Abstract
Background: The real-world outcomes of the use of the BASILICA (Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction) transcatheter technique in Europe have not been described.
Aims: We sought to evaluate the procedural and one-year outcomes of BASILICA in patients at high risk for coronary artery obstruction (CAO) undergoing transcatheter aortic valve implantation (TAVI) in a multicentre European registry (EURO-BASILICA).
Methods: Seventy-six patients undergoing BASILICA and TAVI at ten European centres were included. Eighty-five leaflets were identified as targets for BASILICA due to high risk for CAO. The updated Valve Academic Research Consortium 3 (VARC-3) definitions were used to determine prespecified endpoints of technical and procedural success and adverse events up to one year.
Results: Treated aortic valves included native (5.3%), surgical bioprosthetic (92.1%) and transcatheter valves (2.6%). Double BASILICA (for both left and right coronary cusps) was performed in 11.8% of patients. Technical success with BASILICA was achieved in 97.7% and resulted in freedom from any target leaflet-related CAO in 90.6% with a low rate of complete CAO (2.4%). Target leaflet-related CAO occurred significantly more often in older and stentless bioprosthetic valves and with higher implantation levels of transcatheter heart valves. Procedural success was 88.2%, and freedom from VARC-3-defined early safety endpoints was 79.0%. One-year survival was 84.2%; 90.5% of patients were in New York Heart Association Functional Class I/II.
Conclusions: EURO-BASILICA is the first multicentre study evaluating the BASILICA technique in Europe. The technique appeared feasible and effective in preventing TAVI-induced CAO, and one-year clinical outcomes were favourable. The residual risk for CAO requires further study.
Introduction
Coronary artery obstruction (CAO) is a rare but serious complication of transcatheter aortic valve implantation (TAVI), with mortality rates that can reach up to 50%1. It occurs when the diseased aortic valve leaflet is pushed aside towards one or both coronary ostia by the newly implanted transcatheter heart valve (THV) causing direct obstruction of the ostium or sequestration of the corresponding sinus. The highest risk for CAO has been observed following valve-in-valve (ViV) procedures for degenerated surgical tissue valves, especially when these are stentless or with externally mounted leaflets. Further risk factors include a low coronary ostial height, a narrow sinus of Valsalva, a low sinotubular junction (STJ) height and a virtual valve-to-coronary (VTC) distance <3-4 mm23.
Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction (BASILICA) is a procedure to prevent CAO by transcatheter electrosurgical leaflet splitting immediately before TAVI. It is performed by slicing the native or bioprosthetic leaflet in front of the coronary artery ostium, so that it splays open after TAVI to preserve coronary artery flow2. Initially, the BASILICA Investigational Device Exemption (IDE) Trial from the early pioneering centres showed a technical success rate of 93% with no coronary obstruction in all 30 included patients3. Recent results from a multicentre study, including 214 patients from 25 centres almost exclusively in North America, showed a procedural success rate of 86.9% and a 30-day mortality rate of 2.8%4. Target leaflet-related CAO was reported in 4.7% of patients.
In Europe, a single-centre study of 21 patients at a high-volume centre showed a similarly high technical success rate and effective prevention of CAO in 90% of cases, with only one case of partial coronary obstruction5. Since further data on the feasibility, safety and efficacy of BASILICA beyond the boundaries of high-volume pioneering centres are necessary before this treatment option can further expand, we sought to report procedural and one-year outcomes of the procedure from a multicentre European registry.
Methods
Study population
EURO-BASILICA is a multicentre registry that collected data from patients undergoing BASILICA and TAVI between December 2017 and October 2021 across ten European centres (Supplementary Appendix 1). All patients were considered at prohibitive risk for surgical aortic valve replacement and had a high risk for CAO when undergoing TAVI. Informed consent was obtained from all patients before the procedure and the individual anonymised data sharing was performed according to the instructions of the local ethics committee of each participating centre. Patients were retrospectively and prospectively identified by each centre and entered into a dedicated case report form (database), including baseline and periprocedural TAVI characteristics, BASILICA details, as well as in-hospital and follow-up outcomes.
The BASILICA procedure
Computed tomography (CT) was performed to assess anatomical features and preprocedural planning. The final decision to perform TAVI with BASILICA, the use of general anaesthesia and the application of cerebral embolic protection for each individual case was made by the multidisciplinary Heart Team. We included all patients with an attempted BASILICA procedure that were deemed to be at increased risk for CAO from each participating centre.
A minimum of two BASILICA procedures were proctored by an external expert at each centre. A detailed description of the BASILICA technique has been published elsewhere26. In brief, an electrified guidewire is used to penetrate the base of the target leaflet and is snared in the left ventricular outflow tract. Afterwards, a kink with a focally denuded inner surface is created in the guidewire and positioned at the base of the leaflet. Under tension the guidewire is electrified, and the leaflet is lacerated so that is splays open in front of the coronary ostium when TAVI is performed as the next step.
Study endpoints
The study aimed to evaluate the feasibility, efficacy and safety of the BASILICA technique. Feasibility was defined as technical success of BASILICA and included successful leaflet traversal and laceration without injury of the surrounding structures and successful retrieval of the system, measured at the time of leaving the procedure room. Procedural success was based on the recently published Valve Academic Research Consortium 3 (VARC-3) recommendations for composite endpoints addressing short-term procedure-related issues after achieving technical success7 and was modified to accommodate specific outcomes of interest related to BASILICA. It includes successful BASILICA, freedom from target leaflet-related cardiac structural complications (including CAO), freedom from mortality caused by BASILICA and freedom from surgery or interventions related to BASILICA, at the time of discharge.
Early procedural safety was defined according to the updated VARC-3 recommendations, which is a composite of all-cause mortality, all stroke, VARC-defined bleeding type 2-4, major vascular complications, access-related or cardiac structural complications, acute kidney injury (stage 3/4), moderate to severe aortic regurgitation, new pacemaker implantation due to procedure-related conduction abnormalities and valve-related dysfunction requiring re-intervention at 30 days7.
Additional endpoints included periprocedural myocardial infarction, coronary obstruction at 30 days and one year, survival at 30 days and one year, and New York Heart Association (NYHA) Functional Class I/II at 30-day and one-year follow-up.
Statistical analysis
Continuous variables were summarised as mean and standard deviation or as median and interquartile range (IQR), and comparisons between groups were performed by using the unpaired t-test and the Mann-Whitney U test, as appropriate. Categorical variables were presented as counts and percentages and were compared between groups using the chi-squared test or Fisher’s exact test, as appropriate. Kaplan-Meier estimates were performed for survival analysis and group comparisons were made using the log-rank test. A two-tailed p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS, version 28 (IBM).
Results
Baseline characteristics and preprocedural risk assessment
A total of 76 patients were enrolled across ten centres in Europe (Figure 1). Baseline characteristics are summarised in Table 1. Most of the treated valves (92.1%) were surgical bioprosthetic (62.9% externally mounted stented and 14.2% stentless) valves. Failed transcatheter valves were treated in 2.6%. The median age of the degenerated heart valves was 9 (IQR 7-11) years. The mode of degeneration and indication for TAVI was aortic stenosis in 47.4% and aortic regurgitation in 29.0% of patients. A combination of both was present in 23.7%. The effective orifice area was 0.7 (IQR 0.6-1.0) cm2 with a peak and mean gradient of 55 (IQR 43-70) and 33 (IQR 24-44) mmHg, respectively. A detailed description of the initial valve type is summarised in Supplementary Table 1.
A total of 85 leaflets with high predicted risk for CAO were defined as targets for BASILICA. The preprocedural risk assessment is summarised in Table 1. All leaflets had at least one of the following risk factors for TAVI-related CAO: coronary height on the target site <10 mm (87.1%), VTC on the target site <4 mm (68.2%), valve-to-STJ (VTSTJ) on the target site <2 mm (63.5%). The predicted mechanism of CAO was sinus deficiency in 27.1% of patients, sinus sequestration in 25.9% and a combination of both mechanisms in 47.0%.
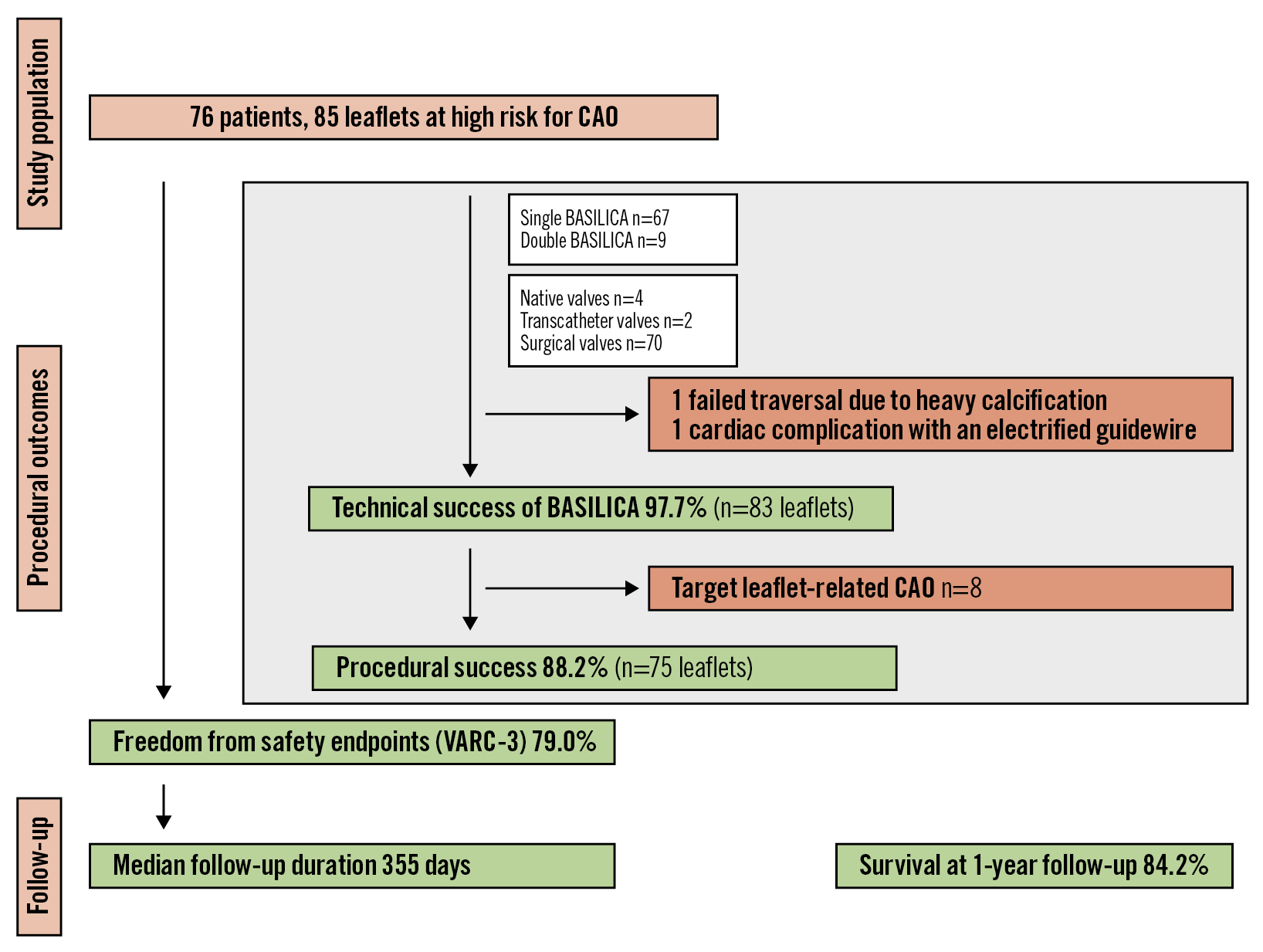
Figure 1. Study flowchart. Seventy-six patients from ten European centres were included in EURO-BASILICA and 85 leaflets were defined as being at high risk for coronary artery obstruction. Patients were evaluated for procedural outcomes, safety endpoints and one-year survival. BASILICA: Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction; CAO: coronary artery obstruction; VARC: Valve Academic Research Consortium
Table 1. Baseline characteristics and preprocedural risk assessment.
| n=76 patientsn=85 leaflets | ||
|---|---|---|
| Age, years | 79.0±5.5 | |
| Female | 46 (60.5) | |
| Comorbidities | STS-PROM score | 4.8 [3-8] |
| Logistic EuroSCORE | 28 [18-40] | |
| EuroSCORE II | 10.2 [7-15] | |
| NYHA Functional Class III or IV | 60 (79.0) | |
| Coronary artery disease | 37 (48.7) | |
| Prior percutaneous intervention | 15 (19.7) | |
| Prior coronary artery bypass surgery | 22 (29.0) | |
| Hypertension | 66 (86.8) | |
| Diabetes mellitus | 15 (19.7) | |
| Peripheral artery disease | 9 (11.8) | |
| Prior myocardial infarction | 7 (9.2) | |
| Prior stroke | 8 (10.5) | |
| Pacemaker or ICD | 11 (14.5) | |
| Pulmonary hypertension | 37 (48.7) | |
| End-stage kidney disease on dialysis | 3 (4.0) | |
| Liver cirrhosis | 3 (4.0) | |
| Oral anticoagulant | 32 (42.1) | |
| Indication for TAVI | Aortic stenosis | 36 (47.4) |
| Aortic regurgitation | 22 (29.0) | |
| Mixed aetiology | 18 (23.7) | |
| Effective orifice area (if stenotic failure), cm2 | 0.7 [0.6-1.0] | |
| Peak gradient, mmHg | 55 [43-70] | |
| Mean gradient, mmHg | 33 [24-44] | |
| Baseline LVEF, % | 55 [45-62] | |
| Diseased aortic valve | ||
| Native | 4 (5.3) | |
| Transcatheter | 2 (2.6) | |
| Surgical | 70 (92.1) | |
| Internally mounted, stented | 16 (22.9) | |
| Externally mounted, stented | 44 (62.9) | |
| Stentless | 10 (14.2) | |
| Age of degenerated valve, years | 9.0 [7-11] | |
| Risk evaluation of coronary obstruction | ||
| Coronary height on the target site, mm | 7.0±2.4 | |
| <10 mm | 74 (87.1) | |
| STJ height on the target site, mm | 14.6 [12.7-16.8] | |
| VTC on the target site, mm | 3.5 [3.0-4.3] | |
| <4 mm | 58 (68.2) | |
| <3 mm | 19 (22.4) | |
| VTSTJ on the target site | 1.5 [0.3-2.25] | |
| <2 mm | 54 (63.5) | |
| <1 mm | 24 (28.2) | |
| Predicted mechanisms of coronary obstruction | Sinus deficiency (VTC <4 mm) | 23 (27.1) |
| Sinus sequestration (VTSTJ <2 mm) | 22 (25.9) | |
| Combined sinus deficiency and sequestration | 40 (47.0) | |
| Values are n (%), mean±standard deviation or median [interquartile range]. EuroSCORE: European System for Cardiac Operative Risk Evaluation; ICD: implantable cardioverter defibrillator; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; STJ: sinotubular junction; STS-PROM: Society of Thoracic Surgeons Predicted Rate of Mortality; TAVI: transcatheter aortic valve implantation; VTC: virtual transcatheter valve-to-coronary distance; VTSTJ: virtual transcatheter valve-to-sinotubular junction distance | ||
Procedural characteristics
Single-leaflet BASILICA was attempted in 88.2% of cases and double-leaflet BASILICA in 11.8% of cases. All procedures were performed under general anaesthesia with transoesophageal echocardiography. The procedural and technical details of BASILICA are presented in Table 2 and Supplementary Table 2. Cerebral protection devices were used in 89.5% of patients. The median duration of BASILICA was 53 minutes (IQR 38-77), and the total procedural time was 130 (IQR 91-166) minutes. There was a need for resuscitation in one patient due to severe aortic regurgitation after laceration and in two patients due to CAO. The latter two patients also required temporary mechanical haemodynamic support. Balloon-expandable SAPIEN 3 valves (Edwards Lifesciences) were used in 15.8% and self-expanding Evolut valves (Medtronic) in 84.2% of treated patients.
Table 2. Procedural characteristics.
| n=76 patients n=85 leaflets |
||
|---|---|---|
| Single-leaflet BASILICA | 67 (88.2) | |
| Target site left | 64 (95.5) | |
| Target site right | 3 (4.5) | |
| Double-leaflet BASILICA | 9 (11.8) | |
| Number of traversal attempts, per leaflet | 2 [1-4] | |
| Guidewire traversal into the left atrium, per leaflet | 8 (9.4) | |
| Guidewire penetration into the septum, per leaflet | 1 (1.3) | |
| Additional coronary protection, per leaflet | 14 (16.5) | |
| Lowest pressure after leaflet laceration | Systolic, mmHg | 100 [80-110] |
| Diastolic, mmHg | 40 [35-50] | |
| Type of THV | Evolut R | 64 (84.2) |
| SAPIEN 3 | 12 (15.8) | |
| Size of THV | 20 mm | 1 (1.3) |
| 23 mm | 50 (65.8) | |
| 26 mm | 18 (23.7) | |
| 29 mm | 5 (6.6) | |
| 34 mm | 2 (2.6) | |
| Cerebral embolic protection device | 68 (89.5) | |
| Transoesophageal echocardiography | 76 (100) | |
| General anaesthesia | 76 (100) | |
| Total procedure time (access to haemostasis), min | 130 [91-166] | |
| Single-leaflet BASILICA, min | 125 [85-151] | |
| Double-leaflet BASILICA, min | 220 [166-320] | |
| BASILICA time (sheath-in to laceration), min | 53 [38-77] | |
| Time from laceration to THV implantation, min | 7 [6-12] | |
| Fluoroscopy time, min | 44 [33-65] | |
| Volume of contrast medium used, ml | 100 [70-162] | |
| Intraprocedural complications | ||
| Target leaflet-related coronary obstruction requiring intervention | 8 (9.4) | |
| Need for increase of vasopressors | 12 (14.1) | |
| Resuscitation | 3 (3.5) | |
| Due to severe AR after laceration | 1 (1.2) | |
| Due to coronary obstruction | 2 (2.4) | |
| Requiring mechanical haemodynamic support | 2 (2.4) | |
| Injury of a non-target structure | 1 (1.2) | |
| Values are n (%) or median [interquartile range]. AR: aortic regurgitation; BASILICA: Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | ||
PROCEDURAL OUTCOMES
Technical success of BASILICA was achieved in 97.7% of leaflets (Table 3). In one patient, guidewire traversal failed because of a severely calcified target leaflet, and the target coronary artery was treated with ostial stenting using the chimney technique. A second patient experienced injury of the target coronary artery by the electrified guidewire resulting in haematoma and myocardial infarction. The patient was treated with orthotopic stenting after BASILICA. Successful laceration could be performed in all successfully traversed leaflets.
The procedural success rate of BASILICA was 88.2%, and procedural failure was mainly driven by partial CAO (Central illustration). Intraprocedural coronary obstruction requiring intervention was reported in ten patients, with eight events attributable to the target leaflet. The rate of periprocedural myocardial infarction was 4.0%. Detailed characteristics of patients who experienced CAO are summarised in Table 4.
Two patients had total target leaflet-related CAO. One had an occlusion of the left coronary artery (LCA) requiring extracorporeal membrane oxygenation for one hour; the occlusion was treated with ostial stenting using the chimney technique. The patient was lost to follow-up. The second patient had a total obstruction of the LCA ostium due to partial avulsion of a bioprosthetic valve leaflet. The obstruction was treated with orthotopic ostial stenting and required Impella (Abiomed) support. The patient experienced a periprocedural myocardial infarction but survived with an NYHA Functional Class I at 405 days.
Five patients were treated with orthotopic ostial stenting due to partial obstruction of the target coronary artery secondary to leaflet prolapse without signs of coronary ischaemia (Supplementary Figure 1). Freedom from any target leaflet-related CAO was 90.6%.
Freedom from the early safety composite endpoint was met in 79.0% of cases. In-hospital mortality occurred in one patient who died on day 22 after haemorrhagic shock due to injury of the right external iliac artery caused by a ruptured post-dilatation balloon. Mortality was adjudicated as a cardiovascular death not related to BASILICA. During the periprocedural period, there was one cardiac tamponade, one major ischaemic stroke (in a patient in whom no cerebral embolic protection was used) and five VARC-3 defined bleedings type 2-4. Other clinically important periprocedural complications included acute kidney injury stage 3-4 in one patient and major vascular complications in two patients. Three patients required implantation of a pacemaker due to conduction abnormalities.
Postprocedural echocardiographic outcomes are summarised in Supplementary Table 3. The effective orifice area at discharge was 1.5 (IQR 1.3-1.7) cm2 with a peak and mean gradient of 20 (IQR 14-29) and 12 (IQR 8-16) mmHg, respectively. Moderate or greater than moderate paravalvular leakage was not observed.
Table 3. Procedural outcomes.
| n=76 patientsn=85 leaflets | |
|---|---|
| Technical success of BASILICA | 83/85 (97.7) |
| Successful leaflet traversal | 84 (98.8) |
| Successful leaflet laceration | 84 (98.8) |
| No injury of surrounding structures | 84 (98.8) |
| Successful retrieval of the system | 85 (100) |
| Procedural success | 75/85 (88.2) |
| Successful BASILICA | 83 (97.7) |
| Freedom from target leaflet-related coronary obstruction | 77 (90.6) |
| BASILICA-related mortality | 0 (0) |
| Freedom from early safety endpoints at 30 days (VARC-3) | 60/76 (79.0) |
| All-cause mortality | 1 (1.3) |
| All-stroke | 2 (2.6) |
| Access-related or cardiac structural complications | 14 (18.4) |
| Coronary events requiring intervention | 10 (13.2) |
| Periprocedural myocardial infarction | 3 (4.0) |
| Major vascular complications | 2 (2.6) |
| Injury of cardiac structures | 1 (1.3) |
| Cardiac tamponade | 1 (1.3) |
| Bleeding type 2-4 | 5 (6.6) |
| Acute kidney injury, stage 3-4 | 1 (1.3) |
| New pacemaker implantation | 3 (4.0) |
| Moderate to severe aortic regurgitation | 0 (0) |
| Conversion to surgery or reintervention | 0 (0) |
| Values are n (%). BASILICA: Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction; VARC: Valve Academic Research Consortium | |
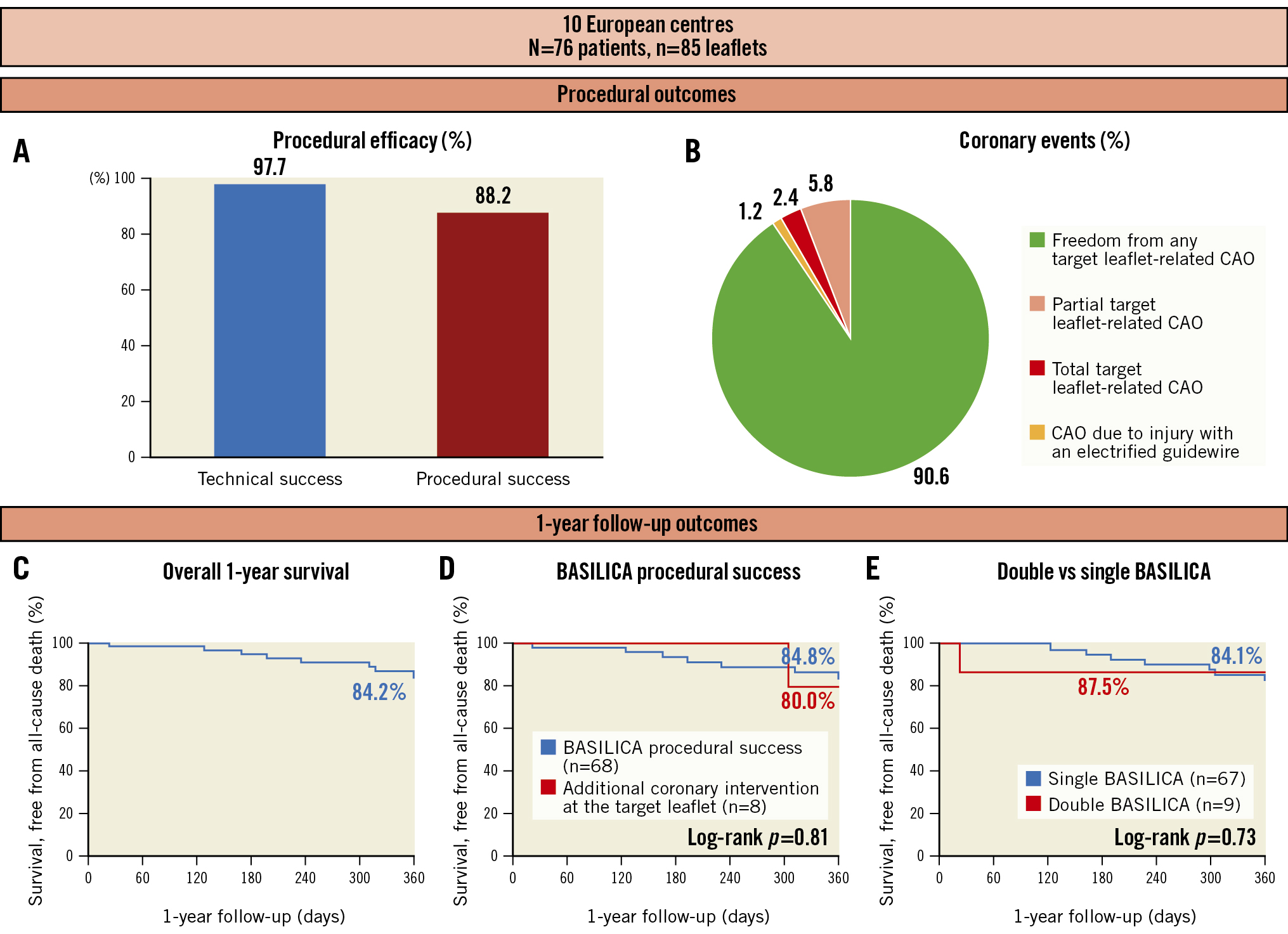
Central illustration. Procedural outcomes and survival after BASILICA in patients at high risk for coronary artery obstruction undergoing TAVI. A) Graphical representation of procedural outcomes showing technical and procedural success rates. B) Pie chart showing the distribution of coronary events and freedom from CAO. C) Kaplan-Meier event curves for one-year survival for the whole cohort. D) Survival according to procedural success of BASILICA (blue line) or the need for additional coronary intervention at the target leaflet (red line). E) Survival according to single-leaflet (blue line) and double-leaflet BASILICA (red line). BASILICA: Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction; CAO: coronary artery obstruction
Table 4. Coronary events or additional coronary interventions.
| Sex | Initial valve | THV | Target coronary | VTC, mm | VTSTJ, mm | Coronary height, mm | STJ height, mm | Details | Target leaflet- related | Additional procedures | Clinical outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | Freedom-Solo 27 mm | SAPIEN 3 29 mm | Left | Lt. 4.0 Rt. 2.9 | Lt. 0.0 Rt. 2.3 | Lt. 8.5 Rt. 12.3 | Lt. 16.2 Rt. 19.6 | Total occlusion of the RCA due to direct coverage by a leaflet not targeted by BASILICA | No | RCA ostial stenting (chimney technique) | No further clinical sequelae, non-CV death (168 days) |
| M | Native valve | SAPIEN 3 23 mm | Left | 5.6 | 1.2 | 7.2 | 17.0 | Partial obstruction due to leaflet prolapse without ischaemic signs | Yes | LMCA orthotopic ostial stenting | No further clinical sequelae, unknown death (309 days) |
| F | Native valve | SAPIEN 3 23 mm | Left | 5.0 | 0.0 | 11.1 | 17.4 | Partial obstruction due to leaflet prolapse without ischaemic signs | Yes | LMCA orthotopic ostial stenting | No further clinical sequelae, survival, NYHA II (185 days) |
| F | Freedom-Solo 21 mm | Evolut R 23 mm | Left | 3.6 | n/a | 10.9 | 15.1 | Partial obstruction due to leaflet prolapse without ischaemic signs | Yes | LMCA orthotopic ostial stenting | No MI, survival, NYHA I (55 days) |
| F | Toronto 27 mm | Evolut R 29 mm | Left | 2.7 | 1.7 | 7.4 | 19.1 | Partial obstruction due to leaflet prolapse without ischaemic signs | Yes | LMCA orthotopic ostial stenting | No further clinical sequelae, survival, NYHA I (355 days) |
| M | Freedom-Solo 25 mm | SAPIEN 3 26 mm | Left | 0.9 | 0.4 | 8.1 | 19.7 | Total obstruction due to leaflet prolapse into the LCA ostium after TAVI | Yes | LMCA orthotopic ostial stenting, Impella for LV unloading | MI, survival, NYHA I (405 days) |
| F | MitroFlow 21 mm | Evolut R 23 mm | Left | Lt. 2.6 Rt. 0.8 | Lt. 0.0 Rt. 0.0 | Lt. 4.5 Rt. 7.7 | Lt. 11.3 Rt. 13.4 | Partial obstruction of the RCA | No | RCA orthotopic ostial stenting | No further clinical sequelae, survival, (78 days) |
| F | MitroFlow 21 mm | Evolut R 23 mm | Both | Lt. 1.8 Rt. 2.0 | Lt. 0.0 Rt. 1.1 | Lt. 0.6 Rt. 4.7 | Lt 7.1 Rt. 10.4 | Partial obstruction | Yes | LMCA orthotopic ostial stenting | No further clinical sequelae, survival, (92 days) |
| F | MitroFlow 21 mm | Evolut R 23 mm | Both | Lt. 3.3 Rt. 2.6 | Lt. 1.3 Rt. 1.5 | Lt. 4.6 Rt. 6.1 | Lt. 8.9 Rt. 12.8 | Total occlusion of target coronary (LCA) requiring ECMO | Yes | LMCA ostial stenting (snorkel technique) | MI, no follow-up |
| F | MitroFlow 21 mm | Evolut R 23 mm | Left | 4.1 | 2.6 | 3.8 | 16.1 | Haematoma around the LMCA due to injury by electrified guidewire with LCx embolus and pericardial tamponade | Yes | LMCA orthotopic ostial stenting, emergency pericardiocentesis | MI, minor stroke, rehabilitation with tracheostomy (34 days) |
| BASILICA: Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction; CV: cardiovascular; ECMO: extracorporeal membrane oxygenation; F: female; LCA: left coronary artery; LCx: left circumflex; LMCA: left main coronary artery; Lt: left; M: male; MI: myocardial infarction; NYHA: New York Heart Association; RCA: right coronary artery; Rt: right; STJ: sinotubular junction; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; VTC: virtual transcatheter valve-to-coronary distance; VTSTJ: virtual valve-to-sinotubular junction | |||||||||||
CLINICAL FOLLOW-UP
Clinical follow-up is summarised in Table 5 and Supplementary Figure 2. Thirty-day follow-up was available for 73 (96.1%) patients. The survival rate at 30 days was 98.7%, and 86.3% of patients presented with NYHA Functional Class I/II. There was one cardiovascular death due to major bleeding, as mentioned above, and two strokes (one major and one minor).
The overall median follow-up duration was 355 (IQR 148-370) days. After one year, there were three additional strokes but no delayed CAO. The one-year survival rate was 84.2% (Figure 1), and 90.5% of patients presented with NYHA Functional Class I/II.
Table 5. Thirty-day and one-year outcomes.
| n=76 patients | ||
|---|---|---|
| Thirty days | All death | 1 (1.3) |
| Cardiovascular | 1 (1.3) | |
| Non-cardiovascular | 0 (0) | |
| Coronary events requiring intervention | 10 (13.2) | |
| Intraprocedural | 10/10 (100) | |
| Target cusp | 8/10 (80.0) | |
| Non-target cusp | 2/10 (20.0) | |
| Delayed (after the index procedure) | 0/10 (0) | |
| Conversion to surgery | 0 (0) | |
| Stroke | 2 (2.6) | |
| Any bleeding | 10 (13.2) | |
| 30-day rehospitalisation | 6 (8.2) | |
| NYHA Functional Class I/II | 63 (86.3) | |
| One year* | All death | 8 (15.8) |
| Cardiovascular | 3 (6.3) | |
| Non-cardiovascular | 5 (10.3) | |
| Delayed coronary events requiring intervention | 0 (0) | |
| Stroke | 5 (11.5) | |
| Dialysis | 2 (4.3) | |
| Pacemaker implantation | 3 (4.2) | |
| Moderate to severe aortic regurgitation | 0 (0) | |
| SVD | 0 (0) | |
| One-year rehospitalisation | 17 (35.3) | |
| NYHA Functional Class I/II | 64 (90.5) | |
| Values are n (%). *calculated from Kaplan-Meier estimates. NYHA: New York Heart Association; SVD: structural valve deterioration | ||
PREDICTORS OF CORONARY OBSTRUCTION
Table 6 demonstrates a comparison of baseline characteristics between patients who experienced target leaflet-related coronary events and those who were free from CAO. There was a significant difference between both groups concerning the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II (p=0.013), the initial valve type (p=0.028), the age of the initial valve (p=0.025) and THV implantation depth (p=0.003). The aortic valves associated with CAO were older, were primarily stentless, and had the THV implanted at a higher (more aortic) position. Risk factors for CAO did not differ significantly between both groups. The majority of cases with CAO (6/8) occurred in centres with limited BASILICA experience.
Table 6. Baseline characteristics of patients with and without target leaflet-related coronary events.
| Coronary event (n=8) | No coronary event (n=68) | p-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | 77±7 | 79±5 | 0.400 |
| Female | 5 (62.5) | 41 (60.3) | 0.704 |
| Height, cm | 163±6 | 165±10 | 0.500 |
| Weight, kg | 73±12 | 76±15 | 0.500 |
| EuroSCORE II | 5.4 [2.6-9.2] | 12.4 [7.8-16.1] | 0.013 |
| STS-PROM | 3.2 [2.4-5.8] | 6.7 [3.2-7.7] | 0.050 |
| Target valve type | |||
| Native valve | 2 (25.0) | 2 (2.9) | 0.028 |
| Surgical bioprosthetic valve | 6 (75.0) | 64 (94.2) | |
| Internally-mounted type | 0 (0) | 16/64 (25.0) | |
| Externally-mounted type | 3/6 (50.0) | 41/64 (64.1) | |
| Stentless type | 3/6 (50.0) | 7/64 (10.9) | |
| Transcatheter heart valve | 0 (0) | 2 (2.9) | |
| Age of the initial valve, years | 11 [10-15] | 9 [7-10] | 0.025 |
| Indication for TAVI | |||
| Aortic stenosis | 4 (50.0) | 32 (47.1) | 0.967 |
| Aortic regurgitation | 2 (25.0) | 20 (29.4) | |
| Mixed aetiology | 2 (25.0) | 16 (23.5) | |
| Procedural characteristics | |||
| Centre with limited BASILICA experience | 6 (75.0) | 27 (39.7) | 0.071 |
| During initial proctoring | 5 (62.5) | 22 (32.4) | 0.124 |
| Implanted THV | |||
| SAPIEN 3 | 3 (37.5) | 10 (14.7) | 0.132 |
| Evolut R | 5 (62.5) | 58 (85.3) | |
| Degree of diameter oversizing, % | 13.5 [4.13-14.45] | 5.00 [-1.72 to 11.13] | 0.153 |
| Implantation depth, mm | 2.7 [0.40-4.33] | 6.80 [5.40-11.7] | 0.003 |
| Bioprosthetic valve fracture | 0 (0) | 11 (16.2) | 0.594 |
| Use of dextrose | 8 (100) | 62 (91.2) | 1.000 |
| Commissural misalignment LCA | 1 (12.5) | 9 (13.2) | 1.000 |
| Commissural misalignment RCA | 1 (12.5) | 12 (17.6) | 1.000 |
| Postdilatation | 3 (37.5) | 35 (51.5) | 0.711 |
| Postdilatation balloon size, mm | 19.3±1.2 | 20.9±1.9 | 0.102 |
| Risk evaluation of coronary obstruction | n=8 leaflets | n=77 leaflets | p-value |
| Coronary height on the target site, mm | 6.7±3.6 | 7.1±2.2 | 0.350 |
| STJ height on the target site, mm | 17.4±2.0 | 14.9±3.6 | 0.070 |
| VTC on the target site, mm | 3.5 [2.0-4.8] | 3.5 [3.0-4.3] | 0.763 |
| VTSTJ on the target site, mm | 1.2 [0-1.7] | 1.5 [0.7-2.4] | 0.289 |
| Predicted mechanisms of CAO | |||
| Sinus deficiency | 2 (25.0) | 21 (27.3) | 0.187 |
| Sinus sequestration | 2 (25.0) | 20 (26.0) | |
| Combined sinus deficiency and sequestration | 4 (50.0) | 36 (46.7) | |
| Values are n (%), means±standard deviation or median [interquartile range]. BASILICA: Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction; EuroSCORE: European System for Cardiac Operative Risk Evaluation); CAO: coronary artery obstruction; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LCA: left coronary artery; RCA: right coronary artery; STJ: sinotubular junction; STS: Society of Thoracic Surgeons Predicted Rate of Mortality; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; VTC: virtual transcatheter valve-to-coronary distance; VTSTJ: virtual transcatheter valve-to-sinotubular junction distance | |||
Discussion
Coronary obstruction is a catastrophic complication of TAVI and is associated with a markedly increased mortality1. BASILICA has been developed to prevent CAO by transcatheter electrosurgical leaflet splitting immediately before TAVI, but the procedure remains complex and requires special training. In addition, results from multicentre studies on feasibility and safety have been scarce and were predominantly limited to centres in the United States. Following the publication of encouraging single-centre results from two European groups58, EURO-BASILICA is the first European multicentre experience evaluating this technique with an extended follow-up up to one year. The study demonstrates a high technical and procedural success, which did not differ between single- and double-leaflet procedures. We also observed an encouraging rate of freedom from any target leaflet-related CAO (90.6%) with a low rate of total coronary obstruction (2.4%) within a high-risk patient cohort. Survival at one year was as high as 84.2%.
Since BASILICA is considered a complex interventional procedure requiring meticulous preprocedural planning, dedicated material and high operator expertise, its applicability in real-world practice remains questionable. The current study included several centres with limited BASILICA experience. Nevertheless, technical feasibility was high (97.7%) and comparable to previously published results, which highlights the importance of initial proctoring and continuous training. Kitamura et al5 and Khan et al4 showed feasibility rates of 95% and 94.4%, respectively. In the current study, one patient suffered from injury by the electrified guidewire, and in one patient guidewire traversal failed because of a heavily calcified leaflet. These findings go hand in hand with the results from other groups35, where traversal failure was described in a few patients with severely calcified leaflets, which underlines specific patient characteristics potentially precluding successful BASILICA. In addition, the study demonstrates an excellent 30-day survival after BASILICA of 98.7% and a one-year survival of 84.2% within a high-risk patient group. Similar to our results, the only study with long-term follow-up reported one-year survival of 83.9%4. Double BASILICA was not associated with an increased risk for complications, which supports results published earlier by Khan et al4.
The main objective of BASILICA is to prevent CAO. Despite the high predicted risk for coronary obstruction in our study population, 90.6% underwent the procedure without any coronary obstruction at the target leaflet, and notably, 97.7% were free from total CAO. However, there were two total occlusions and five partial obstructions at the culprit cusp. Of note, almost all of these cases were orthotopically stented through the struts of the THV, which was potentially enabled by BASILICA, and there were no cardiovascular deaths within this group of patients up to one year. Evidence of myocardial infarction was found in three patients (40% of all CAO), which is similar to the results published by Khan et al4. This reflects the fact that the majority of target leaflet-related coronary obstructions were non-flow-limiting obstructions. Several factors may potentially lead to these partial obstructions, such as an unpredictable mode of displacement in severely degenerated and/or bulky leaflets, an eccentric cut of a heavily calcified leaflet, or mechanical leaflet tear or leaflet avulsion instead of electrosurgical splitting. However, the incidence of this finding could also vary according to the mode of detection, since selective coronary angiography and/or intravascular imaging may be needed for diagnosis, which further raises some doubt on their clinical impact and the proper diagnostic and treatment approach. Importantly, there were no cases of delayed CAO in this study, which was an earlier concern due to potential late leaflet mobilisation9.
An evaluation of predictors for CAO was not an objective of this study, and risk factors for CAO remain incompletely understood. Nevertheless, it is noteworthy that all cases of target leaflet-related CAO occurred in native, stentless or externally mounted valves, which signifies a persistent risk for CAO in these patient groups. These findings are supported by previous studies23 and could be explained by a different mode of valve displacement after splitting. In addition to the type of the initial valve, we identified the age of the initial valve as a potential risk factor for target leaflet-related CAO, which could be related to more advanced thickening and degeneration of the leaflet tissue preventing effective splay after BASILICA. Higher THV implantation was also associated with a higher risk of target leaflet-related CAO, which is an important and potentially modifiable factor. Valves implanted at a high position may directly obliterate the sinus of Valsalva through their sealing skirt, increasing the risk of CAO in spite of an effectively lacerated leaflet. In addition, there was a numerical trend towards an association between CAO and the degree of THV oversizing as well as centre experience. However, it is worth mentioning that the geometric predictors for CAO (VTC and VTSTJ) were not associated with an increased risk for CAO at the target leaflet in this series. Since life expectancy is further increasing and the number of degenerated valves requiring reintervention will further increase, there is an important need for identifying and verifying specific risk factors for CAO. Importantly, identifying particular risk factors for target leaflet-related CAO in patients undergoing BASILICA may help identify patients who need additional measures for coronary artery protection, such as the application of intravascular ultrasound (IVUS), coronary stent placement, balloon-assisted BASILICA, specific devices that combine traversal and laceration10 or even complete leaflet removal11.
The occurrence of stroke is another major concern during BASILICA. Stroke risk may be increased by extensive manipulation of highly calcified and degenerated leaflets. Cerebral embolic protection devices are available for potential prevention of stroke, and in the current study, the use of cerebral embolic protection was high (89.5%). At 30-day follow-up, we observed neurological events in only two patients. Our low stroke rate of 2.6% at 30 days was comparable with the majority of TAVI trials without BASILICA1213. In comparison to that, the BASILICA Trial3 showed a 10% stroke rate, where cerebral embolic protection was used in only 43% of patients. Khan et al recently described a 1% stroke rate in patients who received cerebral embolic protection and a 4.5% stroke rate in those who did not receive cerebral embolic protection. However, the authors could not further comment on whether cerebral embolic protection reduced stroke due to the overall small event rate and a possible selection bias4. Whether to routinely use cerebral embolic protection during TAVI remains a matter of debate, but its application during a complex and lengthy procedure such as BASILICA may seem justified.
Another concern related to BASILICA is the potential for haemodynamic instability mainly caused by severe aortic regurgitation (AR) following laceration together with delayed THV implantation and the use of electrical current. As in previous trials35, haemodynamic instability due to AR was rare in our study population and was observed in only one patient.
Limitations
First, although our study is the largest published multicentre study that included patients from outside the United States, the study was not randomised, the overall number of included patients remains relatively small, and a large proportion of cases were performed at a single centre with a relatively extensive BASILICA experience, which limits real-world applicability and outlines the need for strict preprocedural planning and initial proctoring. Second, even though we performed a risk evaluation of all patients and provided a detailed description of all subjects who experienced target leaflet-related CAO, there is limited knowledge about independently validated, widely accepted confounders and cut-off values for predicting CAO in this population, and the lack of prespecified inclusion criteria represents a limitation. Third, our stroke rate may be underestimated because of missing uniformity in detecting and reporting clinical and subclinical strokes. Further studies are needed to investigate the risk of stroke during BASILICA and the role of cerebral embolic protection for stroke prevention. The fact that VARC-3 has recently published an updated version of standardised definitions has encouraged us to apply these recommendations to our study. However, using these updated definitions may prevent comparability with previously published studies.
Conclusions
In a multicentre European setting, BASILICA appeared to be feasible and safe in real-world practice with initial proctoring support. Clinical outcomes at 30 days and one year were favourable, with no differences between single- and double-leaflet procedures. Larger studies are needed to further evaluate specific risk factors for target leaflet-related CAO in patients undergoing BASILICA.
Impact on daily practice
The results from this first multicentre study evaluating the BASILICA technique in Europe suggest that BASILICA is feasible and safe with favourable outcomes up to one year in a real-world setting when performed with initial proctoring support. The age of the initial valve, the presence of stentless surgical valves and THV implantation depth could potentially increase the risk for target leaflet-related CAO. Future studies are needed to identify risk factors for CAO at the target leaflet for BASILICA and define specific management strategies.
Conflict of interest statement
M.Taramasso is aconsultant for Abbott Vascular, Edwards Lifesciences, Medtronic, Boston Scientific, Shenqi Medical, CoreMedic, Medira, HiD Imaging, VentriMend, Simulands, MTEx, and Occlufit. A.Unbehaun is aphysician proctor for Boston Scientific; and reports speaker honoraria from Medtronic, Boston Scientific, and Edwards Lifesciences. T.K.Rudolph received speaker honoraria from Medtronic, Boston Scientific, and Edwards Lifesciences. F.Ribichini is proctor for Edwards Lifesciences and Medtronic. R.Binderf is aproctor for Medtronic and Boston Scientific; and receives speaker honoraria from Edwards Lifesciences and Abbott Vascular. J.Schofer is aconsultant for Edwards Lifesciences. B.Trejo-Velasco received funding from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) via the 2021 EAPCI Andersen & Cribier Fellowship Program. M. Abdel-Wahab declares that his hospital receives speaker honoraria and/or consultancy fees on his behalf from Boston Scientific and Medtronic. The other authors have no conflicts of interest to declare in relation to this manuscript.
Supplementary data
To read the full content of this article, please download the PDF.

