Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) has revolutionised transcatheter aortic valve implantation (TAVI), enabling the procedure in patients otherwise ineligible because of a risk of coronary artery obstruction1. The technical complexity led some to question its broad applicability outside pioneering centres. However, reassuringly, the real-world North American experience recapitulated the positive results seen in the BASILICA trial2. Successful traversal and laceration were achieved in all attempted leaflets, 30-day survival was 97.2%, and the rate of disabling stroke was extremely low at 0.5%.
In this issue of EuroIntervention, Abdel-Wahab and colleagues detail, for the first time, the real-world outcomes of BASILICA outside North America3. They conducted a retrospective review of the EURO-BASILICA registry, from December 2017 to October 2021, at 10 participating medical centres. In 76 patients at prohibitive risk for surgical aortic valve replacement and high risk for coronary artery occlusion (CAO), BASILICA was attempted on 85 leaflets (67 single- and 9 double-leaflet procedures). Technical success (defined as successful leaflet traversal and laceration without injury to surrounding structures and successful retrieval of the system on leaving the operating room) was reported for 97.7% of leaflets. Laceration was successful in 100% following leaflet traversal. A composite procedural success endpoint was met in 88.2% of leaflets and was driven by partial CAO. Five of the 8 reported intraprocedural CAO without signs of myocardial ischaemia were successfully treated with orthotopic stenting (Figure 1). Only 2 leaflets had definite target leaflet CAO, one required snorkel stenting and the other orthotopic stenting (Figure 1); both patients survived to discharge. Major ischaemic stroke was seen in one patient in whom cerebral embolic protection was not used. Other drivers of the 79% composite early safety endpoint were non-BASILICA-related. Thirty-day survival was excellent in this high-risk cohort, at 98.7%. At 1 year, there was no delayed CAO, there were 3 additional strokes, and survival was 84.2%.
The results reported are reassuring, consistent with contemporary North American experience2 and also with patients undergoing TAVI without risk for CAO4. This is a reassuring signal that excellent outcomes with BASILICA are indeed reproducible outside North America, where expert centres and support from high volume BASILICA operators are more readily available.
Several intriguing findings deserve further discussion. The most important is the relatively high rate of partial CAO which resulted in post-BASILICA coronary artery stenting. It is unclear how this was defined or assessed, and the majority (n=6/8) appear to have occurred in centres with limited BASILICA experience. Angiographic imaging may be misleading in this context – lucent lines from appropriately lacerated leaflets pinned between a transcatheter heart valve (THV) frame and effaced sinus are common and usually benign – and we therefore recommend confirmation of partial CAO with intravascular ultrasound (IVUS) before considering stent placement, especially in the absence of objective ischaemia. Only 2 of 6 partial CAO were assessed with IVUS. Cautious overtreatment is, of course, understandable, and patient outcomes were excellent up to 1 year, with no delayed CAO.
Snorkel stenting behind the THV frame is undesirable and may preclude future coronary access, or lead to stent deformation, fracture, thrombosis or delayed coronary artery obstruction5. In this context, a second important finding from this study is the high reported rate of orthotopic stenting. Orthotopic stenting, through the frame of the THV from the aortic root to the coronary ostium, is preferable, as it allows for “physiologic” stent positioning and is only possible following successful BASILICA (Figure 1). Hence, successful orthotopic stent placement could be included as an indicator of successful BASILICA, with failure being indicated only in cases where snorkel stenting must be employed.
Predicting coronary obstruction during TAVI is challenging. We recently described a simplified risk-scoring algorithm with high sensitivity and specificity that may improve patient selection6. In the present study, the reported mechanism of CAO in most cases was leaflet prolapse. This could be explained by procedural factors that increase the risk of mechanical avulsion rather than “clean” laceration, such as the non-universal use of dextrose during laceration (92.1% of patients) or electrosurgical failure. Dedicated transcatheter electrosurgery devices for BASILICA, currently under investigation, should ensure more reliable and consistent tissue laceration.
The current study adds to the body of evidence that BASILICA is feasible, safe, and reproducible outside expert centres in North America. Indeed, the results presented within may underplay the efficacy of BASILICA through the inclusion of partial obstruction and orthotopic stenting as a failure of the predefined composite procedural success outcome. Non-flow-limiting, partial CAO should be assessed with intravascular imaging to confirm the presence of prolapsing leaflet material within the coronary ostium and to guide decision-making. Future BASILICA studies may choose to include orthotopic stenting, which is impossible without successful BASILICA, as a marker of success rather than one of failure.
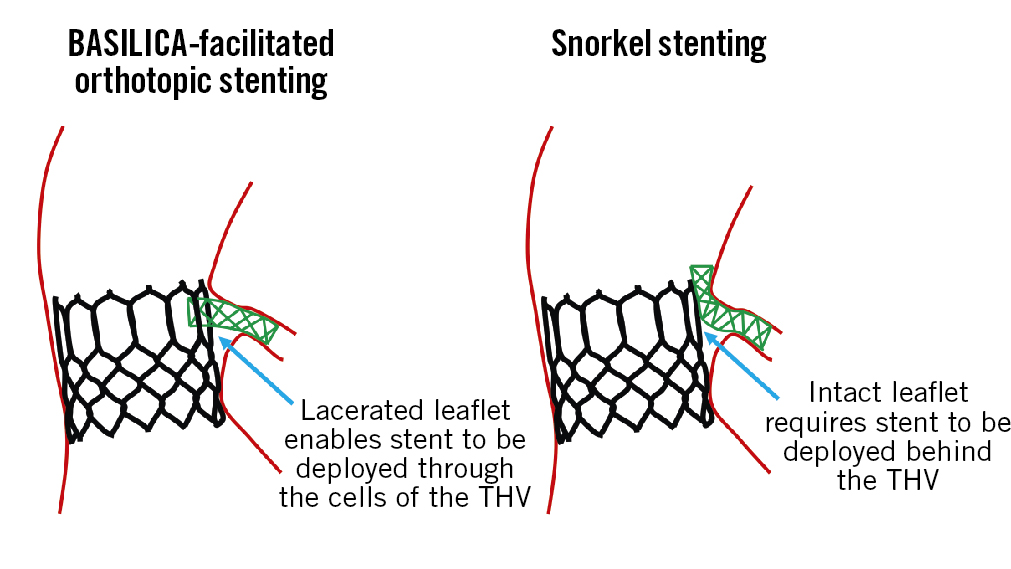
Figure 1. Orthotopic coronary artery stenting indicates successful BASILICA laceration. BASILICA: Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction; THV: transcatheter heart valve
Funding
This work was supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health USA grant Z01-HL006040.
Conflict of interest statement
T.Rogers is aconsultant for Edwards Lifesciences, Medtronic, Boston Scientific, Abbott, and Transmural Systems; serves on an advisory board at Medtronic, Boston Scientific, and Abbott; has equity interest in Transmural Systems; and is aco-inventor on patents, assigned to NIH, for transcatheter electrosurgery devices. C.G.Bruce is aco-inventor on patents, assigned to the NIH, for transcatheter electrosurgery devices.

