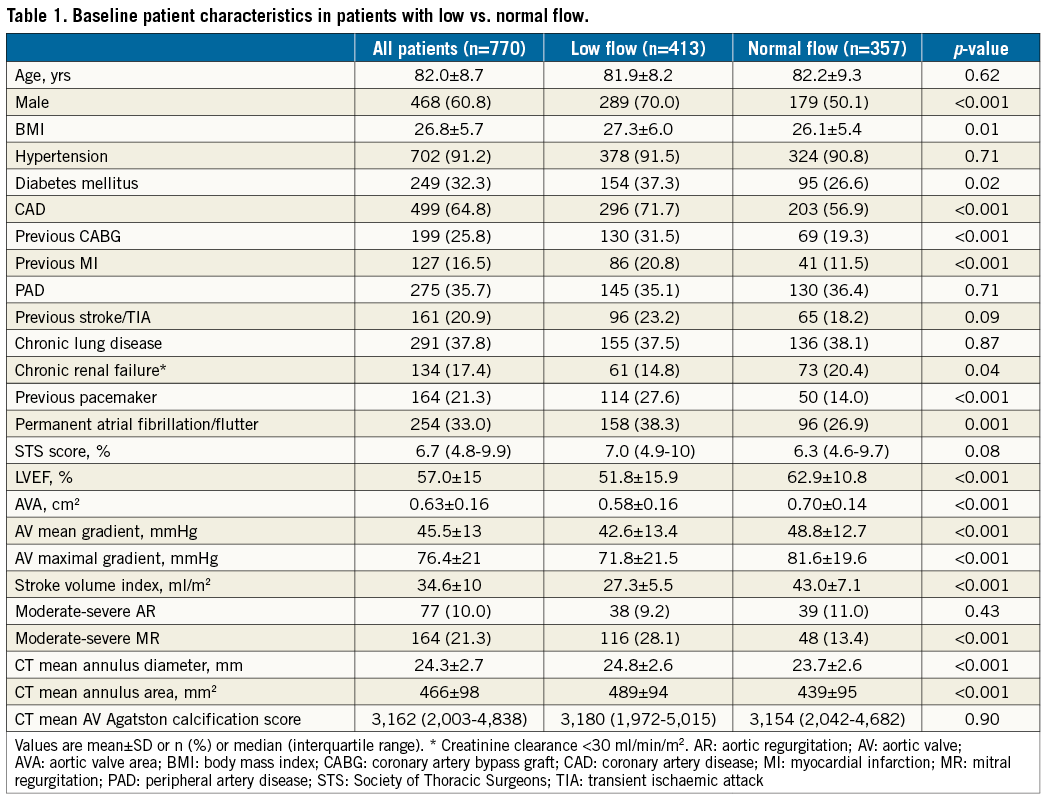
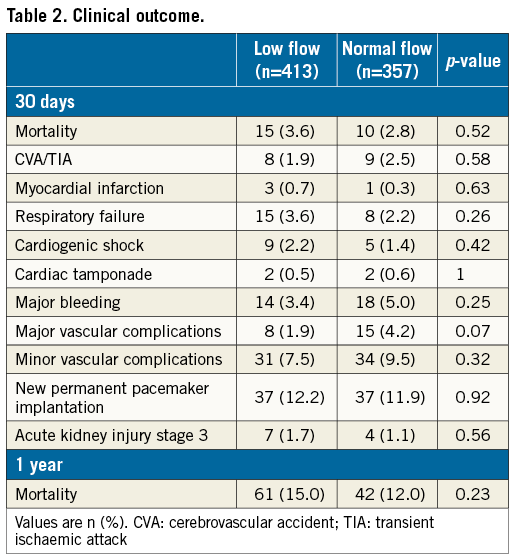
Overall, the transfemoral approach was used in 86.4% of the cases, transapical approach in 5.3%, transaortic approach in 7.0% and subclavian approach in 1.0%. All patients had either Edwards SAPIEN/SAPIEN XT/SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) or Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA) implantation. Device success rate was 95.4% (394 of 413) for patients with LF compared with 95.2% (340 of 357) for patients with NF (p=0.92). There were similar rates of post-procedural paravalvular regurgitation in both groups. Thirty-day complications are elaborated in Table 2. All complication rates were similar between groups.
Patient distribution is shown in Figure 2A. The percentage of males was significantly higher in all three LF subgroups compared to the NF-HG group. Patients with LF-LG-LEF had a significantly higher prevalence of diabetes mellitus, coronary artery disease, and atrial fibrillation compared to patients with NF-HG. Patients with LF-LG had significantly lower aortic valve calcification scores compared to patients with NF-HG and LF-HG.
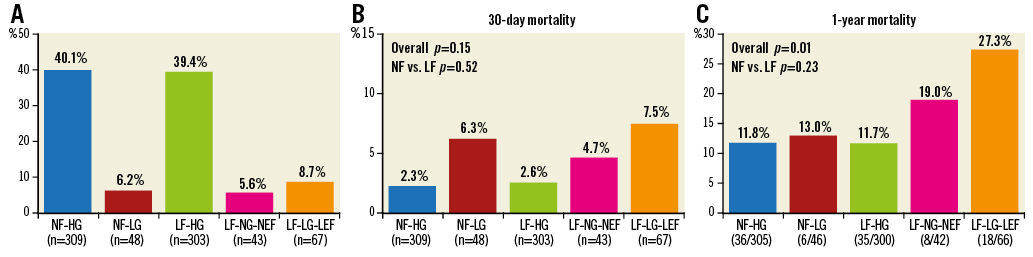
Figure 2. Patient distribution and mortality following TAVR according to flow, gradient, and ejection fraction status. A) Patient distribution according to flow (NF ≥35 ml/m2; LF <35 ml/m2), aortic valve gradient (HG ≥40 mmHg; LG <40 mmHg) and left ventricular ejection fraction (NEF ≥50%; LEF <50%). B) Incidence of 30-day mortality according to flow, gradient, and ejection fraction status. C) Incidence of one-year mortality according to flow, gradient, and ejection fraction status. LF-HG: low flow and high gradient; LF-LG-LEF: low flow, low gradient, and low ejection fraction; LF-LG-NEF: low flow, low gradient, and normal ejection fraction; NF-HG: normal flow and high gradient; NF-LG: normal flow and low gradient
IMPACT OF FLOW, LVEF, AND GRADIENT ON 30-DAY AND ONE-YEAR MORTALITY
Overall, there were 25 deaths at 30 days (3.2%) (Figure 2B). Patients with NF had similar 30-day mortality compared with those in the LF group (10 of 357 [2.8%] vs. 15 of 413 [3.6%], respectively, p=0.52). Among patients with LF-LG, 30-day mortality was not statistically different (p=0.56) between those with NEF (4.7%) and those with LEF (7.5%). One-year mortality follow-up data were available for 759/770 patients (98.6%). Overall, there were 103 deaths at one year (13.6%) (Figure 2C). Patients with NF had similar one-year mortality compared with those in the LF group (42 of 351 [12.0%] vs. 61 of 408 [15.0%], respectively, p=0.23). Patients with LF-LG had higher one-year mortality (24.1%) compared to the other groups (p=0.001). Among patients with LF-LG, one-year mortality was not statistically different (p=0.33) between those with NEF (19.0%) and those with LEF (27.3%).
IMPACT OF FLOW, LVEF, AND GRADIENT ON CUMULATIVE MORTALITY
Overall, there were 209 deaths during a mean follow-up of 21.4±11.2 months and 85 were of cardiovascular cause. Patients with LF had increased all-cause mortality (hazard ratio [HR]: 1.42, 95% confidence interval [CI]: 1.07-1.87; p=0.01) and cardiovascular mortality (HR: 1.85, 95% CI: 1.18-2.90; p=0.01) compared with those with NF (Figure 3). Mortality was also increased in patients with low EF compared with those with normal EF (all-cause mortality HR: 1.75, 95% CI: 1.31-2.33, p<0.001; cardiovascular mortality HR: 3.09, 95% CI: 2.01-4.75, p<0.001) and in patients with LG compared with those with HG (all-cause mortality HR: 1.75, 95% CI: 1.30-2.37, p<0.001; cardiovascular mortality HR: 2.53, 95% CI: 1.63-3.94, p<0.001).
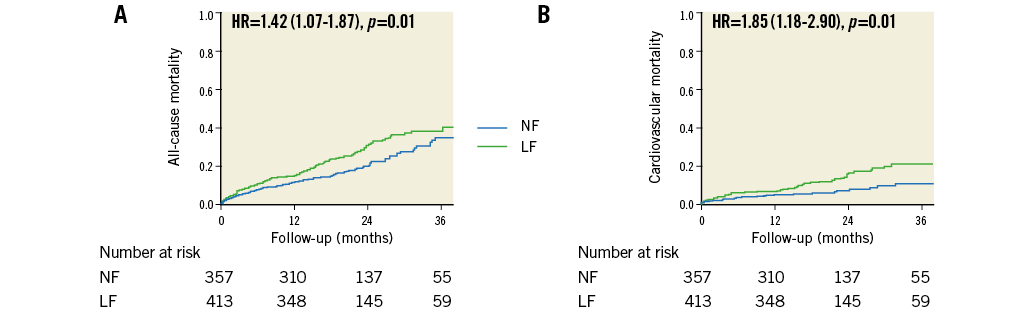
Figure 3. Kaplan-Meier curves following TAVR according to stroke volume indexed to body surface area. Curves for all-cause mortality (A) and cardiovascular mortality (B). LF: low flow; NF: normal flow
Patients in the NF-HG, NF-LG, and LF-HG groups had similar outcomes (all-cause mortality p=0.49; cardiovascular mortality p=0.11), whereas all-cause mortality was significantly higher in the LF-LG group compared with the NF-HG (HR: 2.31, 95% CI: 1.59-3.36, p<0.001), and the LF-HG (HR: 1.89, 95% CI: 1.32-2.72, p=0.01) groups (Figure 4). All-cause mortality was borderline significantly higher in the LF-LG group compared with the NF-LG group (HR: 1.79, 95% CI: 0.96-3.31, p=0.066). Cardiovascular mortality was also increased in the LF-LG group compared with the NF-HG (HR: 3.92, 95% CI: 2.18-7.05, p<0.001) and the LF-HG (HR: 2.40, 95% CI: 1.42-4.07, p=0.001) groups. After further dichotomisation of the LF-LG group according to LVEF, no significant difference was observed between LF-LG-NEF and LF-LG-LEF subgroups with respect to all-cause mortality (p=0.16), but there was increased cardiovascular mortality in patients with classical LF-LG-LEF compared to patients with paradoxical LF-LG-NEF (p=0.04) (Figure 5).
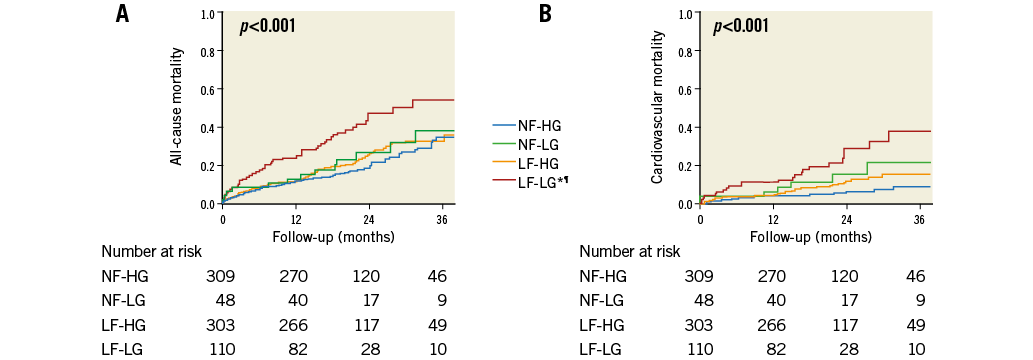
Figure 4. Kaplan-Meier curves for the four groups of patients separated according to flow and gradient levels. Curves for all-cause mortality (A) and cardiovascular mortality (B). *p<0.05 vs. NF-HG. ¶p<0.05 vs. LF-HG. LF-HG: low flow and high gradient; LF-LG: low flow, low gradient; NF-HG: normal flow and high gradient; NF-LG: normal flow and low gradient
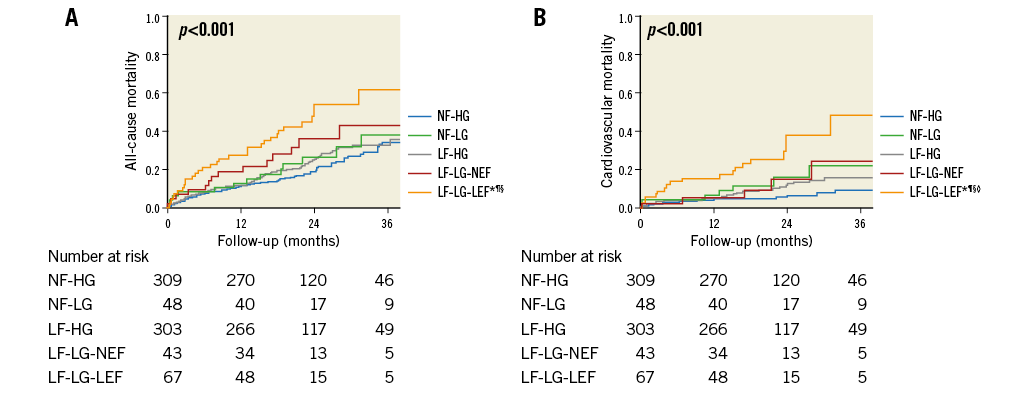
Figure 5. Kaplan-Meier curve analysis after further dichotomisation of the LF-LG group according to left ventricular ejection fraction. Curves for all-cause mortality (A) and cardiovascular mortality (B). *p<0.05 vs. NF-HG. ¶p<0.05 vs. LF-HG. §p<0.05 vs. NF-LG. ◊p<0.05 vs. LF-LG-NEF. LF-HG: low flow and high gradient; LF-LG-LEF: low flow, low gradient, and low ejection fraction; LF-LG-NEF: low flow, low gradient, and normal ejection fraction; NF-HG: normal flow and high gradient; NF-LG: normal flow and low gradient
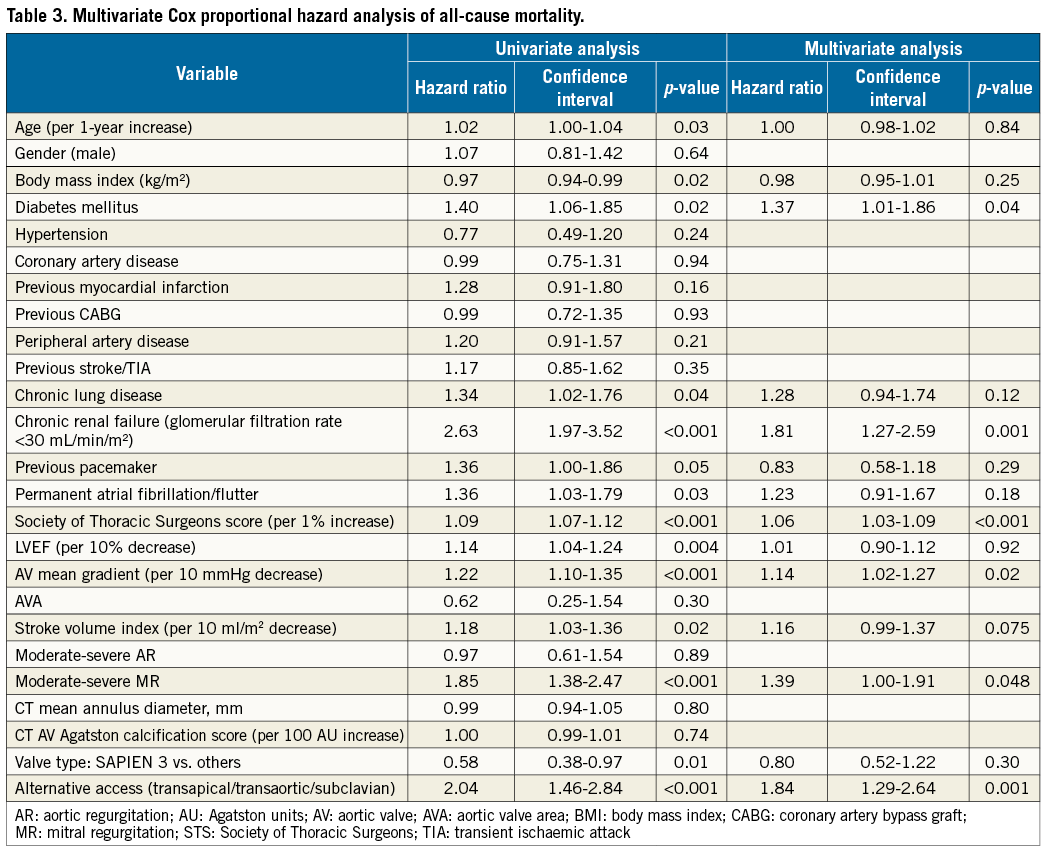
Predictors of all-cause mortality are shown in Table 3. Although in a univariable analysis SVi was found to be a significant predictor of both all-cause and cardiovascular mortality, in a multivariable analysis it was not an independent predictor of both all-cause mortality (HR: 1.16, 95% CI: 0.99-1.37 per 10 ml/m2 decrease, p=0.075) and cardiovascular mortality (HR: 1.21, 95% CI: 0.93-1.58 per 10 ml/m² decrease, p=0.16). Aortic valve mean gradient was an independent predictor of both all-cause mortality (HR: 1.14, 95% CI: 1.02 to 1.27 per 10 mmHg decrease, p=0.02) and cardiovascular mortality (HR: 1.29, 95% CI: 1.07 to 1.54 per 10 mmHg decrease, p=0.001) in multivariable analyses. Although it was a significant mortality predictor in univariable analysis, LVEF was not an independent predictor in multivariable analysis (Table 3). In a similar ancillary multivariable analysis including the five different flow/gradient/EF groups, only LF-LG-LEF was independently significantly associated with increased overall mortality compared to NF-HG (HR: 2.08, 95% CI: 1.32-3.27, p=0.002). LF-LG-NEF was associated with a trend for increased mortality in this model (HR: 1.69, 95% CI: 0.94-3.02, p=0.077).
Discussion
The major findings of this study are as follows. 1) More than half of the patients who had TAVR were in an LF state and about 20% had LG prior to the procedure. 2) Univariable analysis found LF, LEF and LG to be predictors of overall and cardiovascular mortality, but in a multivariable model only LG remained an independent predictor of mortality. 3) Patients with LF-LG had increased mortality following TAVR compared with those with NF-HG, NF-LG, and LF-HG. 4) Among patients with LF-LG, those with LEF (classical LF-LG) had increased cardiovascular mortality compared to those with NEF (paradoxical LF-LG AS).
Standard parameters of AS severity, including AVA and transvalvular gradients, vary with flow16. Patients with AS are subdivided by these parameters combined with their LVEF. These subgroups were found to have different outcomes in the natural course of the disease and/or following therapeutic interventions1,6,17,18. Interestingly, most of the studies that have examined directly the effect of LVEF or transvalvular gradients on the outcome following TAVR did not include stroke volume in their analysis9-11. Two previous studies analysed the impact of LF, LG and LEF on mortality following TAVR12,13. Both of these studies reported that LF and LG are significantly associated with mortality in a univariable analysis, but only reduced SVi remained statistically significant in a multivariable model. The results of the present study are different from these two studies. In the present analysis, LG was found to be an independent predictor of overall and cardiovascular mortality but LF and LEF only predicted mortality in a univariable model.
A possible explanation for the discrepancies in the results of the present study and the previous two studies presented above may derive from a different time period in which TAVR was performed. While the previous studies reported outcome of patients who underwent TAVR between 2005 and the beginning of 201212,13, all patients in the present study underwent TAVR between 2012 and 2014. Advances in technology providing new generations of devices with reduced profile sizes and improved navigability, utilisation of CT for better annular sizing and increased operator and anaesthesiologist experience have reduced short-term mortality dramatically. While Le Ven et al12 reported increased 30-day mortality in patients with LF vs. NF (11.4 vs. 5.9%, p=0.01), we found a substantially lower 30-day mortality, similar between LF and NF patients (3.6% and 2.8%, respectively, p=0.52). This low mortality rate is compatible with recent contemporary short-term mortality reports19. With the marked reduction in overall periprocedural mortality and morbidity with TAVR during the past 10 years, the association between LF and mortality, and especially short-term mortality, may have become less important in the most recent series, including the present one.
Study limitations
The main limitation of the present study is that it represents a retrospective, single-centre experience. We analysed stroke volume using the Doppler-derived two-dimensional LVOT diameter and VTI without confirmation of invasive haemodynamic measurements. The definition of LF is not standardised in the literature, but we chose a definition (SVi ≤35 mL/m2) that has been commonly used1,10,12,13. Moreover, patients with LF had more comorbidities and higher STS scores at baseline which, despite multivariable analysis, may have biased the results. Future studies with a larger number of patients, longer follow-up and the use of different valve types may further clarify this subject.
Conclusions
LF is a common finding in patients with severe AS. Patients with NF-LG have excellent outcomes following TAVR, something which is similar to those of patients with HG AS, whereas those with LF-LG AS have an increased risk of mortality. Among patients with LF-LG, those with LEF (classical LF-LG) have increased mortality compared to those with NEF (paradoxical LF-LG AS). In contrast to previous reports, LG but not LF or LEF is an independent predictor of late mortality in high-risk patients with severe AS undergoing TAVR.
| Impact on daily practice Patients with NF-LG have excellent outcomes following TAVR, similar to those of patients with HG AS, whereas those with LF-LG AS have an increased risk of mortality. In contrast to previous reports, the present study demonstrated that LG, but not LF or LEF, is an independent predictor of late mortality following TAVR. Future studies should evaluate whether earlier intervention is indicated to reduce the adverse effects of LG and/or LF. |
Funding
The study was funded by internal departmental resources.
Conflict of interest statement
R. Makkar has received grant support from Edwards Lifesciences and St. Jude Medical and is a consultant for Abbott Vascular, Cordis, and Medtronic. P. Pibarot has received a research grant from Edwards Lifesciences for echocardiography core lab analyses. The other authors have no conflicts of interest to declare.

