Abstract
Coronary obstruction is a life-threatening complication of transcatheter aortic valve replacement. BASILICA (bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction) seems to be an effective method for preventing that complication. In this article, we describe how to assess patients for their risk of coronary obstruction and how to evaluate for BASILICA, including defining coronary cusp fluoroscopic projections and analysing coronary ostia eccentricity.
Abbreviations
| BASILICA | bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction |
| CT | computed tomography |
| MPR | multiplanar reconstruction |
| STJ | sinotubular junction |
| TAVR | transcatheter aortic valve replacement |
| THV | transcatheter heart valve |
| VTC | virtual transcatheter heart valve to coronary ostium distance |
| VTSTJ | virtual transcatheter heart valve to sinotubular junction distance |
Introduction
Clinical outcomes of transcatheter aortic valve replacement (TAVR) continue to improve, and younger and lower-risk populations are being offered a less invasive therapy1,2. Nevertheless, catastrophic adverse events, including coronary obstruction, occasionally occur. The incidence of coronary obstruction during TAVR is 0.4-0.5% in the Transcatheter Valve Therapy (TVT) registry data and 2.3% in the Valve-in-Valve International Data (VIVID) registry, with associated in-hospital mortality of approximately 50%3,4. Although imaging enables recognition of anatomical characteristics associated with coronary obstruction, effective and safe methods to prevent coronary obstruction are lacking4,5,6,7. BASILICA (bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction) is a novel approach to prevent coronary obstruction which seems to be effective8,9. In this article, we describe how to assess the risk for coronary obstruction following TAVR and how to evaluate patients for BASILICA.
MECHANISMS OF CORONARY OBSTRUCTION FOLLOWING TAVR
Coronary obstruction following TAVR can result from several mechanisms and aetiologies (Figure 1). Commonly, the displacement of the native or bioprosthetic valve leaflet by the newly implanted transcatheter heart valve (THV) is the main cause of the obstruction. In natural conditions, aortic valve leaflets are in a closed position during diastole and the widely opened sinus of Valsalva enables a large pool of blood to be delivered to the coronary arteries. TAVR creates an abnormal condition in which a deflected leaflet does not close in diastole and thus the flow to the sinus of Valsalva can diminish. This is clinically relevant in cases where the deflected leaflet rises above the origin of the coronary artery and/or when the gap between that deflected leaflet and the sinus wall is narrow. It is therefore clear that patients with low coronary ostia origin and narrow sinus of Valsalva are prone to coronary obstruction.
The anatomical relation of the aortic root to the coronary ostium can be divided into three main types, according to the location of the coronary ostia and size of the aortic valve complex. These are important for defining the risk for coronary obstruction. In Type I, the coronary ostium lies above the top of the deflected native or bioprosthetic aortic valve leaflet (Figure 1, Type I). In that condition, the deflected leaflet will not be able to cover the flow to the coronary artery, even if the sinuses are extremely narrow. In Type II, the coronary ostium lies below the top of the deflected leaflet. In these cases, the risk of coronary obstruction will depend on the capacity of the sinuses to accommodate the deflected leaflet (Figure 1, Type II). If the sinus is wide (Figure 1, Type IIA) then coronary obstruction will not occur. However, if the sinus is effaced (Figure 1, Type IIB), then coronary obstruction can happen after TAVR. Patients after surgical or transcatheter aortic valve replacement occasionally have implanted leaflets that can extend above the sinotubular junction (STJ) when deflected. This is especially common in supra-annular transcatheter valves. These conditions may not be at risk for coronary obstruction after TAVR if both the sinuses and STJ are wide (Figure 1, Type IIIA). However, coronary obstruction may occur by the deflected leaflet if either the sinuses or the STJ are narrow. It should be emphasised that the non-effaced sinuses of some patients may obstruct the inflow to the coronaries if the leaflets can be deflected above the STJ level and positioned close to the aortic wall (Figure 1, Type IIIC). Anatomies at risk for coronary obstruction include Type IIB, Type IIIB, and Type IIIC (Figure 1). These conditions may require protection from coronary obstruction with BASILICA, if TAVR is considered.
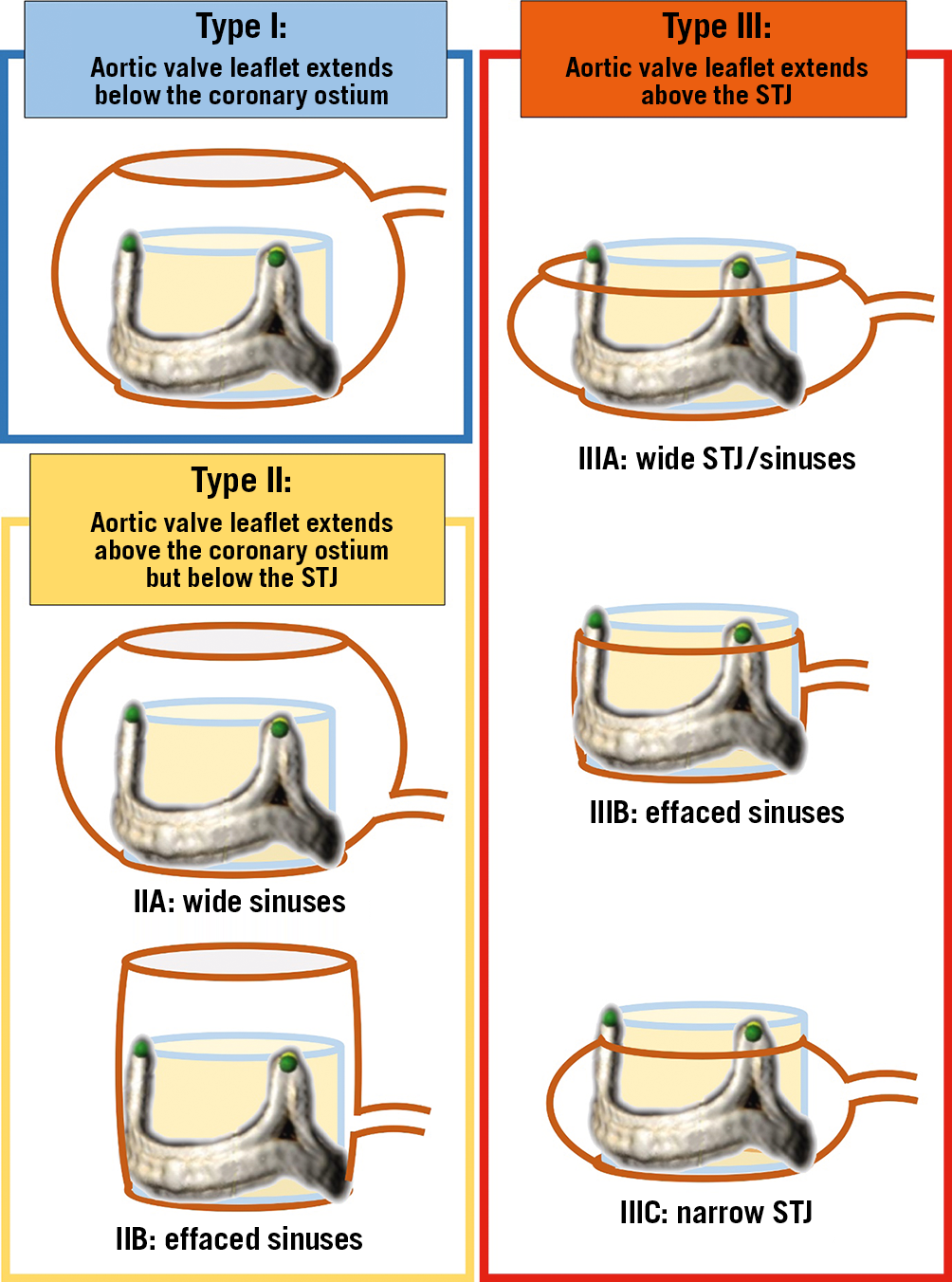
Figure 1. VIVID classification of coronary ostia and aortic root morphologies influencing the risk for coronary obstruction after TAVR. STJ: sinotubular junction
Assessment for the risk of coronary obstruction
CATH LAB ASSESSMENT
Although computed tomography (CT) is the gold standard in assessing the risk for coronary obstruction, conventional coronary angiography and aortography that are performed in the catheterisation lab can convey important basic screening information for the risk of coronary obstruction7 (Figure 2). During conventional pre-TAVR coronary assessment, this simple method can enable qualitative assessment of the location of the coronary ostia in relation to the annulus and of the capacity of the sinuses to accommodate deflected leaflets without causing coronary obstruction. The stented bioprosthetic surgical valve’s frames with visible fluoroscopic markers can commonly reveal whether the coronary ostia originate below or above the potential location of the deflected leaflets after a valve-in-valve procedure. These simple methods can separate cases in which both coronary ostia originate above the top of the bioprosthetic stent posts (Figure 1, Type I), which are not associated with increased risk of obstruction, and those where at least one of the stent posts rises above one of the coronary ostia or above the STJ (Figure 1, Type II, Type III, respectively) that can potentially be at risk for coronary obstruction. Another important aspect of coronary angiography for the assessment of coronary obstruction is that it can identify the size of the territory at risk, if obstruction occurs. Patency of bypass grafts, significant collateral flow and coronary dominancy may all alter the clinical significance of coronary obstruction.
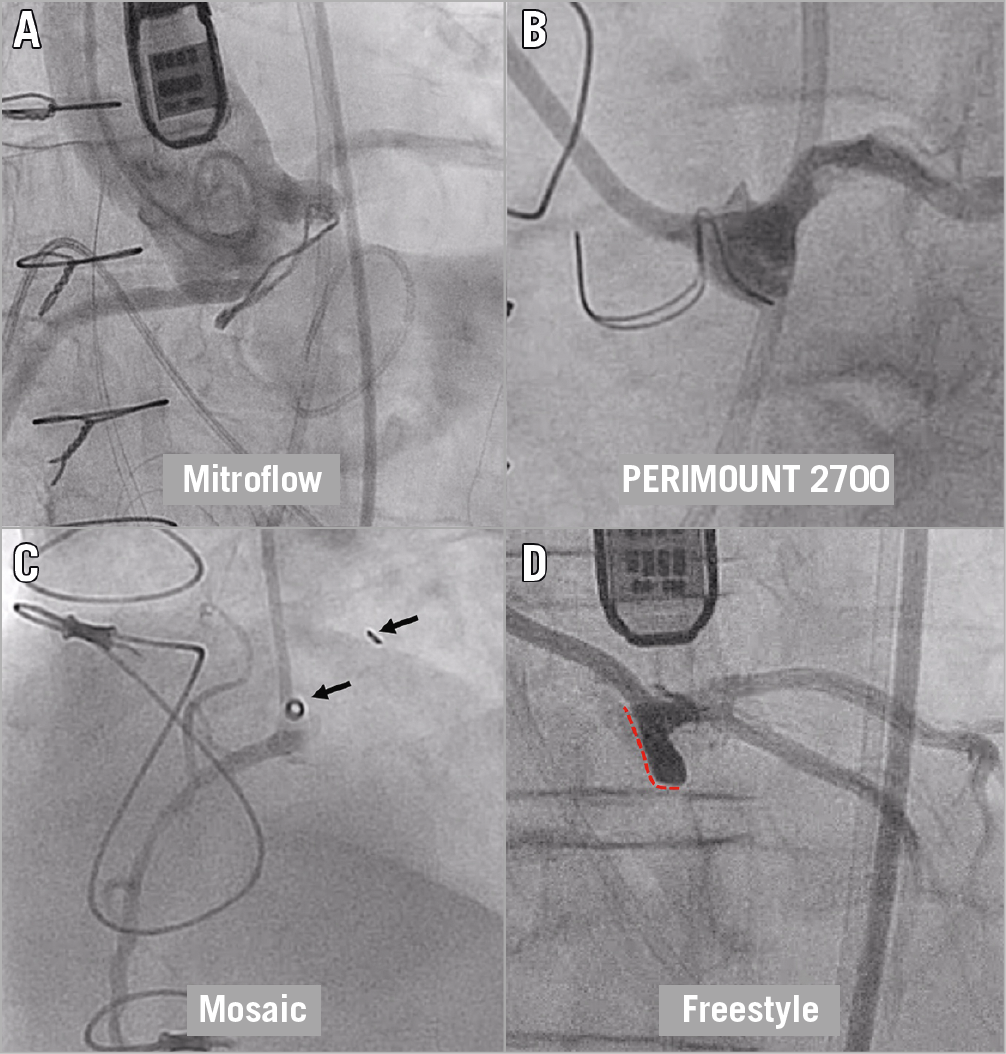
Figure 2. Evaluating the risk for coronary obstruction in the cath lab. A) Very low coronary ostia in relation to the Mitroflow ring. B) Side view (“1-2”) post alignment of the left cusp of a PERIMOUNT 2700 revealing that the tops of the posts reach the level of the STJ. C) Side view (“1-2”) post alignment of the right cusp of a Mosaic valve revealing that the tops of the posts reach above the coronary inflow and that the sinus is severely effaced. D) Side view of the left cusp of a stentless Freestyle valve. The panel shows how the leaflet (red dotted line) can cover the left main coronary artery while deflected and also the very narrow sinus.
Poor contrast opacification in the aortic root is relatively common in patients with a regurgitant bioprosthetic surgical valve. Semi-selective injection of contrast in the coronary ostia or solely into the coronary sinus base may improve assessment of the geometric relationship between the failed surgical valve and the coronary ostia with minimal contrast.
Injection should be performed in a projection that will be perpendicular both to the coronary ostium and to the bioprosthetic surgical valve when it is visible (e.g., stented surgical valve). Determining the optimal plane, perpendicular to the bioprosthetic surgical valve, can usually be accomplished by finding a fluoroscopic projection where the radiopaque components of the circular bioprosthetic basal ring appear as a straight line or where the radiopaque components of the stent posts are at the same height. A simple manoeuvre that provides perpendicularity to a coronary ostium is using the “1-2” technique (obtaining the “side view” projection). The coronary ostium of the left main coronary artery is commonly mid distance between two posts; as a result, a projection perpendicular to the left main ostium is usually achieved when the two adjacent posts are perfectly superimposed (Figure 2B).
CT ASSESSMENT
Computed tomography (CT) is the gold standard tool for assessing the risk of coronary obstruction before TAVR. Although the optimal methodology is in evolution, there are already many advances. CT allows three-dimensional (3D) assessment of the aortic root’s anatomy including the height of the coronary ostia in relation to the aortic annulus, the width and height of the sinus of Valsalva, and the STJ width. In addition, CT delineates valve tissue characteristics, its bulkiness and calcification, which can alter both the risk of obstruction and the efficacy of performing BASILICA. Examples of measurement steps and schemes for determination of the need for BASILICA are shown in Figure 3 and Figure 4, respectively. The height of coronary ostia can be measured from the vertical length of the aortic annulus to the bottom of the coronary ostia. Since the measurement of coronary height is sensitive and needs to be accurate, this should be measured in a multiplanar reconstruction (MPR) image. Difficulty in defining the ostium with a trumpet-shaped inflow occasionally occurs. In such cases, using MPR images, following the sinus wall may help to define that location better. Scrolling up the annulus plane in an axial image can identify the STJ location. Importantly, STJ and annulus planes are not always parallel; therefore, the STJ plane should be defined using MPR images as well. Because of this, occasionally, merely scrolling up the annulus plane in an axial image can mimic the real anatomical relationship with the STJ and coronary ostia. In addition, the deflection of long stented surgical valve bovine leaflets is markedly different from the short deflection of stented porcine leaflets. These in turn are very different from the deflection of different types of failed TAVR valve, some of which can be very long. Measuring leaflet lengths by CT is commonly challenging as there are limitations in terms of spatial and temporal resolution. However, in many cases the commissures are visible and can be used as surrogates for leaflet height. Bioprosthetic valve leaflets do not commonly deflect above their commissures. If full cardiac phase images are available, these should be assessed in systolic and diastolic phases where the diastolic phase sometimes provides better leaflet and commissure imaging, but without showing potential leaflet deflection.
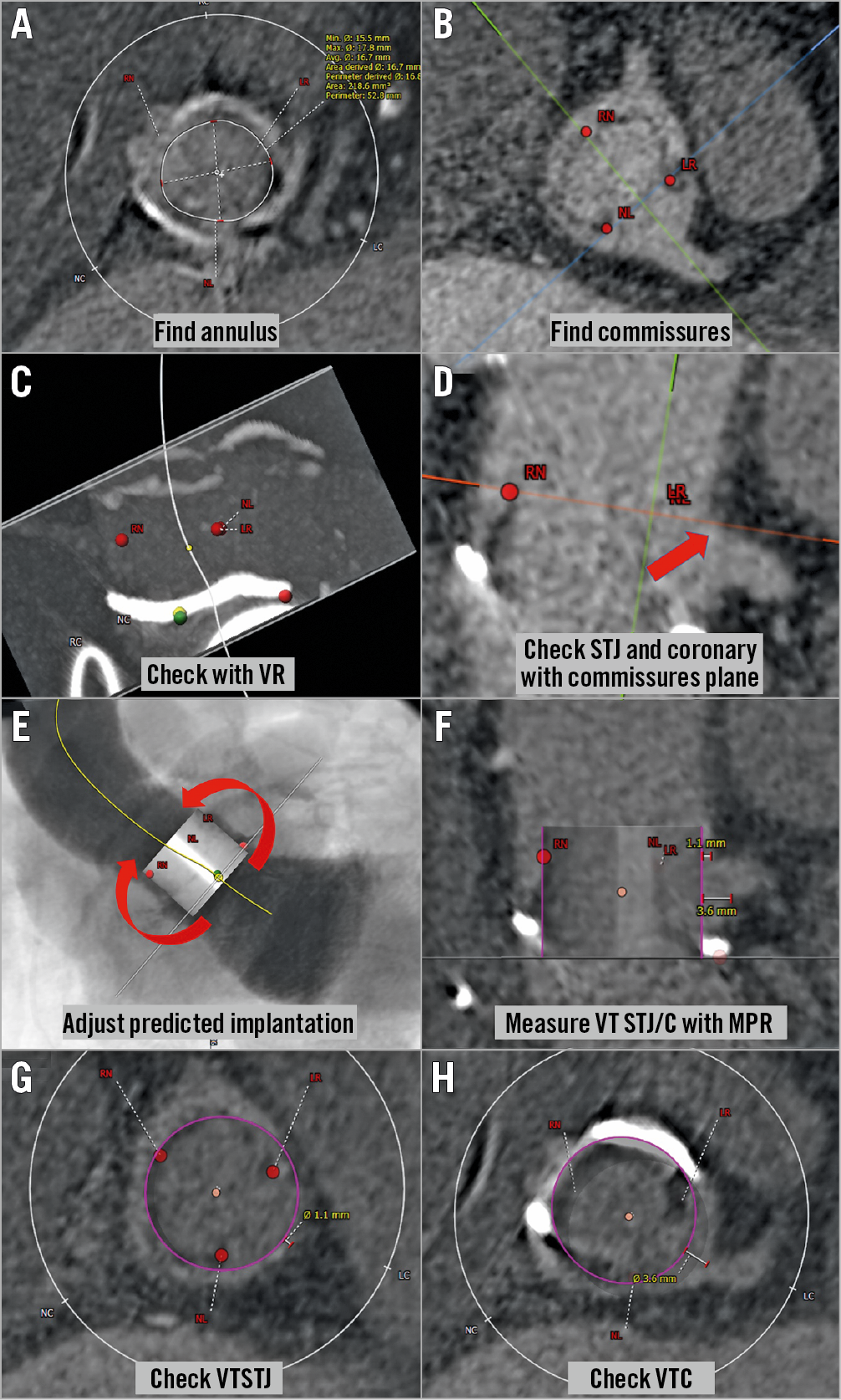
Figure 3. Steps in the assessment of the risk for coronary obstruction using CT in a Mitroflow 21 mm valve. A) Find the annulus plane of the surgical valve. B) Find and mark each stent post (LR: left-right commissure; NL: non-left commissure; RN: right-non commissure) with confirmation in an MPR image. C) If necessary, check and adjust the position of each of the three marked points of the annulus and stent post in a volume-rendering image. D) In an MPR image, draw the plane which includes all three stent posts and check the relationship with the coronary artery and STJ. E) Insert virtual valve (20 mm circle in this case) and adjust the position if necessary. F) Measure the VTSTJ and VTC in an MPR image. G) & H) Check the measurements with axial images. MPR: multiplanar reconstruction; STJ: sinotubular junction; VR: volume rendering; VTC: virtual transcatheter heart valve to coronary ostium distance; VTSTJ: virtual transcatheter heart valve to sinotubular junction distance
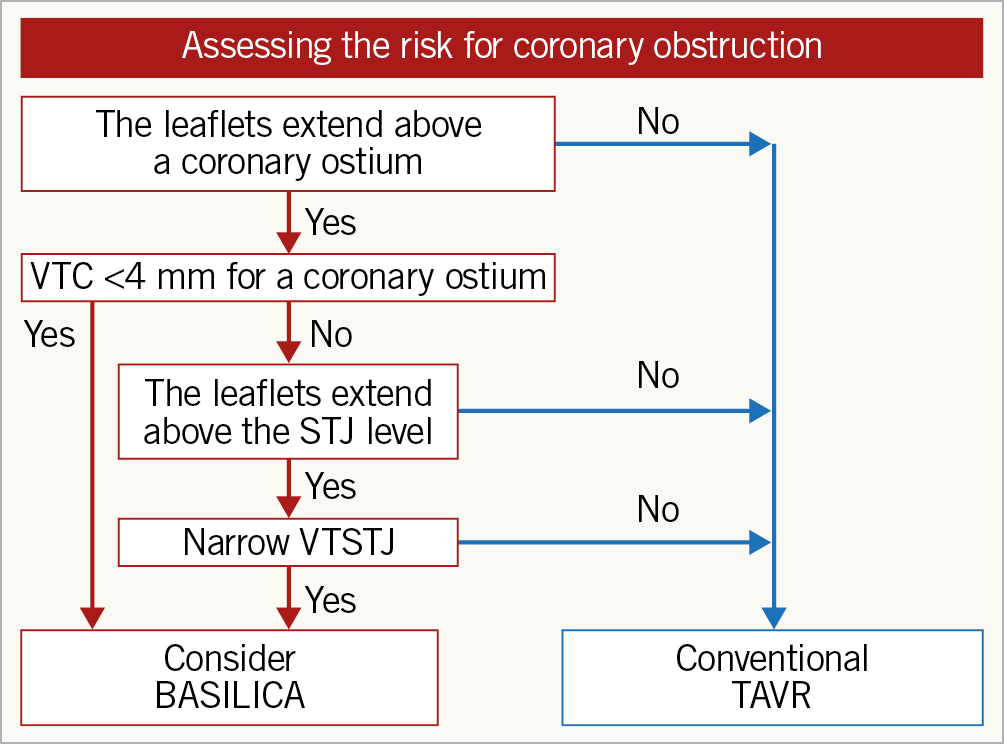
Figure 4. Assessment of the risk for coronary obstruction and BASILICA procedure consideration. Assessment starts with observing the relationship between the coronary ostia and leaflet commissures. If the coronary ostia originate above the commissures, the leaflet will not reach and cover the coronary artery. If the commissure comes above, measure the VTC for the coronary ostia and, if it is less than 4 mm, consider BASILICA. When the VTC is >4 mm, check the risk for STJ-inflow obstruction by evaluating the relationship between the STJ and the leaflet commissures. If the VTSTJ is small, then consider BASILICA. However, there are insufficient data to suggest a high risk for obstruction in cases with a small VTSTJ. The operator also needs to assess leaflet bulkiness, and the position of stent posts in case they are in front of a coronary ostium. STJ: sinotubular junction; TAVR: transcatheter aortic valve replacement; VTC: virtual transcatheter heart valve to coronary ostium distance; VTSTJ: virtual transcatheter heart valve to sinotubular junction distance
In certain surgical valve conditions, canting of the bioprosthesis can result in significantly higher risk for coronary obstruction than would be predicted by coronary height and sinus width measurements7. As a result, after identifying the sewing ring plane or the basal ring, it is essential to evaluate the geometrical axis of the surgical prosthesis at the level of the coronary artery ostia, which is commonly divergent from the long axis of the aortic root. In cases where a coronary ostium originates below the top of the deflected native or bioprosthetic valve leaflet (Type II/III) (Figure 1), the anticipated distance of the failed valve’s deflected leaflet to the coronary ostia should be measured (virtual transcatheter heart valve to coronary ostium distance [VTC]). The method of VTC assessment has been described before. Using this CT-derived parameter seems to be a meaningful tool to predict coronary obstruction5. This is optimally performed by superimposing a virtual ring simulating the diameter of the anticipated expanded THV centred along the geometrical centre of the surgical prosthesis followed by a calliper measurement from the ring towards each coronary ostium. The size of the virtual THV should be according to the size of the THV imposed leaflet deflection at that region. Commonly, for SAPIEN devices (Edwards Lifesciences, Irvine, CA, USA), the size of the deflection is the actual size of the device (unless the balloon is planned to be underfilled or the region is very close to a non-distended ring), while in Evolut (Medtronic, Minneapolis, MN, USA) cases that deflection size is between the waist of the device (20 mm for Evolut #23 and 22 mm for Evolut #26) and its nominal size, depending on coronary height and implantation depth. Future calculators will define better the amount of deflection in Evolut cases according to coronary height and presumed implant depth. In addition, SAPIEN devices have the potential to “flare out” above the surgical valve ring, potentially exacerbating the risk of coronary obstruction, while the Evolut devices are less likely to do so. In cases where the top of the deflected leaflets can cover the STJ, the distance between the deflected leaflet and the STJ wall should be measured as well (virtual transcatheter heart valve to sinotubular junction distance [VTSTJ]). VTC and VTSTJ quantify the capacity of the aortic valve complex to accommodate the THV while maintaining flow to the coronary arteries and account for the frequent eccentric position of the native or surgical prosthesis within the aortic root. Shorter VTC distances confer, at least mechanistically, an increased hazard for coronary obstruction (Figure 5). Data from the VIVID registry reveal that a VTC <4 mm is associated with a high risk of coronary obstruction with high sensitivity and specificity (85% and 89%, respectively)4, albeit with low positive predictive value related to the low incidence of coronary obstruction in clinical practice. There are no clinical data yet regarding the accurate VTSTJ length threshold that puts patients at risk for coronary obstruction and whether this should be different from the 4 mm indicated by VTC. Flow to a coronary sinus can be indirect in borderline cases with a VTSTJ of 3-4 mm and sufficient.
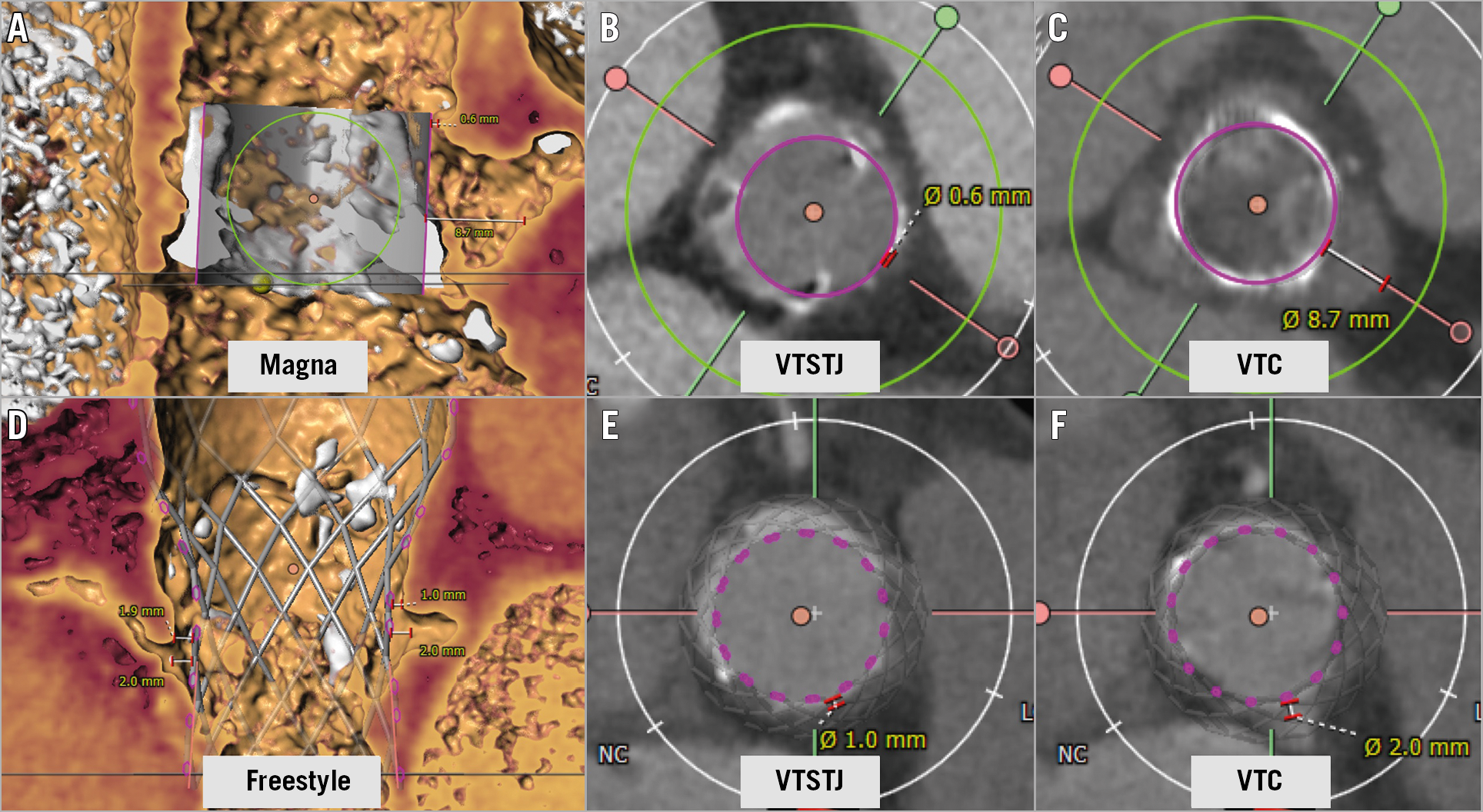
Figure 5. Examples of cases at risk for coronary obstruction in a Magna valve and a Freestyle surgical valve. A) Measurements of left VTSTJ and VTC of a Magna surgical valve with SAPIEN valve in volume rendering image. B) Very narrow VTSTJ and C) wide VTC. This case should be considered high risk for coronary obstruction after TAVR at the STJ level (Type IIIC). D) Measurements of left and right VTSTJ and VTC of Freestyle surgical valve with Evolut valve in volume rendering image. E) Narrow VTSTJ and F) narrow VTC in axial images. This case should be considered high risk for coronary obstruction at both the STJ level and coronary ostium level on both right and left coronary arteries (Type IIIB). VTC: virtual transcatheter heart valve to coronary ostium distance; VTSTJ: virtual transcatheter heart valve to sinotubular junction distance
Assessing the relationship between the deflected leaflet and ostium of a coronary artery or inflow of a coronary cusp is commonly challenging. It is difficult to predict how much that leaflet will be deflected after TAVR. In addition, it is not clear where the exact position of the top of these leaflets will be and whether they can go above the coronary ostial level. Other challenges include how to account for leaflet calcification and bulkiness, how to consider the risk in non-dominant or post-bypass vessels and where to consider the location of the anatomical coronary ostium. There has already been significant progress in this field in recent years and there will surely be more to come.
ECHOCARDIOGRAPHIC ASSESSMENT
There is only limited clinical experience of utilising echocardiography in assessing the risk for coronary obstruction in candidates for TAVR. Advantages of echocardiography include its ability to assess in real time the movement of valve leaflets which may, in some cases, enable better appreciation of whether these leaflets can deflect above the plane of a coronary ostium. Echocardiography does not require contrast in comparison to CT, which is clearly beneficial in patients with advanced renal failure. VTC assessments from echo images are rarely performed. On the other hand, real-time fusion imaging of fluoroscopy and echocardiography may have merits both in assessing the risk for obstruction and in supporting BASILICA procedures.
Leaflet orientation and coronary ostia eccentricity
DETERMINATION OF CATH LAB PROJECTION ANGLES FOR BASILICA
BASILICA requires deeper anatomical understanding of coronary cusps and leaflets to achieve precise traversal and laceration. There are several concepts which are novel, since no previous interventional procedures required accurate orientation of a single coronary cusp in the cath lab. For the procedure, two perpendicular fluoroscopic angles are usually required to understand the catheter position for wire traversal. These are the “front” and “side” projection angles, that are unique for each coronary cusp. To capture these views, a process called “commissural alignment” is utilised during CT analysis (Figure 6).
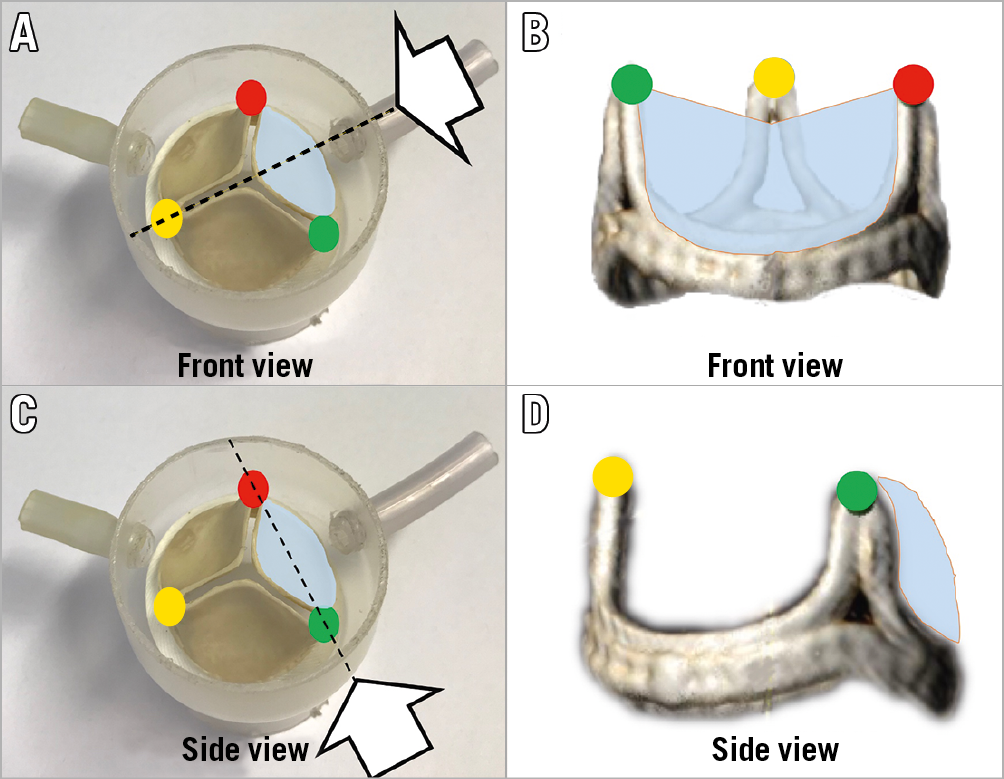
Figure 6. Illustration of commissural alignment. Illustration of “front” (A & B) and “side” (C & D) views of an aortic valve leaflet. Each commissure is indicated by a red, yellow or green circle. A coronary leaflet is highlighted in blue. The white arrows indicate the front view (B) and side view (D) of a bioprosthetic surgical valve.
In order to align the three commissures using CT, each native aortic valve or bioprosthetic valve commissure (right-left commissure, left-non commissure and non-right commissure) is marked. To mark each commissure, several cardiac phases should be carefully scanned to find the best phase to see the commissure. Once the three marks are located, the operator needs to rotate the image to assess the front and side views of the target coronary cusp in 3D evaluation, such as volume rendering. The fluoroscopic angles for each view are typically very close to the conventional S-shaped curve of the annulus plane (Figure 7). Given the anatomical characteristics of the aortic valve complex, the right cusp front view is close to anteroposterior (AP) (or mildly LAO/CRA) and the left side view is in an LAO/CRA projection. Typically, the left cusp front view is in a significant RAO/CAU projection. The right cusp side view is in an even more severe RAO/CAU projection or in an extreme LAO/CRA projection. The feasibility of these angles in the catheter laboratory could be categorised as “achievable”, “challenging” and “unachievable” (Figure 8A). If a projection is unachievable in the cath lab, an alternative projection can be used instead. To obtain an alternative projection, the operator moves the C-arm to a less acute angle (Figure 8B, Figure 8C). There is currently no clear method that enables an optimal alternative projection. In some cases, the right cusp side view is enabled during the procedure only by transoesophageal echocardiography (TEE) guidance or by tilting the patient to the left on the cath lab table.
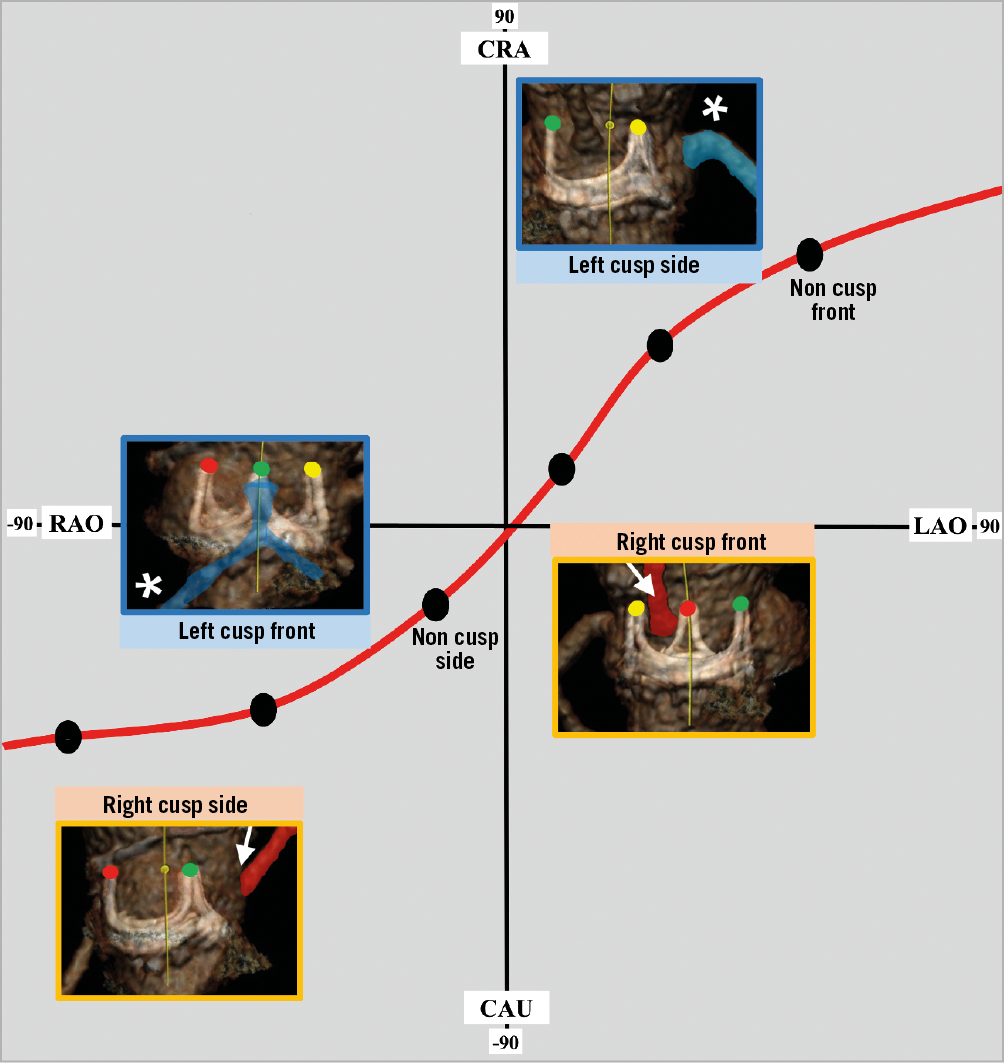
Figure 7. Leaflet projection graph. Plane of perpendicularity of the aortic valve and locations of different aortic valve leaflets – “front” and “side” views. Right cusp side view is commonly not achievable in the cath lab because of severe angulation. Red sigmoid line indicates the plane of aortic valve perpendicularity. White arrow indicates right coronary artery and white asterisk indicates left coronary artery. Each bioprosthetic valve’s commissures are marked with red, green and yellow circles. CAU: caudal; CRA: cranial; LAO: left anterior oblique; RAO: right anterior oblique
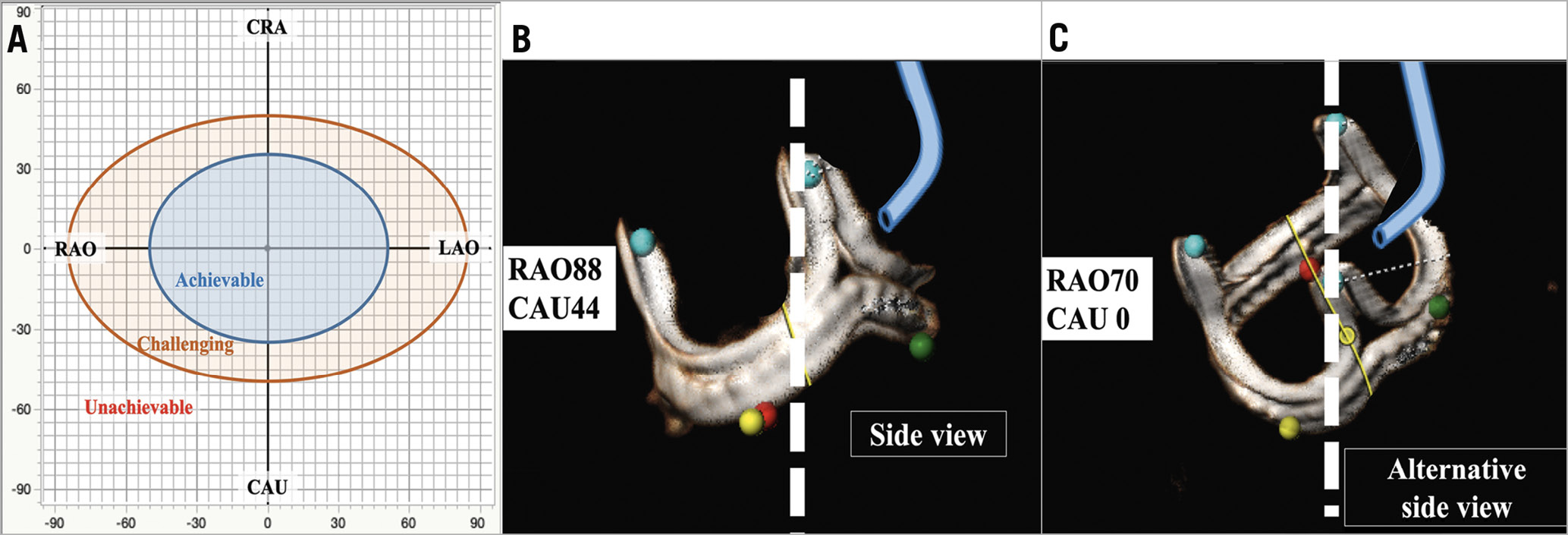
Figure 8. Feasibility assessment and an example of alternative view. Based on BASILICA case experiences, the feasibility of C-arm projections can be categorised as achievable (within LAO/RAO±50, CRA/CAU±35), challenging (within LAO/RAO±85, CRA/CAU±50) and unachievable (outside of achievable and challenging) (A). An example of a non-achievable right cusp side view (B) and an alternative angle for right cusp side view will be useful in this condition (C). CAU: caudal; CRA; cranial; LAO: left anterior oblique; RAO: right anterior oblique
ASSESSMENT OF CORONARY OSTIUM ECCENTRICITY
Coronary heights in relation to leaflet cusps are routinely assessed before TAVR procedures. However, the eccentric location of the two coronary ostia in relation to the coronary cusp is rarely evaluated. This is especially important in cases where BASILICA is performed in very narrow sinuses (e.g., VTC <2 mm). This is because one of the parts of the lacerated leaflets can still directly obstruct the coronary ostium when deflected. In addition, coronary ostial eccentricity can hinder coronary blood flow even after a precise centreline leaflet laceration and can make future coronary access challenging, when a guide is disturbed by a leaflet in front of the coronary artery ostium (Figure 9). Coronary ostial eccentricity angles can be assessed in short-axis images by measuring angles of the coronary ostium from the mid-cusp line. Left main ostia commonly originate in front of the centre of the left cusp. Right coronary ostia commonly deviate towards the commissure between the right cusp and non-cusps. This right coronary deviation is commonly mild (less than 15 degrees) and not clinically relevant. However, occasionally the right coronary artery originates far from the centre of the cusp (Figure 9C). These eccentric coronary ostial locations are more common in bicuspid aortic roots, in those after surgery for bicuspid valves and naturally in cases with anomalous origin of a coronary ostium. Unfortunately, we currently do not have sufficient data to support what should be done for patients with severe coronary eccentricity. Eccentric leaflet laceration could be considered in these cases.
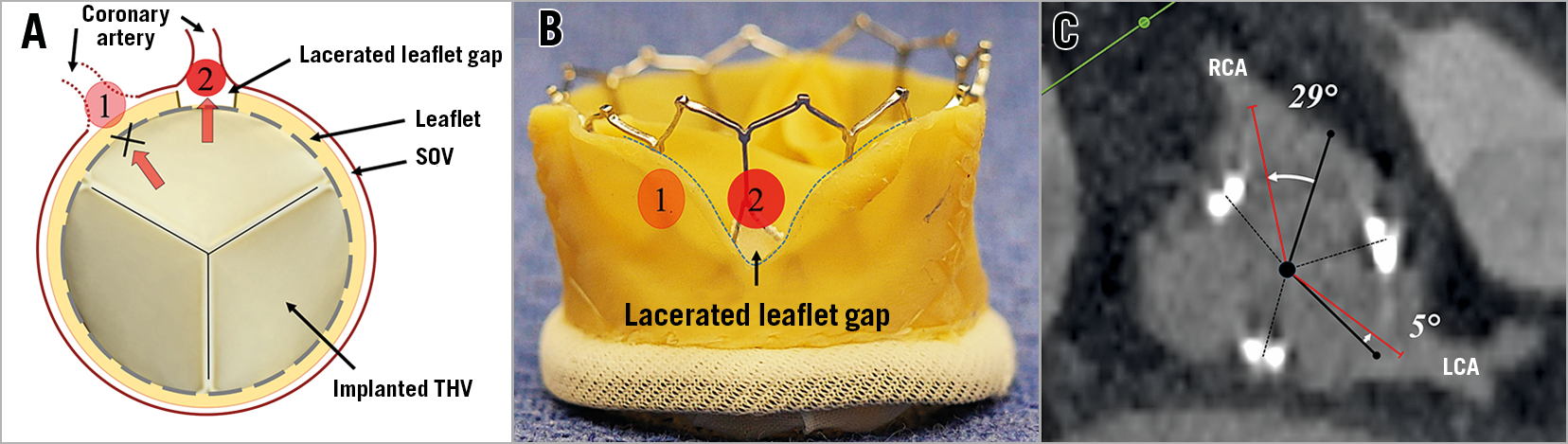
Figure 9. Illustration of coronary ostium eccentricity. Illustration of blood flow into coronary artery depending on its ostial angle (A & B) and an example of a central position of the left main ostium and an eccentric right coronary ostium (C). Coronary blood flows directly into the artery when lacerated space comes in front of the coronary ostium (number 2 in panels A & B). LCA: left coronary artery RCA: right coronary artery; SOV: sinus of Valsalva; THV: transcatheter heart valve
Conclusion
Imaging has an important role in evaluating patients for TAVR procedures. With the growth of TAVR towards younger and lower-risk populations, the need to minimise complications is more important than ever. Assessing the risk for life-threatening coronary obstruction has become more advanced than merely evaluating coronary heights and sinus widths. We propose a new nomenclature for the types of coronary ostia relationship with coronary cusps that identify cases at risk for coronary obstruction. Novel calculations, such as VTC, predict the risk for coronary obstruction and can identify patients in need of preventive measures, such as BASILICA. Orientation views of aortic valve leaflets and understanding new concepts, such as coronary ostial eccentricity, will have a more important role in the future. Assessment of coronary obstruction is work in progress. There are still uncertainties related to the prediction of implantation such as actual post-implantation THV diameter, position, shape and canting, all of which are influenced by numerous factors that are difficult to predict before TAVR. Our suboptimal ability to assess the neo-sinus size post TAVR, and hence the risk for obstruction, should be viewed in relation to BASILICA procedural risk. We believe that in the coming years methods to assess the risk for coronary obstruction will continue to evolve. These will enable us to predict the size of the neo-sinus after TAVR more accurately and to define whether this anatomy can enable sufficient coronary flow after the procedure, or whether prevention techniques, such as BASILICA, will be required.
Conflict of interest statement
D. Dvir is a consultant to Edwards Lifesciences, Medtronic, Gore, JenaValve, and Abbott. The other authors have no conflicts of interest to declare.

