Transcatheter aortic valve intervention (TAVI) has become a relatively mature procedure. With more than 17 years of experience, over 500,000 procedures performed, and over 8,000 published reports, the risks and benefits of this procedure are reasonably well understood. Arguably, TAVI may soon be accepted as the default strategy for the majority of patients with severe aortic stenosis, regardless of whether surgical risk is high or low1,2. Increasingly, the role of the Heart Team may evolve to determine which patients are at high risk of early or late adverse outcomes with TAVI. In some “high TAVI-risk” patients, surgery might be warranted, while in others newer less invasive approaches might be considered.
In this issue of EuroIntervention, Komatsu and colleagues describe the technical steps involved in one such innovative strategy for patients with failed surgical bioprostheses – Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA). The BASILICA procedure uses “catheter electrosurgery” to split the offending bioprosthetic leaflet, creating a triangular space that facilitates blood flow to the coronary artery3,4,5. The BASILICA technique addresses one area of concern in “high TAVI-risk” patients, namely those at risk of coronary ostial obstruction. In addition to preventing potentially fatal coronary obstruction, there may be other benefits in terms of preserving access to the coronary ostia for diagnostic angiography or percutaneous coronary intervention. Although speculative, Khodaee et al suggest the reasonable possibility of normalising leaflet washout with the potential for reducing leaflet thrombus and durability5.
The risk of coronary obstruction is substantial in patients with failed surgical bioprosthetic valves, where diseased tissue leaflets can be displaced in such a way as to exclude a coronary ostia6. Fortunately, we can now reliably identify patients at risk of coronary obstruction; the CT and coronary angiographic predictors are well described7,8. Important factors include the characteristics of the failed surgical valve (leaflets that are long, bulky, supra-annular, or externally mounted), the aortic root (coronary height, sinus and sinotubular dimensions), the new transcatheter heart valve (THV) (expanded diameter, supra-annular position), and the coronary anatomy (dominance, collaterals, and bypass grafts). In current practice, when coronary obstruction occurs, this really should not be a surprise.
The most common transcatheter approach to coronaries at risk has been “protected TAVI”, where preparations are made for ostial “chimney” stenting should coronary flow be compromised9. While this can be lifesaving, the long-term reliability of this approach is questionable10,11. Another promising approach is to utilise a THV that clips or restrains the offending bioprosthetic leaflet12,13. However, the most obvious way to mitigate this risk is to not perform TAVI at all; if surgical risk is not prohibitive, then surgical aortic valve replacement may be the most desirable option.
TAVI in failed surgical bioprostheses is increasingly common. However, with time TAVI in failed transcatheter bioprostheses (THV-in-THV) may become even more common. Of some concern is that the risk of coronary obstruction may be even greater with some failed transcatheter, as opposed to some failed surgical, aortic valves. When a THV is implanted such that its leaflets extend below or far from the coronary ostia there is relatively little risk that repeat THV implantation will obstruct coronary flow. However, when the leaflets of a failed THV extend above the coronary ostia, a redo procedure may be associated with a risk of coronary obstruction.
The risk of coronary obstruction with repeat TAVI will largely depend on the height and position of the coronary ostia and the specific root anatomy (sinus and sinotubular junction dimensions). Importantly, the risk will also depend on the depth of implantation and specific characteristics (supra-coronary vs. intra-annular leaflets) of the failed THV. The presence of unfavourable aortic root anatomy with low coronaries should prompt concerns about whether the selected THV can be implanted far enough from the coronary ostia to allow a subsequent THV-in-THV procedure (Figure 1). As we offer TAVI to lower surgical risk patients with the potential for longevity, implanters will need to think of whether their procedure will be repeatable.
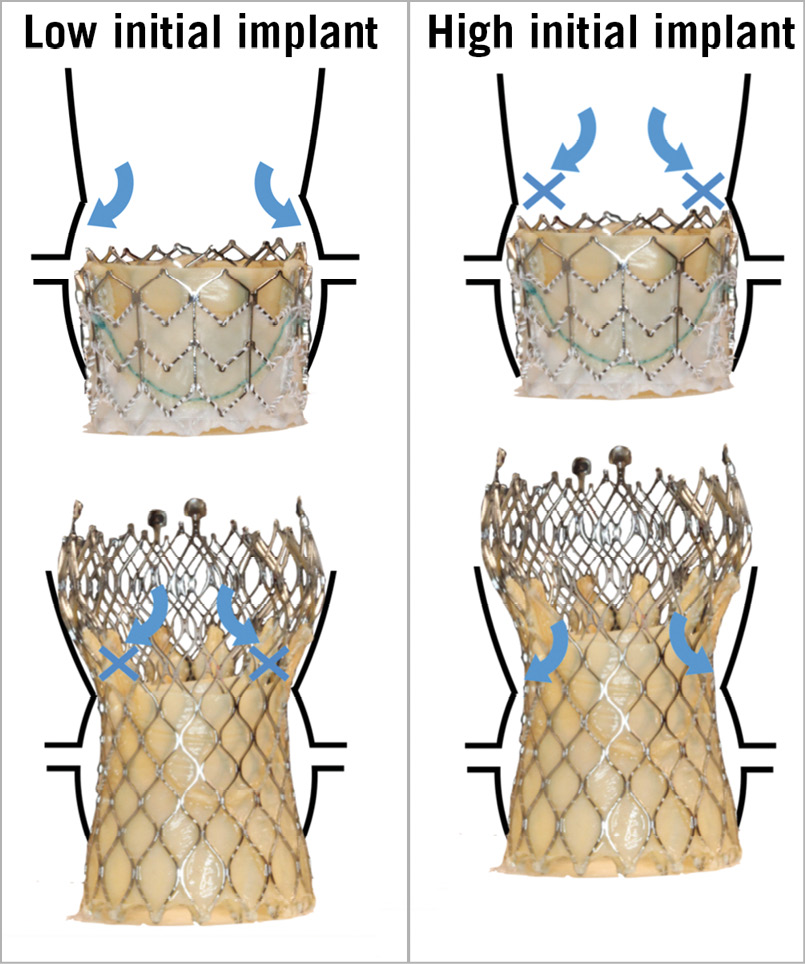
Figure 1. Repeat TAVI in varying root anatomy. Shown are an intra-annular SAPIEN 3 implanted within a SAPIEN XT THV (Edwards Lifesciences, Irvine, CA, USA), and a supra-annular Evolut R implanted within a CoreValve THV (Medtronic, Minneapolis, MN, USA). STJ: sinotubular junction
Surgical valves are typically implanted such that the valve posts are positioned either side of the coronary ostia, with the tissue leaflet directly in front of the ostia. However, current THVs are not anatomically aligned to the native commissures. Consequently, the THV leaflet is unlikely to be centred over the coronary ostia. In fact, the THV commissural attachments are as likely to overlap the coronary ostia. A simple leaflet-splitting BASILICA procedure is unlikely to be a satisfactory strategy to preserve coronary access and perfusion. As with failed surgical bioprostheses, sometimes surgical valve replacement may be the preferred option.
Experience with the BASILICA procedure is growing rapidly, as there are many patients with failed bioprosthetic surgical valves for whom surgery is unappealing. While very promising, evidence for safety and efficacy remains anecdotal. While routine TAVI is becoming increasingly simple, if anything can be learned from these reports it is that this procedure is not simple. It seems likely that in future there will be an increasing variety of even more complex adjunctive procedures. Hopefully, these procedures will be undertaken by high-volume centres with the skills and expertise necessary to achieve optimal outcomes.
Conflict of interest statement
J.G. Webb is a consultant to, and has received research funding from, Edwards Lifesciences, Abbott Vascular, Boston Scientific and ViVitro Labs. J. Sathananthan has received speaking fees from Edwards Lifesciences. D.A. Wood has received research funding from Edwards Lifesciences and Abbott Vascular and is a consultant to Edwards Lifesciences, Abbott Vascular, Medtronic, and Gore Medical.

