
Abstract
An ageing population and increased utilisation of tissue valves in younger patients imply that the number of patients receiving transcatheter aortic valve implantation within failed bioprostheses will continue to increase. There are two major adverse events associated with aortic valve-in-valve procedures that may temper the enthusiasm for these appealing interventions. Residual stenosis is the “Achilles’ heel” of aortic valve-in-valve, while coronary obstruction is an uncommon but life-threatening adverse event. Prevention of these adverse events is essential. Emerging tools and techniques enable operators to manipulate existing devices and to implant new ones inside them safely. Considering the available evidence, it seems that bioprosthetic valve ring fracture and bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) may enable some solution. Until we have prosthetic valves that are both very durable and non-thrombogenic, we can expect that techniques and tools chosen to treat failed bioprosthetic valves effectively will continue to be designed and utilised.
Abbreviations
AS: aortic stenosis
BASILICA: bioprosthetic aortic valve intentional laceration to prevent iatrogenic coronary artery obstruction
BVF: bioprosthetic valve ring fracture
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
ViV: valve-in-valve
VIVID: Valve-in-Valve International Data
Introduction
Valve-in-valve (ViV) procedures are an attractive alternative to open heart reoperation in patients with failed aortic bioprosthetic valves1. An ageing population and increased utilisation of tissue valves in younger patients imply that the number of patients receiving transcatheter aortic valve implantation (TAVI) within failed bioprostheses will continue to increase.
Patients undergoing aortic ViV procedures have similar clinical outcomes compared to those having TAVI for native aortic stenosis (AS)2. Contemporary aortic ViV procedures are associated with low mortality and stroke rates. Pacemaker implantation and paravalvular leakage are less common than in TAVI for native AS and these patients show remarkable symptom improvement3.
Nevertheless, there remain two major adverse events associated with aortic ViV procedures that may temper the enthusiasm for these appealing interventions. Residual stenosis is the “Achilles’ heel” of aortic ViV procedures and data from the Valve-in-Valve International Data (VIVID) and other registries report residual moderate or severe stenosis in 25% of all patients undergoing ViV, and in up to 50% of those with small surgical bioprosthetic valves. Suboptimal haemodynamic results limit the efficacy and durability of aortic ViV procedures and are associated with increased mortality and a much lower likelihood of symptom resolution. The second, and more dramatic and life-threatening, adverse event with these procedures is coronary artery obstruction. Strikingly, this is five to 10 times more common after aortic ViV than TAVI for native AS4. Prevention of this catastrophic complication is essential.
Herein, we describe several novel strategies and techniques that may enable safer and more effective aortic ViV procedures.
Residual stenosis
Residual AS is a major drawback of aortic ViV procedures. In the VIVID Registry, elevated (≥20 mmHg) mean gradients were recorded in 27% of patients4. These were especially common in patients receiving balloon-expandable transcatheter heart valves (THV) in small (label size ≤21 mm) surgical valves5. In such cases, expansion of the THV frame is constrained by the bioprosthetic surgical valve ring, with consequent underexpansion of the THV leaflets, yielding greater obstruction to flow and suboptimal haemodynamic conditions6,7 (Figure 1A, Figure 1B). In addition, residual stenosis may alter leaflet architecture, increase leaflet stress, and decrease THV durability8,9. It is essential to recognise the factors associated with residual stenosis (Table 1). Operators should be familiar with the technical aspects of ViV procedures, especially as ViV is expanded to younger and lower-risk populations where durability is a fundamental concern and where the long-term effects of residual gradients can be more meaningful.
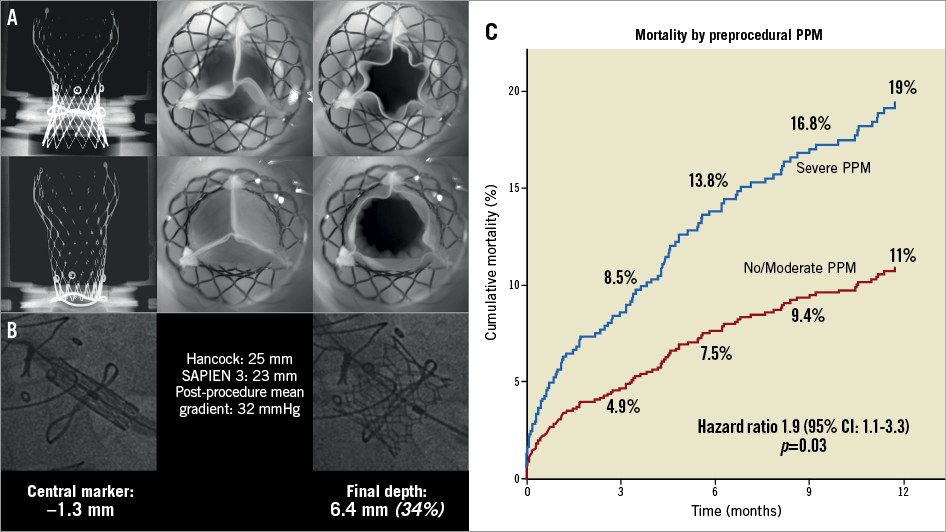
Figure 1. Implantation depths and periprocedural patient-prosthesis mismatch. Residual AS is associated both to lower depth of implantation and moderate/severe preprocedural patient-prosthesis mismatch. A) In vitro implantation of a deeply implanted CoreValve Evolut 23 mm device in a Hancock 21 mm surgical valve versus a highly implanted similar combination, with noted improved coaptation of leaflets. B) Case example of a deeply implanted SAPIEN 3 with significantly elevated mean gradients. C) Association between preprocedural patient-prosthesis mismatch and survival after valve-in-valve. PPM: patient-prosthesis mismatch
The effects of preprocedural patient-prosthesis mismatch (PPM) on ViV outcomes are an important consideration10. Patients with severe PPM at baseline have greater one-year mortality than those without upfront PPM (Figure 1C). Elevated transvalvular gradients are seen in more than 70% of patients who present with baseline PPM if treated with balloon-expandable THVs10. We suggest including the assessment of preprocedural PPM with standardised tables in the work-up of ViV cases. When severe PPM is present, a self-expanding device in a supra-annular position should be the preferred treatment strategy.
Some potential solutions for high residual gradients have been demonstrated to be effective. Optimal positioning of the THV within the surgical bioprosthesis reduces the risk of residual stenosis11. As the pathophysiological cause of the phenomenon is underexpansion of the THV within the “annulus” of the surgical valve, implantation of the transcatheter valve in a supra-annular position could be of benefit. This higher position may allow greater THV expansion, which is needed especially in small bioprostheses. The relationship of THV positioning and final device expansion has been clearly shown in in vitro studies12,13 (Figure 1A). Evidence from the VIVID Registry suggests that this phenomenon may also be true for the SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA)14 (Figure 1B). Data suggest an implant depth of up to 3 mm for the Evolut™ (Medtronic, Minneapolis, MN, USA) and up to 20% frame depth for the SAPIEN 3.
Another solution aims at increasing the potential expansion of the surgical valve ring. This is done through bioprosthetic valve ring fracture (BVF) using high-pressure balloons and is an emerging alternative or complementary strategy to manage elevated ViV gradients. While surgical valve rings may look metallic under fluoroscopy, most are made of plastic and can be broken. In 2015, the earliest reports of this technique surfaced15, but only recently has the procedure gained traction with accumulating bench and clinical data supporting laudable haemodynamic results16.
Bioprosthetic valve fracture
BVF is achieved using high-pressure inflation of a non-compliant balloon to fracture the sewing ring of a stented surgical bioprosthesis intentionally. This allows further expansion of the implanted (or about to be implanted) THV, increasing the valve area that can be achieved with ViV and reducing the likelihood of PPM. Patients with small surgical valves and high residual gradients after ViV seem to benefit most from BVF. It is possible, however, that, by optimising THV expansion and theoretically reducing leaflet stress and a substrate for structural valve failure, most patients undergoing ViV might benefit from BVF, irrespective of the size of their surgical valve or indeed the residual gradient following THV implantation.
To date, two published series have demonstrated that a variety of surgical valves with carbon or alloy frames can be fractured with high-pressure inflations using the non-compliant Atlas® GOLD or TRUE® balloons (Bard Peripheral Vascular Inc., Tempe, AZ, USA) of the same size as or 1 mm greater than the labelled size of the surgical bioprosthesis16,17. However, the Hancock® II (Medtronic) and Trifecta™ (St. Jude Medical, St. Paul, MN, USA) valves which have metallic frames cannot be fractured using currently available balloons18,19. Clinical experience has demonstrated that later-generation PERIMOUNT valves (Edwards Lifesciences), which resemble Magna valves (Edwards Lifesciences) under fluoroscopy, can be fractured. While it appears that older-generation PERIMOUNT or Carpentier-Edwards valves cannot be fractured, their surgical sewing rings can be significantly stretched, or remodelled, with a high-pressure balloon inflation. Similarly, the posts of the Trifecta valve can be flared with a high-pressure inflation to allow further THV expansion.
The procedural and haemodynamic results of patients treated with ViV and BVF have been reported in two case series encompassing 30 cases16,17. In 15 cases in which BVF was performed after ViV, the mean gradient was reduced from 41.9 mmHg to 20.5 mmHg with ViV, and further reduced to 6.7 mmHg following BVF (p<0.001). Accordingly, the effective orifice area increased from 0.6 cm2 to 1.0 cm2 with ViV, and then up to 1.7 cm2 following BVF (p<0.001). Long-term follow-up data are needed, however, to understand whether BVF improves clinical outcomes except for the haemodynamic benefit observed.
To perform BVF, a non-compliant balloon is positioned across the bioprosthesis. During rapid ventricular pacing, an initial hand inflation is used to fill the balloon with dilute contrast, then a standard coronary indeflator is used to increase the inflation pressure to the threshold for valve fracture. Valve fracture is confirmed by a sudden drop in inflation pressure, a visible release of the balloon waist, and/or an audible snap (Figure 2, Figure 3). Given the prolonged nature of the “pacing run” that is required, general anaesthesia could be considered to avoid patient distress.
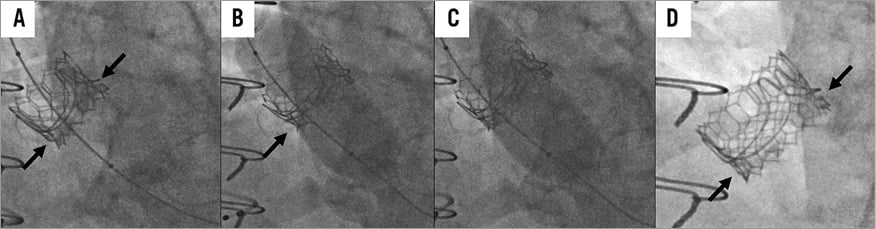
Figure 2. Sequential demonstration of bioprosthetic valve fracture (BVF) in a balloon-expandable ViV procedure. A) Baseline appearance of a 23 mm SAPIEN 3 valve deployed in a 23 mm Magna valve. The arrows indicate the constrained waist of the valve. B) Initial balloon inflation during BVF, with arrow indicating the balloon waist. C) Visible release of the balloon waist compared to panel B. D) Final appearance after ViV TAVI and BVF. The arrows indicate no constrained waist, and the valve has noticeably foreshortened as is expected with further expansion.
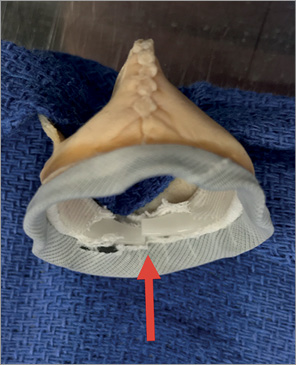
Figure 3. An example of bioprosthetic valve ring fracture of a Mitroflow valve.
Observed and theoretical risks posed by BVF are listed in Table 2. In the clinical series published to date, complications were few; however, anecdotal reports of major complications have occurred and the clinical experience with BVF is still early. It seems that there is still much to learn about clinical and anatomic features that could predispose to complications. The high-pressure balloon inflation used to perform BVF can cause structural damage to the surgical bioprosthesis or to the implanted THV, resulting in acute valvular regurgitation, or possibly long-term durability issues. When BVF is performed after CoreValve® (Medtronic) implantation, disruption of the valve frame can be avoided by using a balloon no more than 2 mm larger than the constrained segment and by positioning the balloon lower (i.e., more ventricular) than the constrained segment, where the leaflets are anchored to the frame18.
Further research is needed to define the optimal procedural technique for performing BVF. Bench testing has demonstrated that the maximum gain in diameter that can be achieved with BVF is between 3 and 4 mm18,19. Therefore, the initial THV size selection should be based on the expected final internal dimension of the fractured surgical valve. However, there remain situations in which optimal THV selection remains unclear; for example, if the maximal achievable internal valve dimension is 24 mm, it is not known whether a partially constrained 26 mm THV results in better haemodynamics than a fully expanded 23 mm THV.
Whether BVF is best performed before or after implantation of the THV is not known. Performing BVF before ViV has the potential benefit of sparing the THV from the high-pressure balloon inflation and the theoretical concern of subclinical damage to the prosthetic leaflets, which might impact on long-term THV durability. On the other hand, if BVF is performed after ViV, this offers the advantage of assessing haemodynamics and residual valve gradient prior to deciding whether BVF should be pursued. Additionally, a high-pressure inflation within the THV prosthesis may ensure optimal expansion of the THV and result in superior haemodynamic results.
In bench testing, balloons sized 1 mm larger than the labelled surgical valve size were utilised. Smaller balloons can be used as well, but higher pressures may be required to achieve valve fracture. While the fracture thresholds of larger bioprostheses have not been systematically tested, based on clinical experience it appears that such valves can be fractured with balloons sized 1 mm larger than the labelled valve size, at similar pressures.
Coronary obstruction
Coronary obstruction remains an uncommon but potentially life-threatening complication of TAVI. The coronary arteries obstruct when the failing leaflets are pushed towards the coronary ostia after TAVI. While in native aortic valves the incidence of obstruction has been systematically <1%, a much higher incidence in the context of ViV procedures has been reported, ranging from 1 to 4%1,20-22. In a recent analysis from the VIVID Registry, including 1,612 aortic ViV procedures, this complication was more common in stented bioprostheses with externally mounted leaflets or stentless bioprostheses than in the more commonly encountered stented bioprostheses with internally mounted leaflets23. The externally mounted bioprostheses have relatively long leaflets outside the stent in comparison with internally mounted leaflets. Likewise, delayed coronary obstruction, occurring hours or even days after the procedure, is ~fourfold more frequent after ViV procedures compared to native valve TAVI. There has been no association shown between type of THV and risk of immediate coronary obstruction. However, there is a higher risk of delayed coronary obstruction with self-expanding devices22.
Coronary obstruction during ViV presents clinically with abrupt and persistent severe hypotension or electrocardiographic changes, such as ST-segment deviation and/or ventricular arrhythmias, immediately or shortly after valve implantation21,24. The presence of regional left ventricular dyskinesia on echocardiography should also prompt immediate assessment for coronary obstruction. The diagnosis is confirmed with selective coronary angiography and/or aortography. Bail-out coronary stenting is feasible in most patients: the stent is deployed from the proximal portion of the coronary artery alongside the THV to keep the bioprosthetic leaflet away from the ostium (chimney stenting). The longer-term outcomes of these bail-out techniques are suboptimal, with potential concerns including stent thrombosis, restenosis, and near-impossible re-access to the coronary artery25,26. Emergent coronary artery bypass surgery is another option in cases where bail-out stenting is not possible.
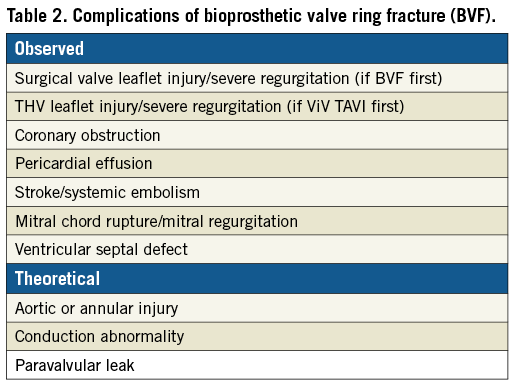
Identification of patients at risk is crucial to avoiding this rare but potentially fatal complication of TAVI. The virtual transcatheter heart valve to coronary distance (VTC) is a CT-obtained parameter that combines risk factors of coronary height, sinus width, and transcatheter heart valve size, and also accounts for bioprosthetic valve tilt in the annulus (Figure 4)26,27. The VTC is measured by first identifying the basal ring plane and the geometric centre of the surgical valve. Then, a virtual cylinder with the estimated nominal size of the THV is placed in the middle of the basal ring. The centres of the basal ring and of the cylinder are aligned. Finally, the horizontal distance between the edge of the cylinder and the ostia of the coronary arteries is measured with a calliper measurement tool of the CT imaging software27. This measurement should be repeated at the upper sinus (the level of the top of the surgical valve posts) to account for inflow obstruction risk. It has been shown that a short VTC distance predicts coronary obstruction, with an optimal cut-off level of <4 mm best identifying those patients at risk with high sensitivity and specificity21. In high-risk cases, it is reasonable to implement additional security measures during ViV procedures, such as coronary protection with a guidewire or an undeployed balloon/stent in the coronary artery, which can be brought back to stent the coronary ostium if necessary20,21,24-26. However, this technique of stent protection is associated with the risk of stent jailing, suboptimal stent durability and challenging repeat access to the coronaries. More recently, an intentional laceration of the failing surgical bioprosthetic leaflet to maintain access to the coronary ostia has been proposed to avoid this complication. This novel “BASILICA” technique will be discussed in the next section28.
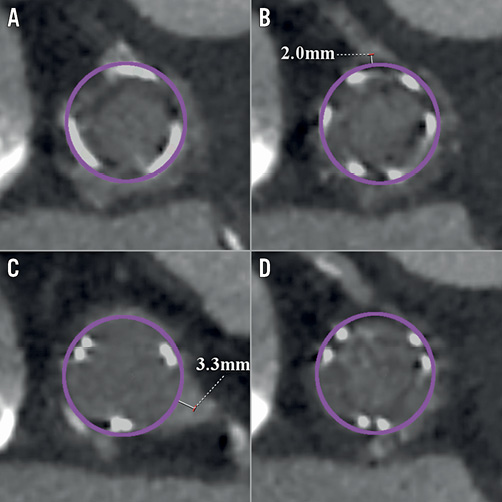
Figure 4. Multidetector computed tomography evaluation pre-TAVI showing the measurement of the distance between a virtual transcatheter ring and a size of the implanted device at the level of each coronary ostium (VTC) with a case example of short VTC for both coronary arteries. A) Virtual ring. B) VTC to the right coronary artery ostium (RCA). C) VTC to the left coronary artery ostium (LCA). D) Effaced sinus of Valsalva.
BASILICA
Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) directly addresses the pathophysiology of coronary artery obstruction by lacerating the leaflet in front of the threatened coronary artery. The concept of BASILICA is that the intentionally sliced leaflet splays after TAVI and creates a triangular space that allows blood flow towards the sinus and from it to the coronary artery, which otherwise would be occluded (Figure 5).
Benchtop testing, animal studies and an initial seven cases, including both native aortic valve and bioprosthetic valve, and both left, right, and double leaflet BASILICA, were reported recently28. The procedure utilises “catheter electrosurgery” techniques previously employed29,30. The clinical procedural and 30-day outcomes showed favourable results with all targeted leaflets successfully lacerated, without coronary obstruction or stroke. BASILICA is currently being prospectively evaluated in a clinical trial in the USA (NCT03381989).
Figure 5. Pictures of transcatheter heart valves (A & B: 23 mm SAPIEN 3; C & D: 26 mm Evolut PRO) implanted in a 25 mm Mitroflow. A) & B) Without BASILICA. C) & D) With BASILICA.
The BASILICA procedure steps for right cusp laceration are shown in Figure 6. A multipurpose guiding catheter with a combination of 300 cm 0.014” guidewire and microcatheter is advanced to the right coronary cusp, and a snare catheter is positioned in the left ventricle outflow from the opposite femoral access (Figure 6D, Figure 6E). Accurate traversal is guided by fluoroscopy, using “front” and “side” views of the bioprosthetic valve leaflet (Figure 7) and transoesophageal echocardiography. After confirming the orientation towards the target traversal location and angle by fluoroscopic image and transoesophageal echocardiogram, the cusp is penetrated with an electrified wire and snared out (Figure 6F, Figure 6G). Later on, the mid-shaft of the guidewire is focally electrified while applying gentle traction to lacerate the aortic leaflet. A conventional TAVI procedure is then performed and patency of the right coronary artery confirmed (Figure 6H, Figure 6I).
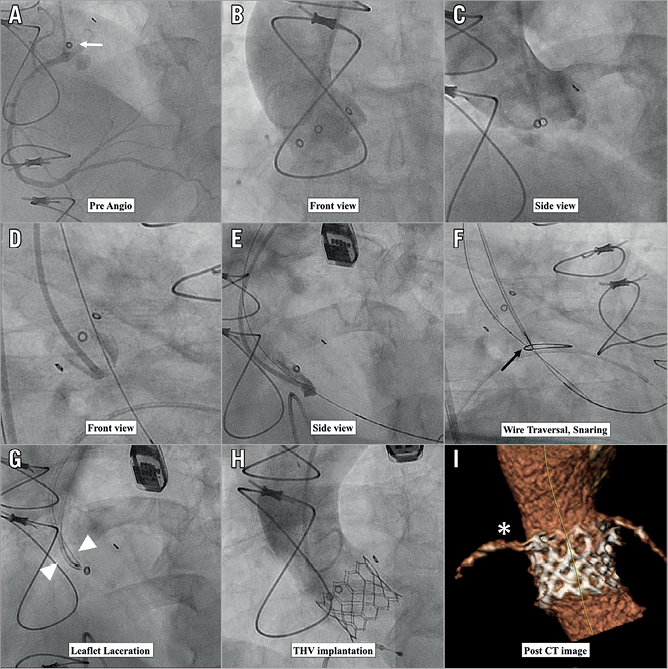
Figure 6. Procedural sequence of right cusp BASILICA. A) Preprocedural right coronary angiogram. The coronary ostium is lower than the stent post of the previously implanted Mosaic surgical valve (white arrow). B) - E) Front view and side view of right coronary cusp with pigtail and multipurpose guiding catheter injections to visualise the traversal location. F) Wire traversal and snaring (black arrow). G) Leaflet laceration (white arrowhead). H) & I) Post transcatheter heart valve implantation and post-procedural CT image indicate patent right coronary artery (white asterisk).
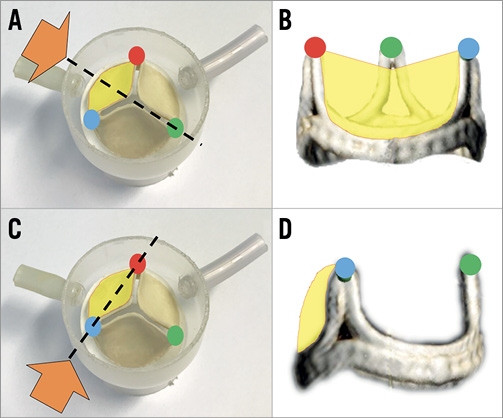
Figure 7. Illustration of cusp front/side view (the target cusp is yellow). A) & B) Front view. Using this view, the right-left orientation towards the traversal target is visualised. C) & D) Side view can show the wire traversal height and direction (attack angle).
Conclusion
Bioprosthetic valves are prone to structural degeneration and ultimately failure. Of concern is the potential for an upcoming pandemic of bioprosthesis failure, particularly as younger patients are treated. Novel surgical bioprosthetic valve designs include effective fluoroscopic markers and are amenable for simple dilatation with TAVI inside them (INSPIRIS; Edwards Lifesciences). Current devices and techniques facilitate successful treatment of most degenerated surgical valves. The main limitations of aortic ViV are directly related to the lack of space in the aortic root (i.e., residual stenosis) and to mechanical complications related to deflection of failed device tissue (i.e., coronary obstruction). Emerging tools and techniques enable operators to manipulate existing devices and to implant new ones inside them safely. Considering the available evidence, it seems that BVF and BASILICA may enable some solution in preventing these adverse events; however, research will need to continue. Other issues relate to the growing need to treat failed THV devices. THV-in-THV have been successfully performed but, given the design of current-generation THVs, THV-in-THV procedures will probably be associated with high rates of coronary occlusion or yield challenging access to the coronary ostia to treat de novo coronary artery disease. Novel strategies to remove or displace the failed leaflets of current THVs to facilitate THV-in-THV and enable coronary access are in development. Careful preprocedural screening and ever-improving operator understanding and technique will result in further improvements in clinical outcomes for patients undergoing ViV.
Until we have prosthetic valves that are both very durable and non-thrombogenic, we can expect that techniques and tools chosen to treat failed bioprosthetic valves effectively will continue to be designed and utilised.
Conflict of interest statement
D. Dvir is a consultant for Edwards Lifesciences and Medtronic Inc. H. Ribeiro is a consultant for Edwards Lifesciences and Medtronic Inc. D. Wood reports grants and consulting fees from Edwards Lifesciences and Medtronic Inc., as well as consulting for Boston Scientific and Abbott. J. Leipsic: Institutional core lab- Edwards Lifesciences; Abbott, Medtronic. J. Webb is a consultant for Edwards Lifesciences and Abbott. The other authors have no conflicts of interest to declare.

