
Abstract
Aims: The objective of this study was to examine the impact of guideline-defined subtypes of severe aortic stenosis (AS) on long-term outcomes after TAVI.
Methods and results: Four hundred (400) consecutive patients who underwent TAVI (203 transapical, 197 transfemoral) at our institution 8/2008-3/2013 were followed systematically (for up to seven years). One hundred and forty-seven (147) individuals suffered from NEF-HG AS (LV-EF ≥50%, high Pmean ≥40 mmHg), 63 from LEF-HG AS (LV-EF <50%, high gradient), 77 from PLF-LG AS (LV-EF ≥50%, low gradient, stroke volume index [SVI] <35 ml/m²), and 81 from LEF-LG AS (LV-EF <50%, low gradient). LEF-LG status was associated with the highest all-cause and cardiovascular mortality and MACCE rate, whereas NEF-HG patients exhibited the best outcome (i.e., median survival 5.1 years in NEF-HG vs. 1.3 years in LEF-LG, p=0.0006; or vs. 3.3 years in PLF-LG, p=0.02). In multivariate analysis, LEF-LG status emerged as the outcome predictor with the highest hazard ratio for all-cause mortality (HR 2.86, p=0.003), cardiovascular mortality (HR 6.53, p<0.0001), and MACCE (HR 2.44, p=0.007), whereas neither baseline EF nor SVI <35 ml/m² independently predicted these endpoints.
Conclusions: These findings suggest that an assessment of LV-EF alone for outcome prediction after TAVI is inadequate; it is the guideline-defined subtype of AS that determines outcome.
Abbreviations
AR: aortic regurgitation
AS: aortic stenosis
AVA: aortic valve area
AVAi: indexed aortic valve area
CKD: chronic kidney disease
COPD: chronic obstructive pulmonary disease
GFR: glomerular filtration rate
LEF-HG AS: low/reduced ejection fraction, high-gradient aortic stenosis
LEF-LG AS: low/reduced ejection fraction, low-gradient aortic stenosis
LV-EF: left ventricular ejection fraction
MR: mitral regurgitation
NEF-HG AS: normal/preserved ejection fraction, high-gradient aortic stenosis
PAsP: pulmonary artery systolic pressure
PCI: percutaneous coronary intervention
PLF-LG AS: paradoxical low-flow, low-gradient aortic stenosis
Pmean: mean transvalvular gradient
SVI: stroke volume index
VARC: Valve Academic Research Consortium
Introduction
During the last decade, it has been recognised that an important proportion of patients with symptoms of severe aortic stenosis (AS), aortic valve area (AVA) <1.0 cm² and indexed AVA (AVA/body surface area [BSA]) <0.6 cm²/m² present with mean transvalvular gradients (Pmean) <40 mmHg. Therefore, current American and European guidelines1,2 acknowledge the phenomenon of low-flow severe AS which can occur either due to left ventricular (LV) systolic dysfunction with reduced ejection fraction (EF) or due to a small hypertrophied LV with preserved EF but nevertheless low stroke volume (“paradoxical” low-flow AS).
However, evidence addressing the influence of “classic” or “paradoxical” low-flow, low-gradient AS on long-term outcomes after TAVI is still limited. Therefore, the objective of our study was to examine the impact of different AS subtypes (defined according to recent guidelines) on outcomes after TAVI.
Methods
The current study represents a retrospective analysis of prospectively collected data including the first 400 consecutive patients who underwent TAVI at our institution 8/2008-3/2013. All echocardiograms were routinely recorded in a Picture Archiving and Communication System. TAVI procedures were performed as previously described using standard techniques. Two hundred and three (203) patients (51%) were treated via the transapical approach and 197 (49%) via the transfemoral approach. The Medtronic CoreValve (Medtronic, Minneapolis, MN, USA) was used in 32 transfemoral cases, whereas in all other procedures the Edwards SAPIEN (after 5/2010 SAPIEN XT) valve (Edwards Lifesciences Inc., Irvine, CA, USA) was implanted. At discharge, periprocedural complications and mortality rates were evaluated according to VARC-2 definitions3, and echocardiography was repeated. All 400 patients were followed by regular telephone contact (which was last performed around the turn of the years 2015/2016) by using a standardised questionnaire, and medical documents were acquired to investigate causes of death and re-hospitalisations.
CLASSIFICATION OF AS SUBTYPE
All echocardiograms were retrospectively re-evaluated by a single observer using the software of TOMTEC (TOMTEC Imaging Systems GmbH, Unterschleissheim, Germany) and Xcelera R2.2L1 SP2 (Philips Healthcare, Best, the Netherlands). Stroke volume was measured by pulsed wave Doppler in the LV outflow tract and was indexed for body surface area (SVI). Subtype classification was then performed according to a combination of Pmean, LV-EF and SVI. Following current American guidelines1, four subgroups of severe AS were defined (Figure 1):
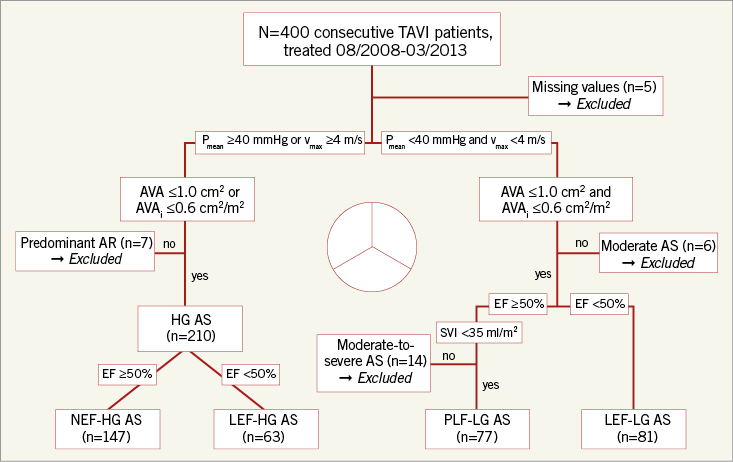
Figure 1. Flow chart summarising patient flow for subdivision into four different entities of AS. AR: aortic regurgitation; AS: aortic stenosis; AVA: aortic valve area; AVAi: indexed aortic valve area; SVI: stroke volume index
1. Normal/preserved ejection fraction, high-gradient AS (NEF-HG AS):
LV-EF ≥50%,
vmax ≥4 m/s or Pmean ≥40 mmHg,
AVA ≤1.0 cm² or indexed AVA ≤0.6 cm²/ m²
2. Paradoxical low-flow, low-gradient AS (PLF-LG AS):
LV-EF ≥50%,
vmax <4 m/s and Pmean <40 mmHg,
AVA ≤1.0 cm² and indexed AVA ≤0.6 cm²/ m²,
stroke volume index (SVI) <35 ml/m²
3. Low/reduced ejection fraction, high-gradient AS (LEF-HG):
LV-EF<50%,
vmax ≥4 m/s or Pmean ≥40 mmHg,
AVA ≤1.0 cm² or indexed AVA ≤0.6 cm²/ m²
4. Low/reduced ejection fraction, low-gradient AS (“classic” low-flow, low-gradient AS) (LEF-LG AS):
LV-EF<50%,
vmax <4 m/s and Pmean <40 mmHg,
AVA ≤1.0 cm²
Divergent from the American definitions, European guidelines2 propose an ejection fraction cut-off of <40% for LEF-LG AS, which leaves low-gradient patients with LV-EF of 40-50% without classification. Therefore, the American definitions1 were chosen and completely adopted for HG AS, PLF-LG AS and LEF-LG AS. The high-gradient cohort was then further subdivided into patients with preserved (NEF-HG) and reduced (LEF-HG) ejection fraction in order to elucidate further the impact of LV-EF itself on prognosis.
ENDPOINTS
All-cause mortality was defined as the primary clinical endpoint according to VARC-2 proposals3. As secondary endpoints, cardiovascular mortality (VARC-2 definition), cardiac-related re-hospitalisation (due to heart failure, acute coronary syndrome, syncope, arrhythmias, and new device implantation), and rate of major adverse cardiovascular and cerebrovascular events (MACCE, comprising a composite of death of any cause, cardiac-related re-hospitalisation and stroke) were chosen.
The study was approved by the local ethics committee, and written informed consent was obtained from all patients.
STATISTICAL ANALYSIS
Statistical analysis was performed with GraphPad Prism, version 4.0 (GraphPad Software, San Diego, CA, USA) and with the Statistical Computing Software R (version 2.15.1; http://www.r-project.org). Continuous variables are presented as mean±standard deviation and were compared using the Mann-Whitney U test for two-group comparison and the Kruskal-Wallis test for multi-group comparison (absence of normality distribution). Categorical variables are presented as absolute numbers and percentages and were compared by Fisher’s exact test for two-group comparison and by Pearson’s chi-square test for multi-group comparison. A value of p<0.05 was considered statistically significant.
Survival analysis was performed on time from date of TAVI to event data for the four predefined endpoints using the “survival” package for R, visualised by Kaplan-Meier plots, and significance was calculated using the log-rank test. In case of multiple testing, the false discovery rate (FDR) approach was applied, and FDR-adjusted p-values were reported. For multivariate models, the Cox proportional hazards model was used. Multivariate survival analyses were performed including all potential outcome predictors for TAVI or conventional heart surgery according to the literature or to pre-analyses of our own cohort (baseline characteristics, echocardiographic measures, AS subtypes), with the only exclusion of surgical risk scores (due to potential multicollinearity). Regarding the subtypes of AS, the factor variable “AS subtypes” including all different subtypes of AS (with NEF-HG AS as reference) was defined.
Results
CLASSIFICATION
Figure 1 summarises the subdivision of patients into the four defined entities of AS. Among the 400 patients, 147 (37%) suffered from NEF-HG AS, 63 (16%) from LEF-HG AS, 77 (19%) from PLF-LG AS and 81 (20%) from LEF-LG AS. Thirty-two patients (8%) who did not fit into any of these subgroups (i.e., missing values, predominant AR) were retrospectively classified as “no severe AS” and excluded from subtype analyses, but included in multivariate analyses.
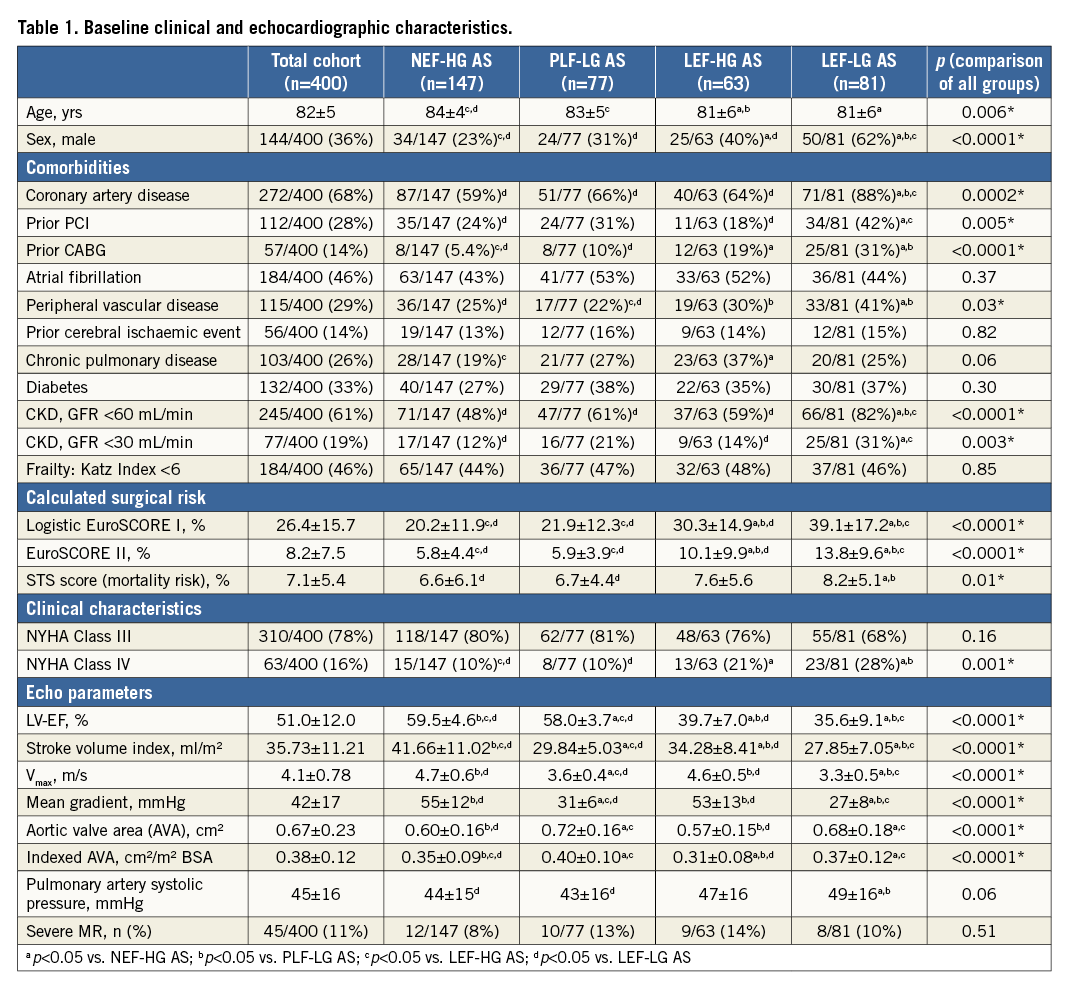
BASELINE CHARACTERISTICS
Baseline clinical and echocardiographic characteristics are displayed in Table 1. The total cohort (36% men, 64% women) was characterised by advanced age (82±5 years) and many comorbidities. Regarding AS subgroups, clear differences in baseline demographics, gender distribution and comorbidities could be detected. Distilled, NEF-HG patients were least likely to be male (23%) and were the “healthiest” among all AS patients, whereas LEF-LG patients (although significantly younger) exhibited the highest burden of comorbidities and were predominantly male (62%).
PROCEDURAL CHARACTERISTICS
The different types of approach, of implanted valve and of anaesthesia were equally distributed among all groups. Similarly, no differences concerning total procedure time, fluoroscopy time or volume of contrast medium were observed.
IN-HOSPITAL OUTCOME
The incidence of specific VARC-defined complications was not significantly different in the four subgroups. However, procedural mortality (death before day 30 or first hospital discharge) was lowest for NEF-HG and highest for LEF-LG patients (8.2% in NEF-HG, 17% in PLF-LG, 13% in LEF-HG and 24% in LEF-LG patients, p=0.01). Additionally, LEF-LG patients were at highest risk of experiencing intraoperative resuscitation (16% vs. 6.1% in NEF-HG, p=0.02), prolonged ventilation of more than 24 hours (15% vs. 5.7% in NEF-HG; p=0.03), and death related to infectious complications (11% vs. 2.7% in NEF-HG, p=0.01). Also, length of hospital stay was longest in this cohort (median, 17 vs. 11 days, p<0.0001).
Interestingly, echocardiography at the time of discharge revealed a significant increase in LV-EF in both groups with reduced EF, although this finding was more pronounced in LEF-HG (48.1±7.7 vs. 39.7±7% at baseline, p<0.0001) than in LEF-LG patients (40.0±10.8 vs. 35.6±9.1% at baseline, p=0.0004) (analysis of matched data only).
LONG-TERM OUTCOMES
Median follow-up period was 3.0 years (minimum: until death; maximum: 7.1 years), and the last systematic follow-up was performed at least 2.8 years after TAVI. Exact one-year mortality was 26% (104/400) in the total cohort, 14.3% (21/147) in NEF-HG AS, 31.2% (24/77) in PLF-LG AS, 22.2% (14/63) in LEF-HG AS, and 43.2% (35/81) in LEF-LG AS (p<0.0001). Also, long-term survival differed significantly between subgroups (comparison of all subtypes: p Coxph <0.0001; see Kaplan-Meier curves and median survival in Figure 2A): NEF-HG patients survived significantly longer in comparison with PLF-LG (HR 1.59, FDR-adjusted p=0.02) and LEF-LG patients (HR 2.70, FDR-adjusted p=0.0006), but not in comparison with LEF-HG patients (HR 1.20, FDR-adjusted p=0.44). On the other hand, prognosis of LEF-LG patients was significantly worse if compared to all other subgroups (HR 1.70, FDR-adjusted p=0.01 compared to PLF-LG; HR 2.14, FDR-adjusted p=0.0006 compared to LEF-HG). To exclude the influence of early mortality, we also performed a landmark analysis with exclusion of all deaths until day 30, which did not change the statistical result.
Survival analysis with regard to cardiovascular mortality revealed the same finding (Figure 2B), indicating that excess mortality especially in LEF-LG patients was mainly driven by cardiovascular causes. Regarding exclusively causes of death in patients discharged alive after TAVI, cardiovascular mortality was 13% (18/135) in NEF-HG, 22% (14/64) in PLF-LG, 25% (14/55) in LEF-HG and 39% (24/62) in LEF-LG patients (p=0.0001). To a large extent, this finding was driven by a higher incidence of sudden unexpected deaths in LEF-LG patients (3.0% in NEF-HG, 3.1% in PLF-LG, 5.5% in LEF-HG and 13% in LEF-LG; p=0.03), whereas the incidence of deaths due to terminal heart failure did not differ significantly (9.6% in NEF-HG, 16% in PLF-LG, 15% in LEF-HG and 19% in LEF-LG; p=0.28).
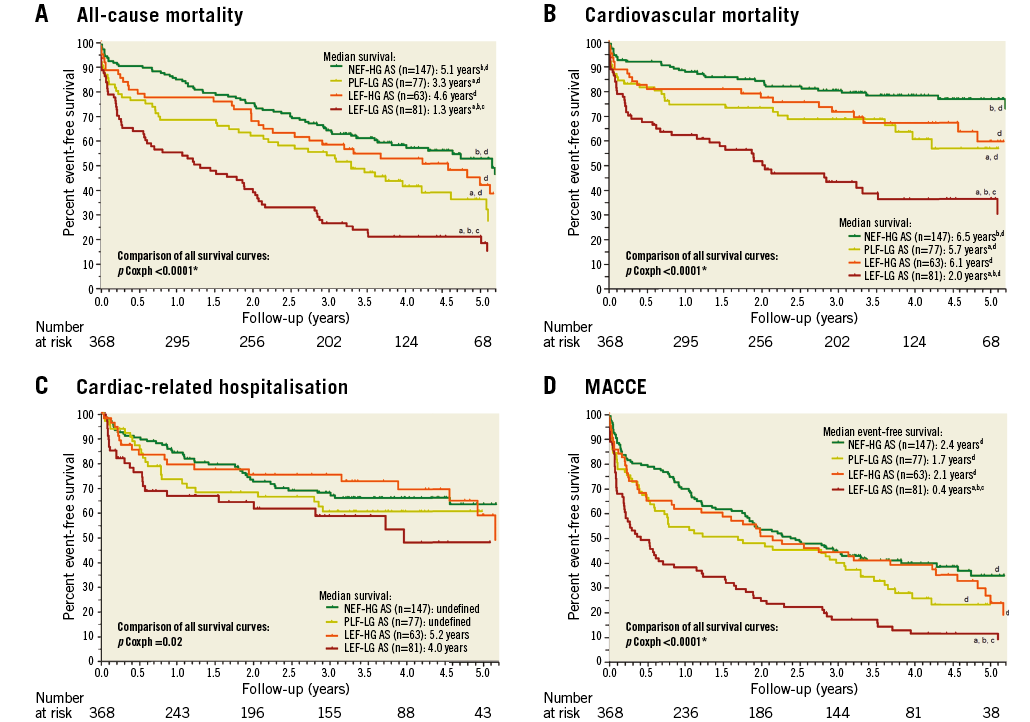
Figure 2. Kaplan-Meier curves in the subgroups of AS. A) All-cause mortality. B) Cardiovascular mortality. C) Cardiac-related hospitalisation. D) MACCE. a: FDR-adjusted p<0.05 vs. NEF-HG AS; b: FDR-adjusted p<0.05 vs. PLF-LG AS; c: FDR-adjusted p<0.05 vs. LEF-HG AS; d: FDR-adjusted p<0.05 vs. LEF-LG AS. LEF-HG AS: low/reduced ejection fraction, high-gradient AS; LEF-LG AS: low/reduced ejection fraction, low-gradient AS; NEF-HG AS: normal/preserved ejection fraction, high-gradient AS; PLF-LG AS: paradoxical low-flow, low-gradient AS
Freedom from cardiac-related re-hospitalisation (Figure 2C) differed only slightly among the four subgroups (p Coxph=0.02). Considering MACCE as a combined endpoint, the most striking fact was the devastating median event-free survival of 0.4 years in the LEF-LG cohort (Figure 2D). Cumulative event rates at the time of last follow-up are presented in Figure 3 for all predefined endpoints.
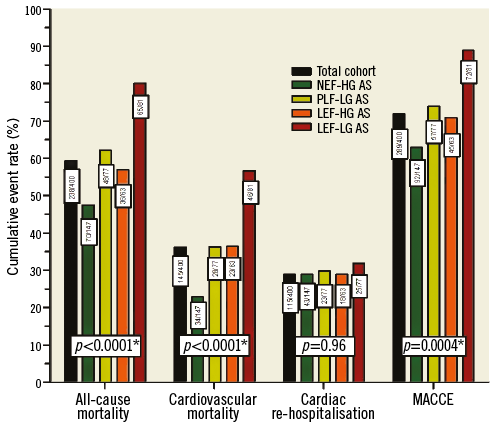
Figure 3. Cumulative event rates at time of last follow-up in the total cohort and AS subtypes; total event numbers are indicated in boxes.
In summary, LEF-LG patients exhibited the worst outcome with regard to all-cause mortality, cardiovascular mortality (especially pronounced), and MACCE.
INDEPENDENT PREDICTORS OF ALL-CAUSE MORTALITY, CARDIOVASCULAR MORTALITY, CARDIAC-RELATED HOSPITALISATION AND MACCE
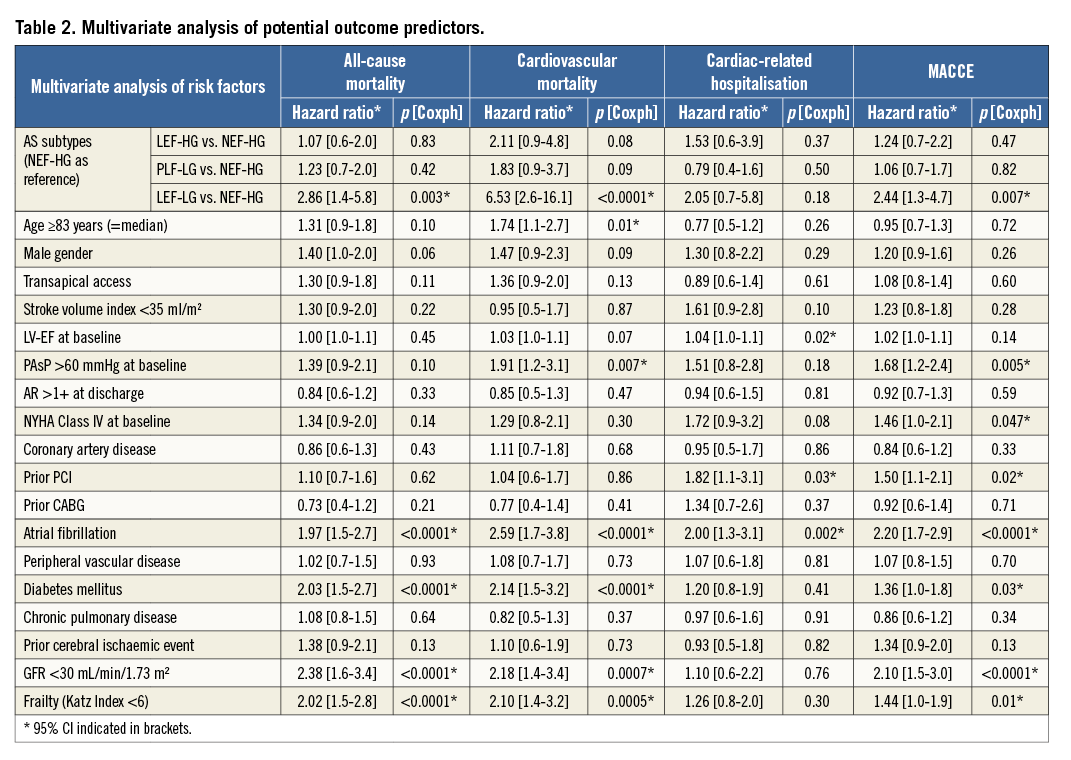
The multivariate model (including hazard ratios and p-values for all inserted variables) is displayed in Table 2. Importantly, multivariate analysis identified LEF-LG status as the outcome predictor with the highest hazard ratio for all-cause mortality (HR 2.86, p=0.003), cardiovascular mortality (HR 6.53, p<0.0001), and MACCE (HR 2.44, p=0.007), whereas neither baseline EF nor SVI <35 ml/m² was predictive. The other important predictors regarding these endpoints were atrial fibrillation, diabetes, renal failure with GFR <30 ml/min/1.73 m³, and frailty defined by Katz Index <6. In contrast, only atrial fibrillation, prior PCI and baseline EF independently predicted cardiac-related hospitalisation during follow-up.
Discussion
To the best of our knowledge, this is the first paper studying the impact of guideline-defined subtypes of AS on long-term outcome during a follow-up period of up to seven years. Our major findings are as follows.
First, LEF-LG status was associated with the worst outcome among all AS subtypes regarding all-cause mortality, cardiovascular mortality and MACCE. In particular, median survival in NEF-HG patients was almost fourfold higher than in LEF-LG patients.
Second, long-term outcome did not differ significantly between NEF-HG and LEF-HG patients, whereas outcome in PLF-LG AS was worse compared with NEF-HG AS.
Third, LEF-LG status emerged as the outcome predictor with the highest hazard ratio for all-cause mortality (HR 2.86, p=0.003), cardiovascular mortality (HR 6.53, p<0.0001), and MACCE (HR 2.44, p=0.007) in multivariate analysis.
Fourth, baseline EF only predicted cardiac-related re-hospitalisations and SVI <35 ml/m² no endpoint at all in multivariate analysis.
CLASSIFICATION
Comparable to other previously published cohorts, only approximately half of our patients who underwent TAVI had high-gradient AS4-7.
BASELINE CHARACTERISTICS AND ECHOCARDIOGRAPHIC MEASUREMENTS
In the literature, there is consensus that LEF-LG patients are younger and more likely to be male, have higher surgical risk scores and exhibit more comorbid conditions in comparison with other AS subtypes4-7 – which is perfectly in line with our own findings. In contrast, the comorbidity profile of PLF-LG patients is not so different from high-gradient patients, but they tend to be older and more often female8,9.
LONG-TERM OUTCOME
The impact of reduced LV-EF itself on outcomes after TAVI has been discussed controversially for many years. Interestingly, two post hoc PARTNER analyses have treated this topic: whereas a significant impact of reduced baseline LV-EF (20-50%) on outcomes could not be identified10, low-flow status (SVI <35 ml/m²) was independently associated with elevated two-year mortality5. Our own findings do not contradict these results: PARTNER only included a few low-gradient patients (Pmean ≥40 mmHg or vmax ≥4 m/s was the inclusion criterion), and NEF-HG and LEF-HG patients exhibited similar outcomes also in our own analysis. In summary, it is likely that findings on the prognostic impact of reduced LV-EF after TAVI strongly depend on the exclusion vs. inclusion of low-gradient patients due to a different pathophysiology of EF decrease. In LEF-HG patients, reduced LV-EF probably results from afterload mismatch (myocardial contractility is temporarily depressed due to lack of adequate preload for a given level of afterload caused by excessive valvular obstruction11), and high gradients suggest at least partly preserved contractility. In such patients, an immediate improvement in LV-EF by aortic valve replacement (causing mechanical unloading of the left ventricle) was already reported 40 years ago11. In contrast, the EF reduction in LEF-LG patients (who exhibited the highest prevalence of CAD, prior PCI and prior CABG of all subgroups in our study) is likely to result from ischaemic scarring to a great extent – with consequent lower potency of recovery. In fact, we observed a rapid increase in LV-EF (+8.4%) at discharge in our LEF-HG patients after TAVI, whereas this recovery was markedly less pronounced (+4.4%) in LEF-LG patients.
However, in contrast to the post hoc PARTNER analysis5 (in which the multivariate model did not include AS subtypes as variables) we could not identify SVI <35 ml/m² as an independent outcome predictor.
Comparison of our post-TAVI outcome data with previously published reports is complicated by the fact that subtype definitions varied: SVI was not always measured, and different EF cut-offs were used. Le Ven et al7 found that patients with paradoxical low-flow, low-gradient AS (SVI ≤35 ml/m², EF ≥50%, Pmean <40 mmHg) and low-EF low-gradient AS (SVI ≤35 ml/m², EF <50%, Pmean <40 mmHg) had similar outcomes, which were both worse than those of normal-flow patients (SVI >35 ml/m², Pmean >40 mmHg). Low-flow, but neither low EF nor low gradient, independently predicted all-cause and cardiovascular mortality. The impact of low-gradient AS was also evaluated in two large patient series enrolled in the German (GARY)6 and French (FRANCE2)12 national TAVI registries. However, SVI was not measured in either study, probably leading to the inclusion of non-severe AS patients with better prognosis in the paradoxical low-flow, low-gradient groups. In GARY, patients with reduced EF (≤40%) and low gradient (<40 mmHg) exhibited significantly elevated one-year mortality, whereas patients with preserved EF and low gradient did not6. Regarding FRANCE2, patients with low-EF, low-gradient AS also had a significantly elevated all-cause and cardiovascular mortality. Preprocedural mean gradient <40 mmHg and impaired LV-EF were independent mortality predictors12. In summary, all three latter studies6,7,12 confirmed the unfavourable course of LEF-LG patients (regardless of LV-EF cut-off) during midterm follow-up after TAVI (although an independent impact could not be found), whereas only the correctly defined PLF-LG status (including SVI <35 ml/m²) was associated with worse outcome7. In our own study, however, we were able to demonstrate an independent impact of LEF-LG status on long-term outcomes after TAVI.
Limitations
Although data acquisition was prospective, our analysis was retrospective and therefore subject to the limitations of an observational study. Also, dobutamine stress echocardiography to assess contractile reserve in LEF-LG patients was not routinely performed. The present investigation represents a single-centre experience with a relatively small sample size. Yet, its strength lies in the complete analysis of consecutive patients, the 100% complete follow-up, and the long follow-up period of up to seven years. Furthermore, 30-day mortality was relatively high. However, this study includes our earliest TAVI experience with 51% transapical procedures and truly inoperable patients.
Conclusions
Our findings suggest that an assessment of LV-EF alone for outcome prediction after TAVI is inadequate; it is the guideline-defined subtype of AS that determines long-term outcome. Guideline-adopted classification helps to identify excellent candidates for TAVI (NEF-HG patients) as well as individuals at elevated risk of adverse early and late outcomes (LEF-LG patients). The latter should be followed very closely after TAVI in order to identify individual modifiable factors of increased risk of death (i.e., early pacemaker implantation in patients with new-onset left bundle branch block and reduced LV-EF, as suggested by Urena et al13).
| Impact on daily practice The AS subtype independently influences long-term mortality, cardiovascular mortality and MACCE after TAVI. In particular, classic low-flow, low-gradient AS (LV-EF <50%, Pmean <40 mmHg) emerged as the outcome predictor with the highest hazard ratio for all-cause mortality (HR 2.86, p=0.003), cardiovascular mortality (HR 6.53, p<0.0001), and MACCE (HR 2.44, p=0.007), whereas neither baseline EF nor SVI <35 ml/m² independently predicted these endpoints. Additionally, outcomes of high-gradient patients with preserved or reduced LV-EF were not significantly different. These findings suggest that the AS subtype rather than LV-EF should be implemented in pre-TAVI risk-to-benefit considerations. |
Conflict of interest statement
C. Jacobshagen has received travel expenses and lecture fees from Edwards Lifesciences and DirectFlow Medical. W. Schillinger has received proctor and lecture fees from Edwards Lifesciences, St. Jude Medical, Abbott Vascular, and DirectFlow Medical. The other authors have no conflicts of interest to declare.

