Abstract
Aims: The study analyses the outcome of patients undergoing transcatheter aortic valve implantation (TAVI) for different subtypes of severe aortic stenosis (AS) based on data from the GARY registry.
Methods and results: Low-EF, low-gradient (LEF-LGAS: EF ≤40%, MPG <40 mmHg), paradoxical low-gradient (PLF-LGAS: EF ≥50%, MPG <40 mmHg) and high-gradient AS (HGAS: MPG ≥40 mmHg) were observed in 11.7% (n=359), 20.8% (n=640) and 60.6% (n=1,864) of the study population, respectively. EuroSCORE I (36.7±20.9 vs. 22.6±15.7 vs. 24.3±17.4; p<0.001) differed significantly among subgroups. In-hospital and one-year mortality were higher in patients with LEF-LGAS compared to HGAS (in-hospital: 7.8% vs. 4.9%; p=0.029; one-year: 32.3% vs. 19.8%; p=0.001). In contrast, mortality in patients with PLF-LGAS was comparable to patients with HGAS (in-hospital: PLF-LGAS: 5.3%; p=0.67; one-year: 22.3%; p=0.192). The rate of TAVI-associated complications was not significantly different among groups. However, postoperative low cardiac output occurred significantly more frequently in patients with LEF-LGAS.
Conclusions: Severe AS with a reduced transaortic flow and gradient is a common finding and is present in >30% of patients undergoing TAVI. Patients with low flow and impaired LV function have a significantly higher mortality within the first year after TAVI. In contrast, the outcome of patients with low flow and preserved EF is comparable to those with a high transvalvular aortic gradient.
Introduction
Based on the current European and American guidelines, severe aortic stenosis (AS) is defined as an effective aortic valve area (AVA) of <1 cm2 (or indexed for body surface area [BSA], AVA/BSA <0.6 cm2/m2) and a mean pressure gradient (MPG) and peak velocity (vmax) of >40 mmHg and 4.0 m/s, respectively1,2. Transcatheter aortic valve implantation (TAVI) is the treatment of choice for patients with symptomatic severe AS and a high or prohibitive surgical risk3-6.
However, in clinical practice there is frequently an inconsistency in diagnostic criteria in patients in whom AS appears to be severe based on AVA (<1 cm2 or <0.6 cm2/m) but moderate or even mild based on transvalvular gradients7-11. This inconsistency is caused by a reduced left ventricular (LV) stroke volume, which leads to a reduction of transaortic flow and gradient. This occurs in patients with severe AS and an ejection fraction (EF) ≤40% (low-EF, low-gradient AS: LEF-LGAS), a subgroup which has a particularly poor prognosis with medical treatment and an increased mortality when undergoing surgical aortic valve replacement or TAVI12-14.
Recently, a second type of low-gradient AS has been reported with low-flow conditions caused by a decreased stroke volume due to a small LV cavity size and a restrictive physiology11. This phenomenon has been described as “paradoxical” low-flow, low-gradient AS (PLF-LGAS), as it is observed in patients with severe AS despite an EF ≥50%8,15-17. The optimal treatment and prognostic impact of PLF-LGAS on early and long-term survival after TAVI are still matters of debate. Therefore, the purpose of this study was to compare the outcome of TAVI in these distinct subgroups of AS based on data from the German Aortic Valve Registry (GARY)18-20.
Methods
REGISTRY DESIGN AND SUBGROUPS
GARY is a prospective, multicentre registry designed to monitor the efficacy and outcome of interventional and surgical aortic valve procedures in Germany. The registry design has been described previously20,21. In brief, data at 78 tertiary cardiovascular centres were collected using standardised case report forms to record demographic and clinical characteristics as well as procedural and follow-up data. The present analysis focuses on data acquired in 2011. Follow-up was obtained at 30 days and at one year based on the medical records and on physician and patient interviews. The investigators had full access to the data and control of the analysis. Ethics approval was obtained from all participating centres, and patients’ written, informed consent was obtained preoperatively.
All patients undergoing aortic valve procedures were eligible for inclusion. Out of 13,860 consecutive patients enrolled in 2011, 3,908 underwent catheter-based aortic valve implantation. At the participating institutions, the decision to perform TAVI was made by a Heart Team in 87.4% of the cases based upon the established criteria2,22. For the present analysis, patients were assigned to one of three subgroups depending on left ventricular ejection fraction (LVEF), mean transvalvular aortic gradient (MPG), and aortic valve area indexed for body surface area (AVA/BSA):
(I) Group LEF-LGAS: EF ≤40%, MPG <40 mmHg, AVA/BSA ≤0.6 cm2/m2 (low-flow, low-gradient AS).
(II) Group PLF-LGAS: EF ≥50%, MPG <40 mmHg, AVA/BSA ≤0.6 cm2/m2 (“paradoxical” low-flow, low-gradient AS).
(III) Group HGAS: MPG ≥40 mmHg, AVA/BSA ≤0.6 cm2/m2 (high-gradient AS).
DEVICES
Patients undergoing TAVI with all commercially available devices were eligible for inclusion. During the period of enrolment, the following devices were commercially available in Germany: the balloon-expandable Edwards SAPIEN XT, a cobalt-chromium stent (sizes 23 mm, 26 mm, and 29 mm) (Edwards Lifesciences, Irvine, CA, USA), and the Medtronic CoreValve™ (sizes 26 mm, 29 mm, and 31 mm) (Medtronic, Minneapolis, MN, USA), a porcine pericardial tissue valve in a self-expanding nitinol stent frame. For implantation of the balloon-expandable device, the Ascendra delivery system (Edwards Lifesciences) was used for transapical access and the RetroFlex delivery system or, more recently, the NovaFlex delivery system (both Edwards Lifesciences) were used for transfemoral access.
The JenaValve™ (sizes 23 mm, 25 mm, and 27 mm) (JenaValve Technology GmbH, Munich, Germany), a natural porcine aortic root mounted on a nitinol stent, and the Symetis ACURATE™ valve (sizes small, medium, and large) (Symetis SA, Ecublens, Switzerland), a porcine pericardial valve mounted on a nitinol frame, were available as transapical devices.
ENDPOINTS
Major clinical endpoints were defined and analysed as reported previously21. The primary endpoint of the present analysis was all-cause mortality at hospital discharge and at one year. Mortality at both time points was further subdivided into cardiovascular and non-cardiovascular mortality as reported in the VARC definitions22. Further endpoints evaluated procedural characteristics (procedural success, device type and access route, device function) as well as the rate of adverse events (postoperative low cardiac output [defined as a CI <2.2 l/min/m2], myocardial infarction, stroke, acute kidney injury, bleeding and vascular complications, requirement for permanent pacemaker)21,22. After device implantation, the degree of aortic regurgitation was classified on a graded scale from 0 to 4, with a higher grade indicating greater severity (grade 0=none; grade I=trace, grade II=mild; grade III=moderate, grade IV=severe).
CALCULATION OF RISK SCORES
The baseline operative risk for cardiovascular surgery was estimated using the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE system), which is calculated by a logistic regression equation (on a scale from 0 to 100%, with higher scores indicating greater risk and a score of more than 20% indicating very high surgical risk). In addition, the German Aortic Valve (German AV) Score, which is based on aortic valve procedures, was calculated by a logistic regression, with higher scores indicating greater risk23. The German AV score is calculated from 15 variables (further details: http://www.bqs-outcome.de/2008/ergebnisse/leistungsbereiche/htc).
STATISTICS
Categorical variables are presented as percentages and values, and continuous data are expressed as mean±standard deviation (SD). The comparison of baseline values among the subgroups was performed by the Kruskal-Wallis test (any differences) or Mann-Whitney U test (pairwise) for continuous variables. Categorical variables were compared by means of the χ2 test and the Fisher’s exact test where applicable. Statistical significance was tested two-sided with the alpha level of 5%. Pairwise results were corrected with the Bonferroni–Holm–Shaffer procedure for multiple comparison.
The vital status of patients already discharged or transferred to a rehabilitation programme was verified by follow-up calls. Survival curves were constructed for time-to-event variables using Kaplan-Meier estimates and compared by the log-rank test.
Backward and forward stepwise logistic regression analysis was performed to identify independent predictors of in-hospital mortality in the overall TAVI population and separately in the three groups. A covariate (LEF-LGAS, PLF-LGAS, HGAS, age >80 years, frailty, diabetes, COPD, CAD-3, pulmonary hypertension, peripheral vascular disease, previous cardiac operations, myocardial infarction, and LVEF ≤30%) was removed from the model if the p-value exceeded 0.10. All p-values <0.05 were considered statistically significant. All statistical analyses were performed using the SPSS statistical package version 19.0.0 (IBM Corp., Armonk, NY, USA). Data management and statistical analyses were performed by the BQS Institute for Quality and Patient Safety, Dusseldorf, Germany.
Results
PATIENT POPULATION
The present study focuses on 3,908 TAVI patients included in the registry between January 1 and December 31, 2011. Complete datasets were available for 3,077 patients which were analysed in this study. Patient demographics and baseline characteristics are detailed in Table 1. In the study population, PLF-LGAS and LEF-LGAS were present in 640 (20.8%) and 359 (11.7%) patients, respectively (Figure 1); 1,864 (60.6%) patients presented with HGAS. Patients with PLF-LGAS were slightly younger than patients with HGAS (80.5±5.6 vs. 81.4±6.1; p<0.001) and older than patients with LEF-LGAS (80.5±5.6 vs. 79.1±6.1; p=0.001). Patients with PLF-LGAS presented with a similar rate of comorbidities compared with patients in the HGAS subgroup. In contrast, the LEF-LGAS subgroup generally had a higher prevalence of comorbidities such as coronary artery disease, pulmonary hypertension, mitral regurgitation, and previous cardiac surgery. This is also reflected by a significantly higher logistic EuroSCORE in the LEF-LGAS group compared to patients with PLF-LGAS and HGAS (36.7±20.1 vs. 22.6±15.7 vs. 24.3±17.4, respectively; p<0.0001).
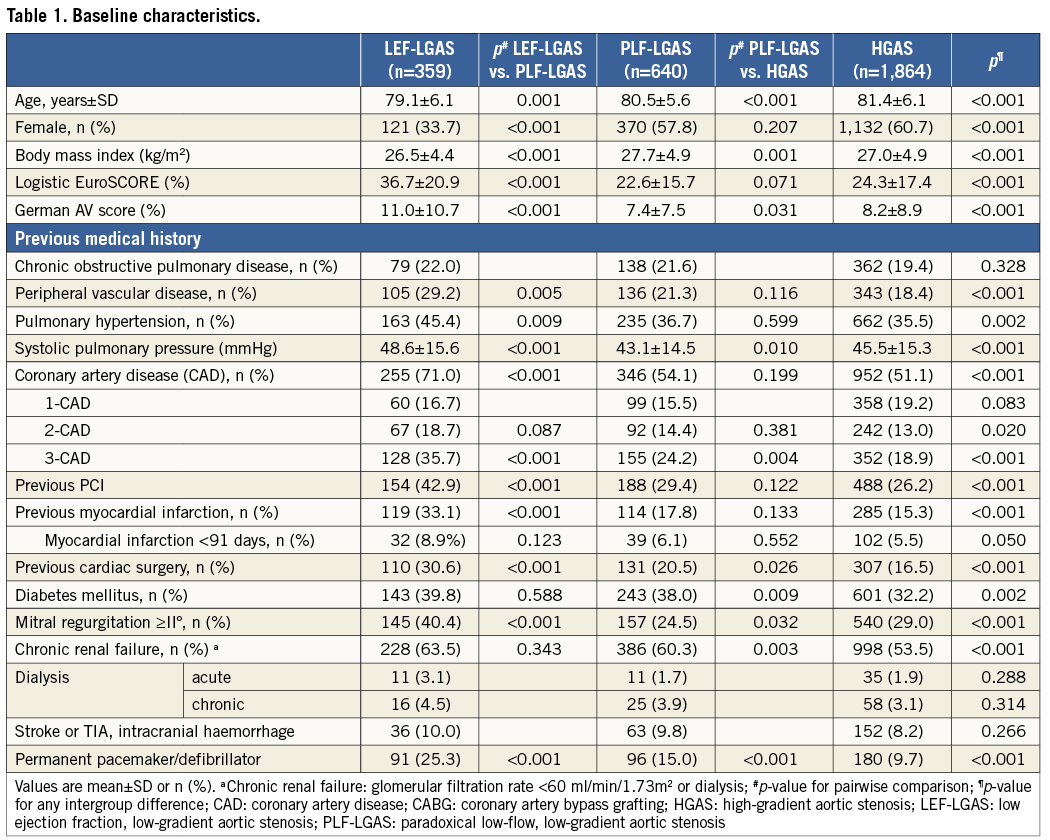
Figure 1. Patient population. AVA: aortic valve area; HGAS: high-gradient severe aortic stenosis; LEF-LGAS: low-EF, low-gradient severe aortic stenosis; PLF-LGAS: “paradoxical” low-flow, low-gradient severe aortic stenosis; TAVI: transcatheter aortic valve implantation
INTERVENTIONAL CHARACTERISTICS
Preoperative haemodynamics and characteristics of the TAVI procedure are presented in Table 2. Prior to device implantation the aortic valve area was severely reduced in all subgroups (PLF-LGAS: 0.73±0.17 vs. LEF-LGAS: 0.70±0.17 vs. HGAS: 0.63±0.17 cm2; p<0.001). As per definition, the mean transvalvular gradient differed significantly among the three groups: PLF-LGAS 30.7±6.5 mmHg vs. LEF-LGAS 26.5±7.3 mmHg vs. HGAS 55.5±13.8 mmHg (p<0.001). There were no differences among the three groups in the access route used. The majority of procedures were performed transfemorally for all subgroups (PLF-LGAS: 68.4% vs. LEF-LGAS: 68.5% vs. HGAS: 71.1%; p=0.343). In patients with LEF-LGAS, self-expandable devices were used significantly more often as compared to patients with PLF-LGAS. The majority of interventions were performed as elective procedures. The rate of TAVI procedures classified as “urgent/emergent” was significantly higher in patients with LEF-LGAS (LEF-LGAS 24.0% vs. PLF-LGAS: 14.5% vs. HGAS: 20.9%; p<0.001). A procedure was classified as urgent when the patient was haemodynamically stable but medical management was not sufficient for symptom control and timely intervention was required.
RATES OF DEATH AND PREDICTORS OF EARLY MORTALITY AFTER TAVI
While in-hospital mortality was higher in patients with LEF-LGAS compared with HGAS (7.8% vs. 4.9%, p=0.029), no significant difference was observed between patients with HGAS and PLF-LGAS (4.9% vs. 5.3%; p=0.67). After one year, the mortality difference was even more pronounced for patients with LEF-LGAS (32.3%) compared with the other two subgroups (PLF-LGAS: 22.3%; p=0.001; HGAS: 19.8%; p<0.001). In contrast, no significant difference in one-year mortality was observed between the PLF-LGAS and HGAS subgroups (p=0.192).
Likewise, the rate of major adverse cardiac and cerebral events (MACCE) at one year (death, MI, and stroke) was significantly higher for patients with LEF-LGAS (LEF-LGAS: 34.5% vs. PLF-LGAS: 27.5; p=0.021 vs. HGAS: 23.8%; p<0.001) (Table 3, Figure 2).
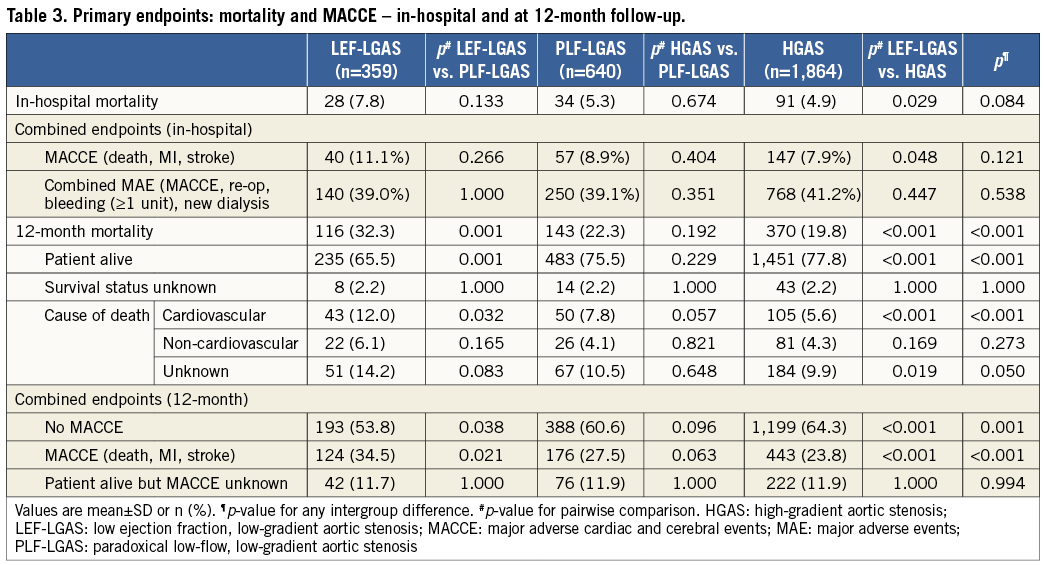
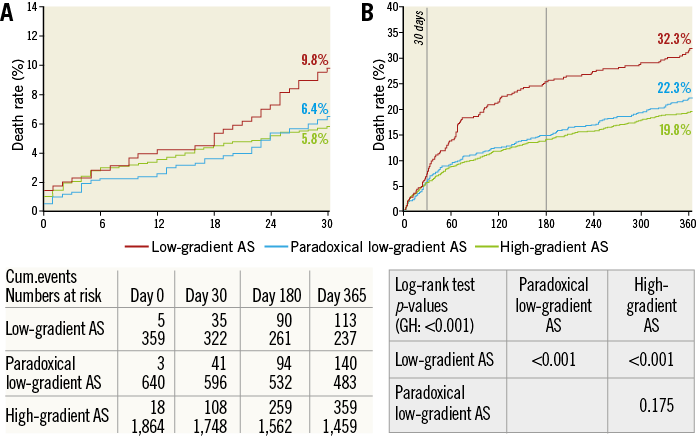
Figure 2. Survival at 30 days and one year after TAVI in low-flow, low-gradient aortic stenosis with preserved and reduced ejection fraction vs. high-gradient aortic stenosis. Kaplan-Meier survival curves for patients with severe AS after TAVI stratified by transvalvular gradient. A) 30-day-mortality and B) one-year mortality curves and numbers at risk after transcatheter aortic valve replacement stratified for patients with low EF-low-gradient (marked red), paradoxical low-gradient (marked blue) and high-gradient aortic stenosis (marked green). In patients with low-gradient aortic stenosis, 30-day mortality is moderately, albeit significantly, higher compared with patients with high-gradient aortic stenosis (log-rank test: p=0.0194).
PREDICTORS OF IN-HOSPITAL MORTALITY
Logistic regression analysis of the overall TAVI population for the three groups was performed to assess the predictive value of the subtypes of AS (LEF-LGAS, PLF-LGAS, and HGAS), age >76 years, frailty, diabetes, chronic obstructive pulmonary disease (COPD), three-vessel coronary artery disease (CAD-3), pulmonary hypertension, peripheral vascular disease, previous cardiac operations, myocardial infarction, and LVEF ≤30% for in-hospital mortality. After stepwise analysis, CAD-3, pulmonary hypertension and peripheral vascular disease remained in the model as an independent predictor for in-hospital mortality in the overall TAVI population. No predictive value of the different subtypes of AS for 30-day mortality was observed (LEF-LGAS vs. PLF-LGAS: OR 0.926, p=0.8; LEF-LGAS vs. HGAS: OR 0.898, p=0.69) in the overall study population.
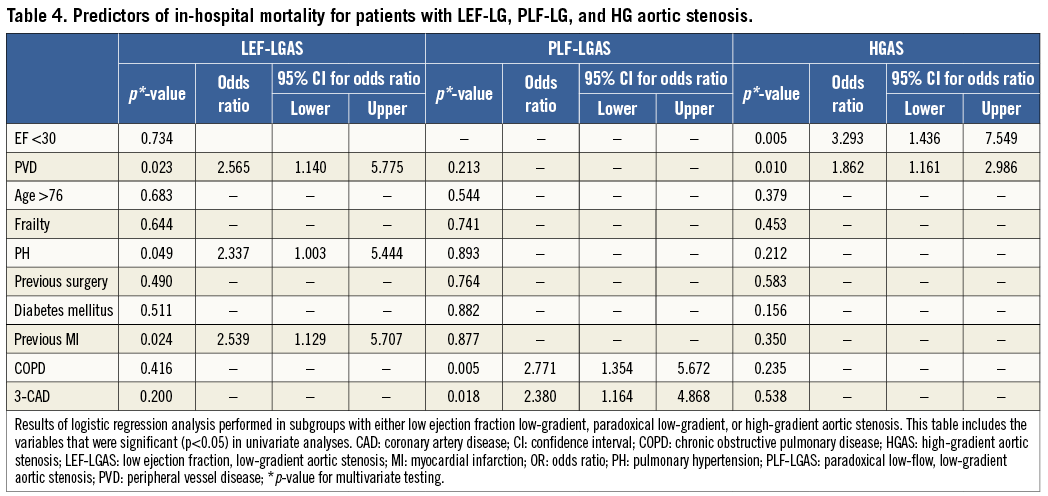
Multiple logistic regression analysis performed for patients with LEF-LGAS identified pulmonary hypertension (OR 2.337, CI: 1.003-5.444, p=0.049), PVD (OR 2.565, CI: 1.140-5.775; p=0.023) and previous myocardial infarction (OR 2.539; CI: 1.129-5.707; p=0.024) as significant predictors of in-hospital mortality. In patients presenting with PLF-LGAS, the presence of three-vessel CAD (OR 2.38, CI: 1.164-4.868; p=0.018) and COPD (OR 2.771, CI: 1.354-5.672, p=0.005) were significant predictors of in-hospital mortality. Among patients with HGAS, PVD (OR 1.862, CI: 1.161-2.986; p=0.010) and EF <30% (OR 3.293, CI: 1.436-7.549, p=0.005) were identified as significant predictors of early mortality (Table 4).
Discussion
Low-flow, low-gradient AS with preserved or reduced ejection fraction is a common finding in patients with aortic stenosis. In GARY, PLF-LGAS is observed in 20.8% of patients and is even more frequent than LEF-LGAS (11.7%). Both subgroups together account for one quarter of all patients with AS selected for TAVI.
The three subgroups of AS show clear differences in demographics, gender distribution, and comorbidities. While LEF-LGAS predominantly occurs in male patients, PLF-LGAS and HGAS are slightly more common in female patients (Table 1). Furthermore, patients with LEF-LGAS present with a higher rate of comorbidities such as CAD, COPD, and peripheral vascular disease, whereas the frequency of comorbidities is comparable between patients with PLF-LGAS and HGAS (Table 1). Patients with HGAS have a significantly smaller valve area compared with patients with PLF-LGAS, a finding that has previously been reported by O’Sullivan et al19. Although the clinical implications of this finding are not yet clear, we have confirmed it here in a larger patient population.
OUTCOME OF PARADOXICAL LOW-GRADIENT VERSUS HIGH-GRADIENT AS
The impact of low transvalvular flow and gradient in patients with severe aortic stenosis on outcome is a matter of debate and published data so far have shown conflicting results14,19. As a main finding of the present study, patients with PLF-LGAS and HGAS have a comparable overall in-hospital (5.3% vs. 4.9%, p=0.672) and one-year mortality (22.3% vs. 19.8%, p=0.192) after TAVI. The rate of MACCE was not significantly different between these two subgroups at hospital discharge (8.9% vs. 7.9%, p=0.4) and at one year (27.5% vs. 23.08%; p=0.063) after TAVI. These results are in line with data from the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial, which reported comparable outcomes in patients with low flow and preserved ejection fraction versus normal flow24. As in the GARY registry, treatment decisions in the SEAS trial were not randomised but based on clinical practice.
However, our observations also contradict some of the findings of earlier studies. In a retrospective analysis of the Placement of Aortic Transcatheter Valves (PARTNER) trial, Herrmann et al observed a significantly higher one-year mortality in patients with a low transvalvular flow (47% versus 34%; hazard ratio 1.5; 95% CI: 1.25-1.89; p=0.006), which is generally defined as a stroke volume index ≤35 ml/m218. In clinical practice, the flow decrease may be caused by a high valvuloarterial impedance and a restrictive filling pattern (as in PLF-LGAS) or by impaired LV contractility (as in LEF-LGAS). In the study by Herrmann et al, a “low-flow” condition was identified as a powerful predictor of early mortality, thus suggesting that a low forward LV output is a more important determinant of outcome than the mechanism of flow decrease18. Thus, in the study by Herrmann et al, the higher mortality among patients with low-flow AS is potentially due to the inclusion of patients with impaired systolic LV function, a subgroup which in clinical practice presents with a higher rate of comorbidities. Another recent study by Le Ven et al confirmed a higher mortality in patients with aortic stenosis and low-flow transvalvular flow without observing an impact of LV systolic dysfunction on mortality in multivariate analysis25. This finding may have been influenced by the smaller number of patients with reduced LV function as well as the comparatively long period of retrospective patient inclusion and the retrospective nature of the study.
Our finding of a comparable outcome between PLF-LGAS and HGAS on the one hand and a significantly higher mortality of LEF-LGAS on the other suggests that the main cause of the increased mortality after TAVI is not decreased flow itself, but rather the reduced ejection fraction as a mechanism for reduced flow and the associated comorbidities of the LEF-LGAS subgroup. This is further supported by the results of the multiple regression analysis, which identified an EF <30% as a predictor of in-hospital mortality among patients with HGAS. Although flow data are not available in the GARY population and PLF-LGAS was defined based on AVA/BSA and MPG, a paradoxical low transvalvular gradient in the setting of severe AS is inevitably the result of a low transvalvular flow.
The subgroup differences in mortality between GARY and PARTNER may be further explained by the study design. While the PARTNER trial was conducted in a highly selected study population and included only balloon-expandable valves, the present analysis reflects the results of TAVI in an unselected, all-comers patient population with paradoxical low-gradient AS and includes all commercially available TAVI devices.
OUTCOME OF LOW-FLOW, LOW-GRADIENT AS
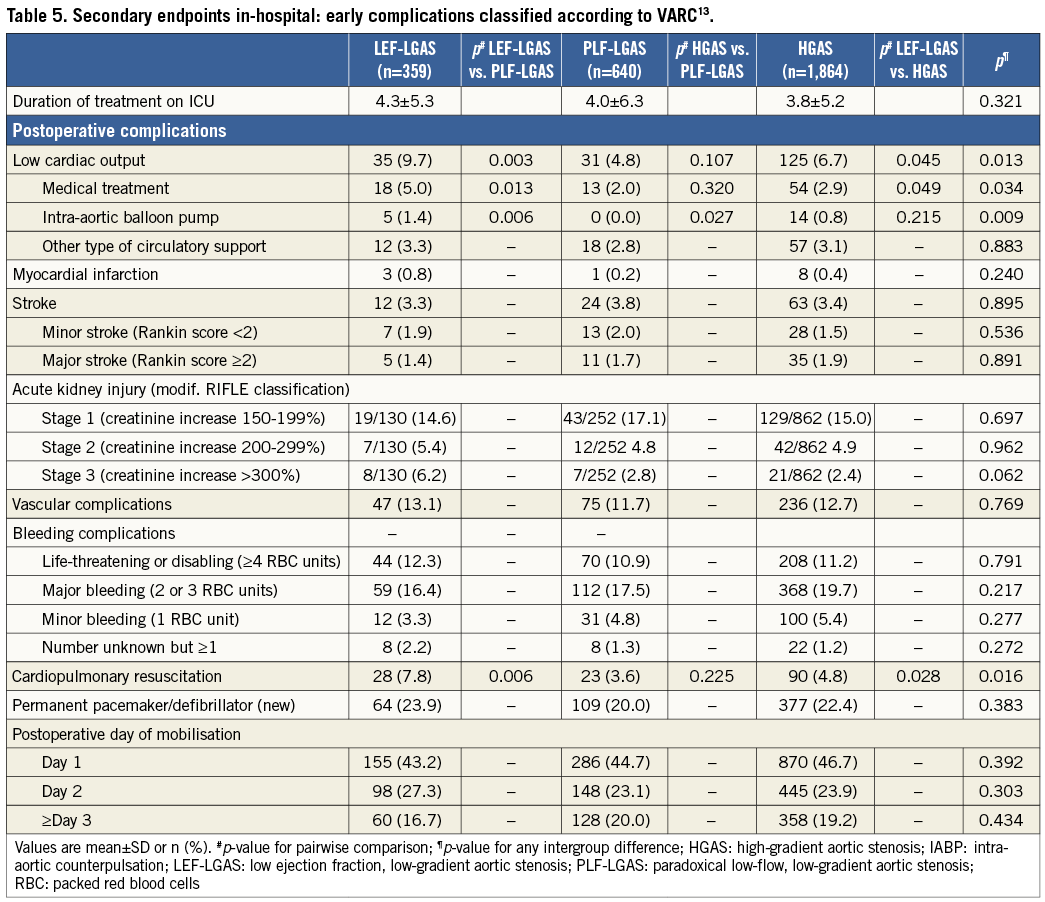
Left ventricular dysfunction is associated with adverse outcomes after surgical aortic valve replacement, but little is known about the impact of LV dysfunction on outcomes after TAVI13,14,26-28. This is confirmed by the GARY data where one-year mortality of LEF-LGAS is significantly higher compared with that of the other AS subtypes (LEF-LGAS: 32.3% vs. PLF-LGAS: 22.3% vs. HGAS: 19.8%; p=0.001). This difference is in part attributable to a higher rate of cardiovascular deaths among patients with low flow and LV dysfunction (LEF-LGAS: 12% vs. PLF-LGAS 7.8% vs. HGAS 5.6%; p<0.001). Furthermore, patients with LEF-LGAS had a higher rate of low cardiac output syndrome and more frequently required cardiopulmonary resuscitation or mechanical circulatory support during the post-interventional period than their counterparts in the other subgroups (Table 5).
The current results confirm a substantial improvement in the early outcome after TAVI among patients with LEF-LGAS compared with earlier, non-randomised data from the German TAVI registry for the period 2009-2010. In this registry, in-hospital and one-year mortalities of 16.1% and 36.9%, respectively, were reported in this subgroup14,29. The current data show a more than 50% decrease in in-hospital mortality (to 7.8%) despite a higher EuroSCORE in the GARY subgroup (GARY: 36.7±20.9 vs. German TAVI registry: 26.8±9.7)14. This improvement in outcome can most likely be attributed to factors such as the increase in operator experience, the downsizing of TAVI devices, and a learning curve in patient selection. However, one-year mortality in the LEF-LGAS subgroup improved only slightly, from 36.9% in the German TAVI registry to 32.3% in the GARY registry. This observation reflects the severity of the underlying cardiac disease in this difficult subgroup which can be only partially reversed by aortic valve replacement and ultimately results in an increased long-term mortality.
Limitations
The present paper reports the largest non-randomised series of patients undergoing TAVI for the different subtypes of AS reported to date. However, as it is based on registry data, several limitations apply. Firstly, only data on AVA, MPG, and EF but not transaortic flow are available for further classification of AS. In patients with both an indexed valve area of ≤0.6 cm2/m2 and an MPG of <40 mmHg, the presence of a reduced transvalvular flow was assumed, and they were therefore allocated to the PLF-LGAS and the LEF-LGAS subgroups. Secondly, particularly for patients with LEF-LGAS, dobutamine stress echocardiography (DES) would be required to exclude patients with pseudostenosis from valve replacement. Since the GARY registry is a nationwide registry of data from cardiology and surgical units, it focuses on a limited range of variables excluding the results of DES. In patients with impaired LV function, further studies should also include data on postoperative recovery of LV function, which may also serve as an indicator for better outcome. Additionally, the EuroSCORE I was used for calculation of perioperative risk, which has been demonstrated to overestimate the 30-day risk of mortality.
Conclusions
In contrast to other studies, the present report summarises a large number of patients and their clinical follow-up from a nationwide registry (nearly all TAVI-performing institutions in Germany). As the time period for inclusion was short, a learning curve and technological changes do not impact on the results. In clinical practice, a low transvalvular flow and gradient despite severe AS is a common finding and is observed in >30% of patients undergoing TAVI. In the GARY registry, paradoxical low-gradient AS is observed in 20.8% of patients and is even more frequent than low-gradient AS with impaired LV function (11.7%). The in-hospital and one-year mortality and complication rates after TAVI among patients with PLF-LGAS are low and similar to the rates for high-gradient AS. In contrast, patients with LEF-LGAS have a significantly higher one-year mortality and a significantly higher rate of low cardiac output after TAVI.
| Impact on daily practice The present study analyses the outcome of patients undergoing transcatheter aortic valve implantation (TAVI) for different subtypes of severe aortic stenosis (AS) based on data from the GARY registry. In this study population a low transvalvular flow and gradient is a common finding and is observed in >30% of patients. A “paradoxical” low-gradient AS despite preserved left ventricular (LV) function is present in 20.8% of TAVI patients and is even more frequent than a low-gradient AS with impaired LV function (11.7%). Early and one-year mortality and complication rates after TAVI for paradoxical low-gradient AS are low and comparable to the rates for high-gradient AS. In contrast, TAVI for low-gradient AS and reduced LV function is associated with significantly higher one-year mortality and rate of MACCE. |
Conflict of interest statement
A. Lauten has received proctor salary and/or consulting fees from St. Jude Medical, JenaValve and Occlutech. C. Hamm has received speakers’ honoraria from Medtronic and Edwards Lifesciences and serves on the advisory board of Medtronic. H. Möllmann has received proctor salary and/or speakers’ honoraria from Abbott, Medtronic, Edwards Lifesciences, St. Jude Medical, and Symetis. H. Figulla is a co-founder of JenaValve and received consulting fees from that company. R. Zahn has received research grants from Medtronic and Edwards Lifesciences. K. Kuck is a consultant for Biosense Webster, St. Jude Medical, Stereotaxis, and Medtronic. M. Böhm has received speakers’ honoraria and scientific support from Medtronic, St. Jude Medical, Boston Scientific, Servier, Boehringer Ingelheim and Menarini. The other authors have no conflicts of interest to declare.

