Abstract
Bioprosthetic heart valves are preferentially selected over mechanical prostheses in the majority of patients undergoing valve replacement surgery. These bioprostheses are prone to structural degeneration, and hence an increasing number of patients are presenting with bioprosthetic failure requiring redo surgery. In selected high-risk cases, successful implantation of a transcatheter aortic valve (TAV) within the failing bioprosthetic surgical aortic valve (SAV) or mitral valve (SMV) has been performed. Herein, we summarise the available evidence, describe the technical challenges, and highlight important procedural considerations for these innovative interventions.
Introduction
In the 1970s, commercialisation of bioprosthetic surgical heart valves revolutionised the treatment of aortic and mitral valve disease. These devices afforded elderly patients and others at considerable risk of bleeding the opportunity for curative surgical aortic or mitral valve replacement. Today, these prostheses are more frequently used than their mechanical equivalent1. Indeed, recent guidelines have recognised bioprosthetic heart valves to be the standard of care for patients ≥60 years of age requiring aortic valve replacement and those ≥65 years old undergoing mitral valve replacement2.
In the last decade we have seen another paradigm shift in the treatment of patients with valvular heart disease. Transcatheter aortic valve implantation (TAVI) has again extended the promise of curative intervention to patients with severe aortic stenosis (AS) at excessive risk for aortic valve replacement3. As with most medical innovations, the remit of this technology has been extended beyond that initially conceived.
Perhaps the most interesting adaptation of TAVI is the treatment of patients with degenerative surgical bioprostheses. Most recently, the Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA) has received Conformité Européenne approval for implantation within failing aortic bioprosthetic valves. This article aims to provide an update on the available evidence supporting this innovative use of transcatheter heart valve (THV) technology.
Surgical bioprosthesis failure
Bioprosthetic heart valves are increasingly preferred to mechanical prostheses as lifelong anticoagulation can be avoided. This advantage is mitigated by structural deterioration of bioprosthetic valves, which have an average lifespan of between 12 and 20 years4,5. Improved bioprosthetic valve design has reduced the rate of structural deterioration and has resulted in these valves being used in younger patient cohorts2. Hence, patients with failing surgical bioprostheses are more frequently encountered. Redo aortic or mitral valve replacement surgery is the treatment of choice for these patients. Operative mortality for an elective redo aortic valve surgery is between 2% and 7%, and is comparable to the primary valve surgery in low-risk cohorts6. However, a significant number of high-risk patients are declined redo surgery, or have protracted postoperative recovery and adverse outcomes7. In these cases, implantation of a transcatheter aortic valve (TAV) within a surgical aortic valve (SAV) or mitral valve (SMV) may offer a less invasive and effective alternative to conventional surgery (Figure 1).
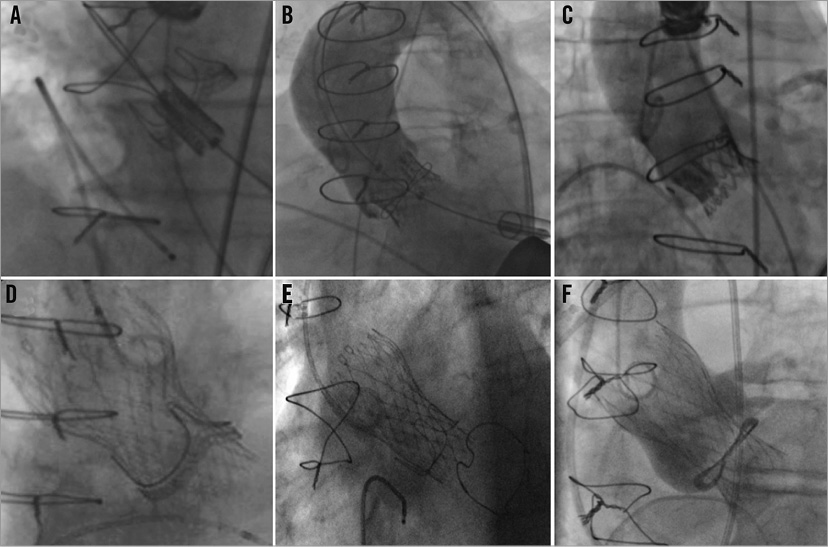
Figure 1. Case examples of TAV-in-SAV procedures. Transapical implantation of an Edwards SAPIEN valve inside: (A) Carpentier-Edwards Perimount; (B) Sorin Soprano; and (C) Medtronic Mosaic. Implantation of a Medtronic CoreValve within (D) Edwards Perimount; (E) Carpentier-Edwards porcine supra-annular valve; and (F) Sorin Soprano.
Classification of surgical bioprosthetic valves
Understanding the construction of surgical bioprostheses and their failure modes is fundamental to successful valve-in-valve (VIV) procedures. These valves may be classified as stented or stentless, with the stented prostheses being more frequently encountered. Stented valves usually have a rigid base ring from which a stent or frame arises to support the valve leaflets (Figure 2)8. The base ring and support frame are covered by pericardium or a synthetic material that protects the frame, and acts as an anchoring suture cuff. The base ring and frame are composed of metal alloys or plastics and can be radiolucent or radiopaque. The valve leaflets are of xenograft or homograft origin, and can be mounted inside (PERIMOUNTTM [Edwards Lifesciences, Irvine, CA, USA], Epic [St. Jude Medical, St. Paul, MN, USA], Hancock II® [Medtronic, Minneapolis, MN, USA]) or outside (Mitroflow® [Sorin Group, Milan, Italy] TrifectaTM [St. Jude Medical, St. Paul, MN, USA]) the frame (Figure 3). Bioprosthetic valves are usually placed at the level of the native aortic annulus though specific supra-annular designs are available (Magna [Edwards Lifesciences], Mosaic® [Medtronic]) to maximise the effective orifice area.
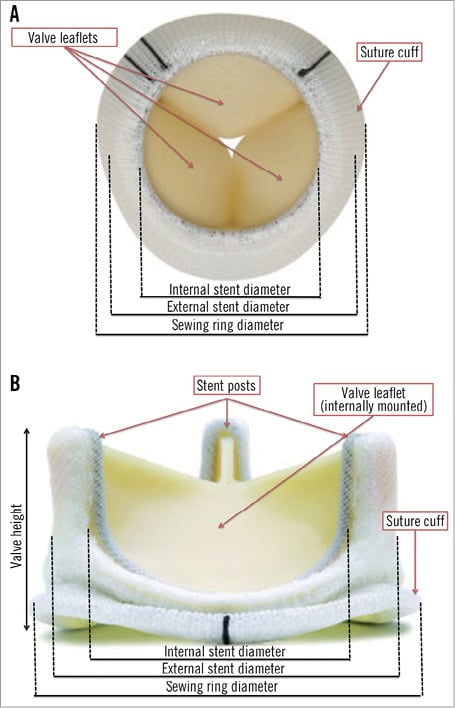
Figure 2. Carpentier-Edwards Perimount Magna Ease aortic valve. A & B) Dimensions and design features of a stented bioprosthetic valve.
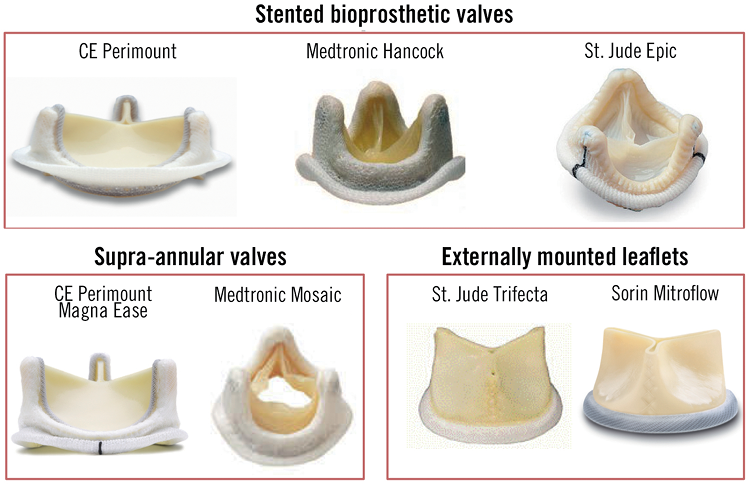
Figure 3. Stented bioprosthetic valves. CE: Carpentier-Edwards.
Stentless valves do not have a base ring or frame to support the leaflets and are of heterograft, autograft or homograft origin (Figure 4). A fabric suture cuff covers the inflow portion of the valve, thereby enabling fixation at the site of the excised native valve.
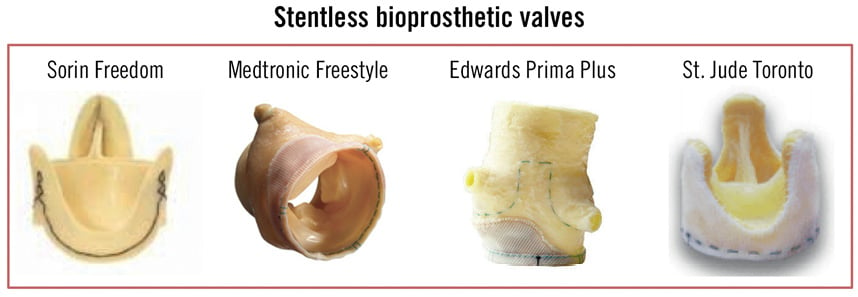
Figure 4. Stentless bioprosthetic valves.
Size labelling of surgical bioprosthesis
The labelled size of stented surgical bioprostheses corresponds to the external diameter of the base ring rather than the internal stent diameter that is relevant for THV sizing for VIV procedures (Figure 2). The internal stent diameter represents the available diameter for THV implantation within the relatively non-distensible base ring. Tables detailing these diameters in all surgical bioprostheses are available9; however, pannus and calcification can lead to a discrepancy between observed and expected inner stent diameters. Thus, pre-implantation multislice computed tomography remains crucial for THV sizing.
Similar to stented valves, the labelled size of stentless valves and homografts usually corresponds to the valve’s outer diameter. The inner diameter of the valve is usually available though the absence of a base ring makes these prostheses more compliant than stented valves. Thus THV sizing can be performed in a manner akin to TAVI for native aortic valve stenosis.
Mechanisms of surgical bioprosthesis failure
Bioprosthetic valve failure results from malfunction of the leaflets or the supporting structures (base ring or frame). Common mechanisms of valve failure include wear and tear, leaflet calcification, pannus formation, thrombosis, and infective endocarditis10. Leaflet tissue deterioration is the most common cause of bioprosthetic valve failure. Although leaflet failure usually occurs insidiously, failing leaflets can tear off the frame and can result in abrupt aortic or mitral insufficiency.
Percutaneous treatment of failing surgical aortic bioprosthetic valves: TAV-in-SAV
Evidence critically evaluating the safety and efficacy of TAV-in-SAV procedures remains limited. While randomised trials comparing redo surgery with THV implantation have not been performed, several small case series and the Global Valve-in-Valve Registry have reported encouraging and intriguing results9,11-17. The latter is a voluntary registry that currently includes 459 TAV-in-SAV cases and 88 TAV-in-SMV cases performed in 55 centres worldwide. In this registry, high-risk patients (mean Society of Thoracic Surgeons [STS] risk of mortality score >11.9%) underwent TAV-in-SAV procedures for bioprosthetic regurgitation (30.7%), stenosis (39.2%), or mixed regurgitation and stenosis (30.1%). Most patients (79.7%) had failing stented surgical bioprosthetic valves.
Clinical outcomes
30-DAY OUTCOMES
Patients successfully discharged from the hospital following TAV-in-SAV experience improvement in symptoms, as demonstrated by a reduction in New York Heart Association (NYHA) functional class17. At 30 days, more than 85% of patients report NYHA Class I/II symptoms14. Reported 30-day mortality rates are between 4.2% and 17%11,15. The Global Valve-in-Valve Registry has reported 30-day rates of all-cause and cardiovascular mortality of 7.6% and 6.5%, respectively (Ran Kornowski EuroPCR 2013, personal communication). These results are comparable to other TAVI cohorts and appear to confirm the safety of these complex interventions18-20. Importantly, physician experience is associated with improved acute procedural results15,17.
LONG-TERM RESULTS
Six-month and one-year survival rates range from 86% to 94% and 84% to 92%, respectively13,14,16,17. In the Global Valve-in-Valve Registry, survival at one year was 85% in Medtronic CoreValve patients and 81.3% in Edwards SAPIEN patients (p=0.44) (Ran Kornowski EuroPCR 2013, personal communication). Transvalvular gradients also appear to be stable out to one-year follow-up14,17. According to the indication for VIV implantation, one-year survival was 91.2% in patients with aortic regurgitation, 83.9% in those with mixed disease, and 76.6% in patients with bioprosthetic stenosis (p=0.01). Indeed, baseline bioprosthetic stenosis was the strongest predictor of mortality at one year (Hazard Ratio: 4.8; 95% confidence interval 1.8 to 12.5, p=0.002) (Ran Kornowski EuroPCR 2013, personal communication).
Longer-term follow-up is required to confirm the efficacy of TAV-in-SAV procedures. This is especially true in patients with high post-procedural transvalvular gradients, as underexpansion of the THV can lead to suboptimal valve function, impaired leaflet coaptation, and increased leaflet stress and dysfunction21,22.
STROKE
The reported incidence of major 30-day stroke ranges from 0% to 2.4%9,13,15,16. Thus, it appears that periprocedural stroke may be less common with VIV procedures than with TAVI for native AS (3.8% to 5.0%)18,19. The lower than expected incidence of stroke in TAV-in-SAV procedures is probably multifactorial: patients tend to be younger than those undergoing TAVI for native AS; bioprosthetic regurgitation usually involves less extensive aortic calcification than native AS; and pre-implantation balloon dilatation is performed more sparingly and less aggressively in TAV-in-SAV procedures.
Procedural outcomes
CORONARY OBSTRUCTION
Coronary obstruction following TAVI for native AS is rare (0.35%)23. In contrast, the reported incidence of coronary occlusion is ten times higher (3.5%) in patients undergoing TAV-in-SAV14. The higher incidence may be explained by: 1) the position of the surgical bioprosthesis (annular or supra-annular: the latter reduces the distance to the coronary ostia); 2) the design features of the bioprosthesis (stented or stentless; internal or external leaflet mounting; leaflet length); and 3) the presence of bulky pannus. In particular, Mitroflow stented valves, which have externally mounted, elongated leaflets, and Freedom stentless valves (Sorin Group) have a higher incidence of left main occlusion14,16,24.
Left main rather than right coronary occlusion predominates due to the lower position of the left coronary ostium relative to the aortic annulus. Rapid haemodynamic instability, ST-segment changes, and ventricular arrhythmias are common23. Coronary occlusion complicating TAVI for native AS can usually be effectively treated percutaneously with success rates of 90% and in-hospital mortality of 8.3%23. In contrast, percutaneous intervention during TAV-in-SAV is more challenging, as the bioprosthetic valve posts or leaflets inhibit guidewire passage (Figure 5)24. In-hospital mortality due to coronary occlusion in the Global Valve-in-Valve Registry was 57.1%14.
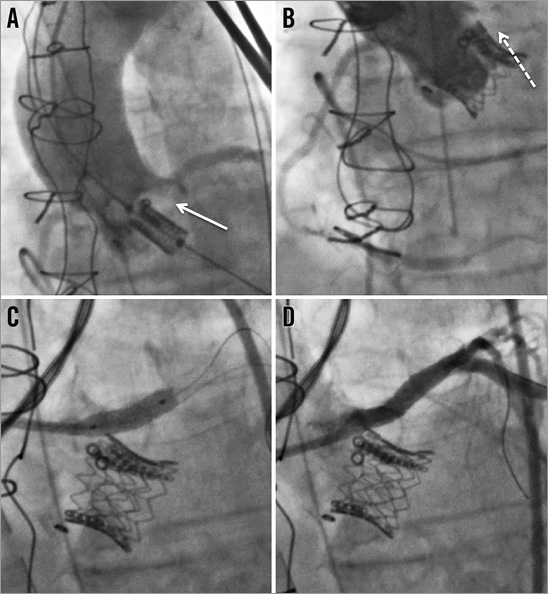
Figure 5. Left main coronary occlusion following TAV-in-SAV. Fluoroscopic images of a stenotic Medtronic Mosaic valve in the aortic position being treated by TAV-in-SAV with an Edwards SAPIEN valve. A) Positioning of the Edwards SAPIEN valve inside the failing bioprosthesis. Note the impressive calcification adjacent to the left main (arrow). B) Aortography following TAV-in-SAV demonstrates left main occlusion (dashed arrow). C) Stenting of the left main. D) Final result of left main stenting.
REQUIREMENT FOR PACEMAKER
Approximately 10-40%25-27 and 4-7% 28,29 of patients require a permanent pacemaker after CoreValve and Edwards SAPIEN implantation for native AS, respectively. The requirement for pacemaker following TAV-in-SAV procedures is lower. In the Global Valve-in-Valve Registry pacemakers were implanted in 12.2% of CoreValve and 4.9% of Edwards SAPIEN recipients, respectively (Ran Kornowski EuroPCR 2013, personal communication). The non-distensible base ring of the surgical prosthesis, paravalvular fibrotic change, and relatively high implantation of the THV probably protect the conduction apparatus from the full distension force of the THV and mitigate against conduction disturbance.
TRANSVALVULAR GRADIENTS
Elevated post-implantation transvalvular gradients appear to be the Achilles heel of TAV-in-SAV procedures. Mean gradients are higher following TAV-in-SAV procedures (≈15-20 mmHg) than TAVI for native AS (≈10 mmHg)9,14,15. High post-procedural gradients (mean gradient ≥20 mmHg) were reported in 28.4% in the Global Valve-in-Valve Registry14. This observation results in a large proportion of cases failing to meet the updated Valve Academic Research Consortium (VARC) definition of acute procedural success30. Elevated gradients may be explained by pre-existing prosthesis patient mismatch, which occurs in up to 52% of patients with a stented aortic bioprosthesis31, or by incomplete expansion of the THV within the rigid base ring of the surgical prosthesis22. This problem is most frequently encountered in those with surgical prostheses of small internal diameter (<20 mm).
The limited number of available THV sizes has resulted in many patients being treated with THVs that would conventionally have been considered too large for the measured internal diameter of the failing surgical bioprosthesis. Thus, the relation of the THV diameter to the surgical bioprosthesis diameter (“prosthesis-to-prosthesis match”32) may be associated with elevated transvalvular gradients. It is interesting to speculate that the introduction of the 23 mm CoreValve and 20 mm Edwards SAPIEN valve may yield a reduction in postprocedural transvalvular gradients, especially in those with small surgical bioprostheses.
Mean postprocedural gradients are also ≈5 mmHg higher with TAV-in-SAV using the Edwards SAPIEN than with the CoreValve (p<0.0001)14. This difference is most apparent inside small surgical bioprostheses, where 43% of Edwards SAPIEN cases had transvalvular gradients >20 mmHg compared to 24% of CoreValve cases (Ran Kornowski EuroPCR 2013, personal communication). It is likely that the supra-annular functionality of the CoreValve provides a larger potential orifice area than the intra-annular position of the Edwards SAPIEN valve. Hence, the CoreValve is the preferred THV for patients with smaller surgical bioprostheses.
Elevated transvalvular gradients and prosthesis-patient mismatch following SAVR are associated with congestive heart failure, perioperative and long-term mortality33. However, the advanced age and comorbid status of patients undergoing TAV-in-SAV may render the long-term consequences of prosthesis-patient mismatch less significant. Close patient follow-up and further study are required to establish the long-term clinical significance of prosthesis-patient mismatch34.
PARAVALVULAR LEAK
Moderate to severe paravalvular leak occurs less frequently with TAV-in-SAV than TAVI for native AS. However, important (grade ≥2) paravalvular leaks continue to occur in approximately 5% of cases14. Moderate paravalvular leak appears to occur more commonly following CoreValve implantation (8.9%) than with the Edwards SAPIEN valve (2.5%). Paravalvular leaks may occur between the THV and the surgical bioprosthesis or between the surgical bioprosthesis and the native annulus.
Procedural considerations
PRE-IMPLANTATION BALLOON VALVULOPLASTY
Opinions remain divided on the role of pre-implantation balloon aortic valvuloplasty (BAV) for TAV-in-SAV procedures. In cases where extensive calcification is present, BAV may be logical. However, the merit of BAV within the non-distensible base ring of stented bioprostheses or in cases of primary aortic regurgitation is uncertain. Surgical bioprostheses are more susceptible to tearing than native aortic leaflets and ensuing haemodynamic instability can render TAV-in-SAV procedures more challenging35. More importantly, intervention on degenerated surgical bioprostheses carries a higher risk of debris embolisation and stroke. Indeed, balloon dilatation of prosthetic left-sided heart valves is contraindicated by societal guidelines2,36. Despite these recommendations, BAV was performed in 27.7% of cases in the Global Valve-in-Valve Registry (16.1% CoreValve; 46.2% Edwards SAPIEN)14.
MALPOSITION OF THE THV
THV malposition during TAV-in-SAV procedures occurs frequently. The Global Valve-in-Valve Registry reported initial malposition in 15.3%, additional manoeuvres to retrieve the CoreValve in 8.9%, and implantation of a second THV in 8.4%14. Malposition occurs more commonly during intervention on stentless surgical bioprostheses and particularly with the Medtronic Mosaic valve14. Several factors may account for the high rates of malposition. Firstly, the large variety of surgical valves with different constructions and fluoroscopic markers creates uncertainty in identifying the optimal position for implantation37. Second, in patients with predominant aortic regurgitation, the elevated stroke volume contributes to prosthesis instability during implantation. Third, the limited number of available THV sizes has necessitated the implantation of relatively larger THVs than in TAVI for native AS, thus making the implant more difficult.
A thorough understanding of the design and fluoroscopic identification of the surgical bioprosthesis is therefore essential. An iPhone app that helps identify the features of currently available surgical bioprostheses is available (http://www.ubqo.com/viv). Rapid ventricular pacing should be considered in patients with significant aortic regurgitation to reduce stroke volume and valve instability. In cases with limited fluoroscopic implantation landmarks, transoesophageal echocardiography should be used for device positioning. It is satisfying to note that the most recent data presented from the Global Valve-in-Valve Registry demonstrate that the requirement for implantation of a second THV has fallen to 4.4% (Ran Kornowski EuroPCR 2013, personal communication).
Percutaneous treatment of failing surgical mitral bioprosthetic valves: TAV-in-SMV
Successful implantation of the Edwards SAPIEN valve within a failing surgical mitral valve was first reported in 200938. In subsequent years, several case reports and small series have demonstrated the feasibility and safety of this technique with both Edwards SAPIEN and Medtronic Melody THVs38-41.
SAFETY AND EFFICACY
Cheung et al have recently reported their institutional experience of 23 transapical TAV-in-SMV cases42. In this high-risk cohort (mean STS score 12.2 ± 6.9%) declined conventional redo mitral valve replacement surgery, the mechanism of bioprosthetic failure was stenosis in 30.4%, regurgitation in 39.1%, and mixed in 30.4%. VARC-defined device success was achieved in 100% of cases and there was no 30-day mortality. The mitral transvalvular gradient significantly decreased from 11.1 ± 4.6 mmHg to 6.9 ± 2.2 mmHg following the VIV procedure, and all patients had no more than mild paravalvular mitral regurgitation. Haemodynamic or structural deterioration was not observed during follow-up, though one patient underwent a further valve-in-valve-in-valve procedure due to atrial migration of the Edwards SAPIEN valve. Heart failure symptoms improved in all but one patient (NYHA Class I/II). At a median follow-up of 753 days the Kaplan-Meier survival rate was 90.4%. Long-term durability and efficacy require further study.
PROCEDURAL CONSIDERATIONS
The majority of TAV-in-SMV cases have been performed using the transapical approach which provides direct and coaxial access to the failing mitral bioprosthesis. Alternative access routes are more technically challenging and include the transatrial approach using a right-sided thoracotomy43, and the transvenous transseptal approach11. As stentless bioprostheses are used very infrequently in the mitral position, THV implantation can usually be performed using fluoroscopy. In cases where the sewing ring is radiopaque, the use of transoesophageal echocardiography is recommended.
Similar to TAV-in-SAV procedures, THV sizing for failing mitral bioprostheses has been guided primarily by the manufacturers’ reported internal stent diameter, as well as screening computed tomography and intraprocedural echocardiography. These latter imaging modalities add important information regarding the failure mode of the surgical valve. In the series by Cheung and colleagues, 10% oversizing of the Edwards SAPIEN valve relative to the surgical bioprosthesis was considered appropriate42.
UNANSWERED QUESTIONS
Although the frequency of VIV interventions is growing, it is important to note that there remain significant gaps in our understanding of these procedures. Long-term efficacy and durability remain unknown, particularly in patients with underexpanded THVs and high transvalvular gradients. A comparative effectiveness analysis comparing VIV with redo surgery has not yet been performed. The optimal degree of THV oversizing inside the surgical bioprosthesis is unknown. Antiplatelet and anticoagulant regimens are untested. In which anatomical or patient groups are these procedures best avoided? Which THV should be preferentially used for annular or supra-annular surgical bioprosthesis? What are the implications of prosthesis-patient mismatch in this patient population? Should specific VARC outcomes be developed for VIV procedures? Further study is required to address these important questions.
Conclusions
Current data support the treatment of patients with failing surgical bioprostheses at high operative risk using THV technology in specialised centres. Although considerable gaps in our understanding of the long-term efficacy of these procedures remain, results to date suggest that these innovative procedures have the potential to become the standard of care for surgical bioprosthetic valve dysfunction.
Conflict of interest statement
N. Piazza is a proctor and a consultant for Medtronic. The other authors have no conflicts of interest to report.

