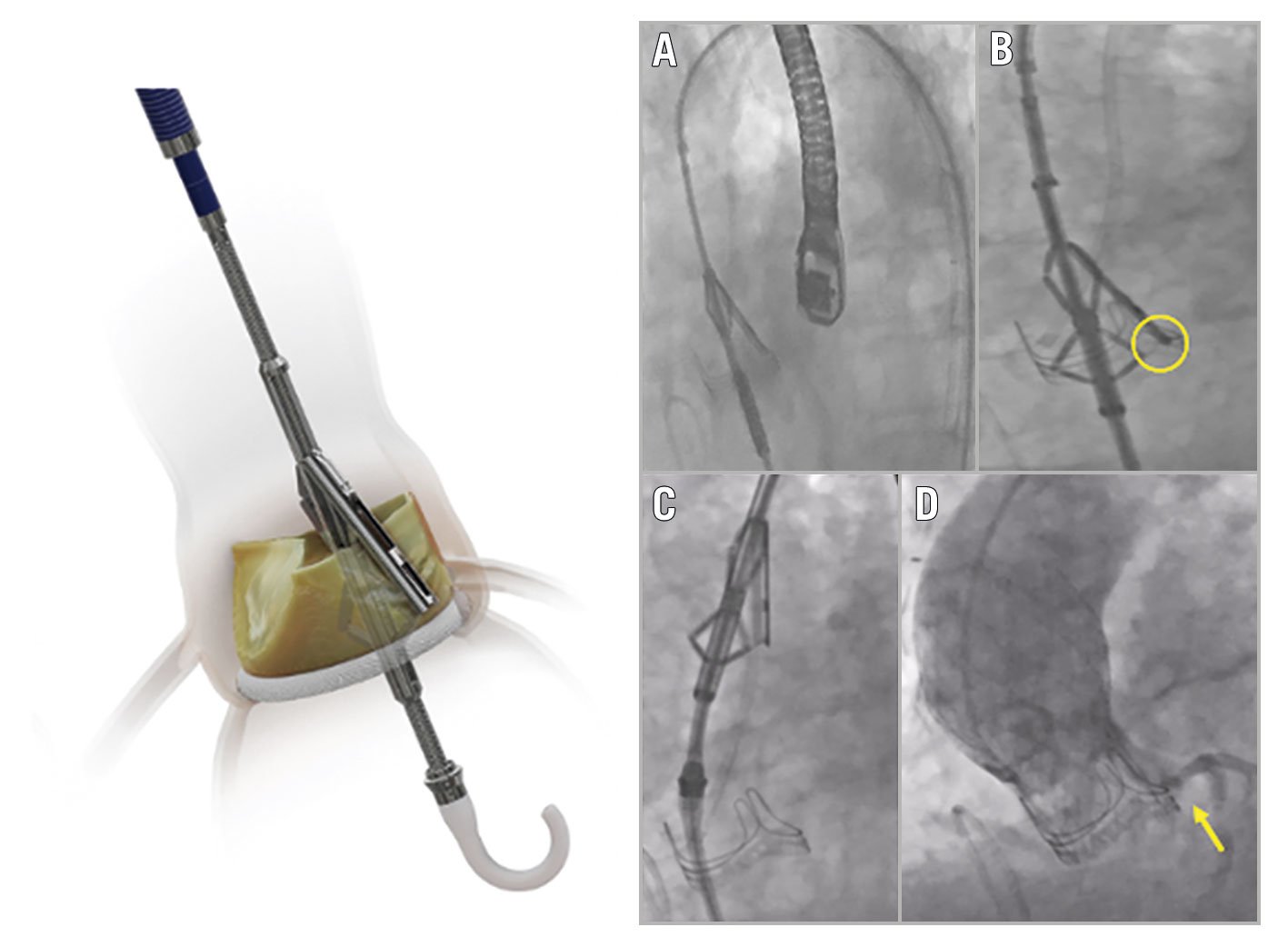
Figure 1. The Pi-Cardia ShortCut device. The ShortCut procedure: A. PA positioning; B. SE activation. The yellow circle shows splitting of the bioprosthetic leaflet facing the left main coronary artery; C. splitting; and D. good coronary flow post-split and TAVR. The arrow points to patency of the left main coronary artery at the end of the procedure. PA: positioning arm; SE: splitting element; TAVR: transcatheter aortic valve replacement
Coronary obstruction is a life-threatening complication that occurs in 2.3% of patients undergoing transcatheter aortic valve replacement (TAVR) for degenerated bioprostheses1. During a valve-in-valve (ViV) procedure, the leaflets of the prior valve are displaced towards the coronary ostia, potentially resulting in an obstruction of coronary blood flow. Multiple reports have demonstrated the feasibility of bioprosthetic valve leaflet laceration utilising an electrified coronary wire to reduce the risk. However, this technique remains technically challenging and is not widely utilised.
The ShortCut (Pi-Cardia) device is designed to split bioprosthetic aortic valve leaflets in patients undergoing ViV-TAVR procedures who are at risk for TAVR-induced coronary artery obstructions. Lifetime management for patients with aortic stenosis is an increasingly important consideration as the use of bioprosthetic valves is rising in younger patients. Therefore, a device such as the ShortCut is necessary to facilitate the treatment of patients requiring multiple valve therapies throughout their lifetime. The device consists of a splitting element (SE) which penetrates the leaflet in a controlled manner in order to perform leaflet splitting, and a positioning arm (PA) that protects the SE throughout the procedure.
The ShortCut procedure is guided by fluoroscopy and echocardiography. During the procedure, the catheter is introduced through a 16 Fr introducer sheath and advanced to the aortic valve over a preshaped guidewire. Once the device is properly aligned at the annular level, the PA is exposed and rotated towards the targeted leaflet (Figure 1A). Once the desired position is achieved, the SE is activated, penetrating in a controlled manner the bottom of the leaflet from the ventricular side (Figure 1B). The PA, situated on the aortic aspect of the leaflet, protects the surrounding tissue from any potential injury and acts as a cover for the activated SE. The leaflet is then split by gently retracting the catheter (Figure 1C). At the end of the splitting sequence, the SE is deactivated and resheathed. If the splitting of a second leaflet is required, the PA can be rotated towards the targeted leaflet and the procedural steps repeated. After the procedure, the ShortCut catheter is resheathed and withdrawn. Following the splitting of the leaflet(s), an echo assessment is performed to visualise the leaflet split. After TAVR, unobstructed coronary flow is confirmed by echo and fluoroscopy (Figure 1D).
The ShortCut device is currently being evaluated globally in a prospective, multicentre, non-randomised, single-arm, open-label clinical study to demonstrate the safety and effectiveness of the device (The ShortCut Study). ClinicalTrials.gov: NCT04952909.
Conflict of interest statement
D. Tchétché is a consultant for Pi-Cardia and principal investigator for the ShortCut study. S. Kodali is a principal investigator for the ShortCut study. He is a consultant (honoraria) for Admedus, Dura Biotech, TriCares, Philips, and TriFlo; has SAB (equity) in Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve Inc., Supira, Admedus, TriFlo, Adona, Tioga, and X-Dot; receives institutional research funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. D. Dvir is a consultant for Pi-Cardia and Principal Investigator for the ShortCut study and is a consultant to Edwards Lifesciences, Medtronic, and Abbott.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Pi-Cardia ShortCut master demo video.

