
Over the last decade, we have witnessed a considerable shift towards the use of surgical bioprostheses for aortic valve repair (irrespective of the patients’ age) due to their lower bleeding and thrombotic risk compared to mechanical prostheses1. Given that bioprosthetic valves tend to degenerate within 10 to 15 years, the number of patients with degenerated bioprostheses requiring re-treatment is likely to rise within the next few years1,2. Conventional redo surgical aortic valve replacement (SAVR), standardly used to treat failed surgical bioprostheses, carries an inherent risk associated with redo open heart surgery, with a perioperative mortality of up to 5%1,2.
In recent years, improvements in transcatheter valve technology have brought an increase in transcatheter valve-in-valve (VIV) usage as an alternative to redo SAVR in patients with failing surgical bioprostheses2. In a large nationwide analysis of matched high-risk adult patients with degenerated surgical bioprostheses (n=6,815), compared to redo SAVR, VIV transcatheter valve implantation (TAVI) was associated with a substantial reduction in 30-day mortality and morbidity risk as well as a decreased length of hospitalisation (Figure 1),2.
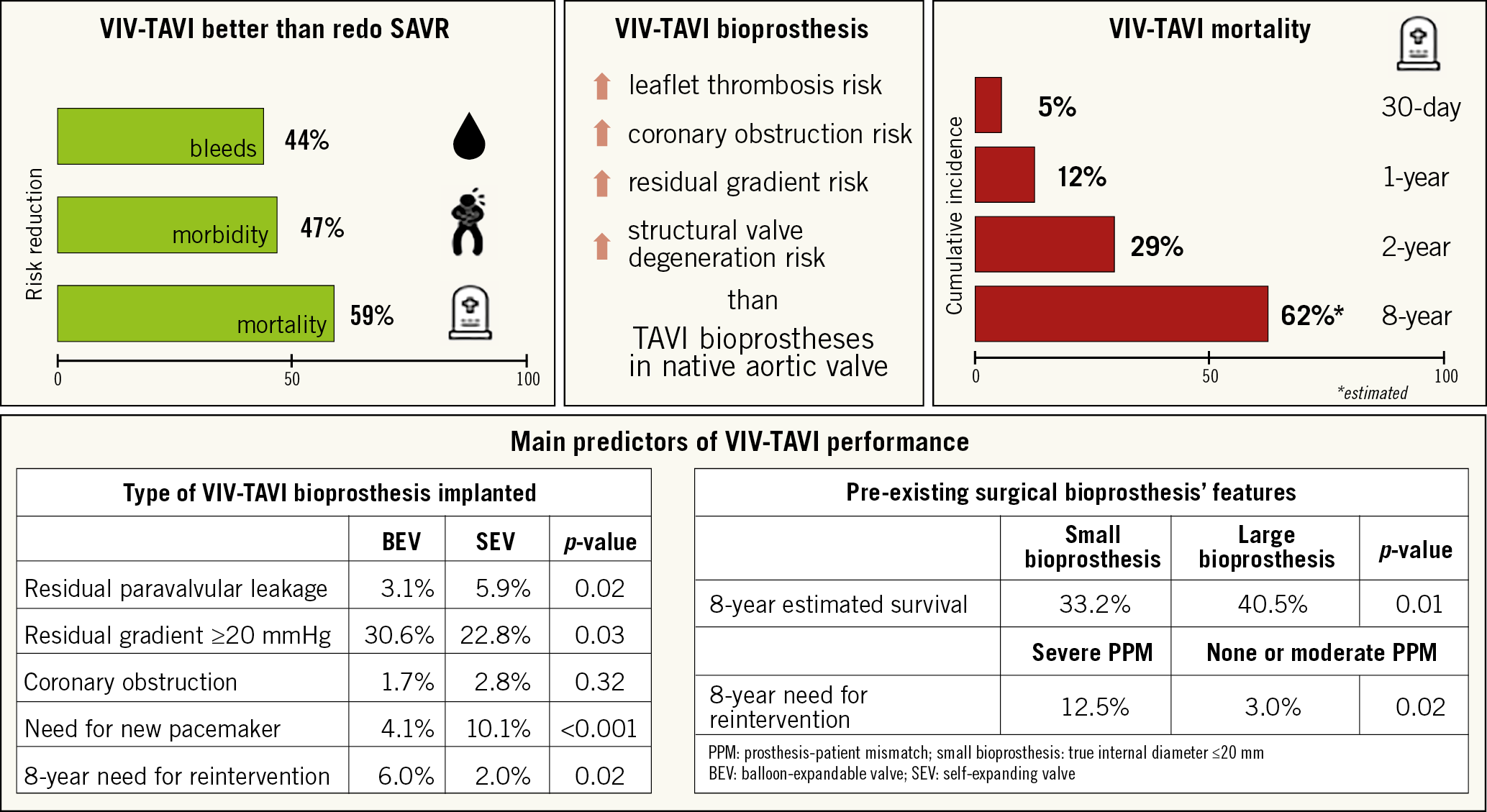
Figure 1. VIV-TAVI procedure fact sheet.
In this issue of EuroIntervention, Mahmoud et al present the results of a systematic review and meta-analysis that they conducted evaluating short-term and midterm outcomes following VIV-TAVI in patients with failed surgical bioprosthetic aortic valves3.
This meta-analysis of 24 studies with a total of 5,553 patients confirmed that VIV-TAVI is associated with a high procedural success rate and favourable outcomes. Despite the inclusion of patients at higher operative risk (mean STS score 9.1), 30-day mortality (5.0%) was comparable to that of unselected patients undergoing VIV-TAVI or redo SAVR reported previously, 2.7% and 5.0%, respectively2. Long-term follow-up was available in only 10% of the population. Three-year mortality was substantially high at 29.0%, reflecting the multi-comorbidities in this high-risk cohort. This is supported by the long-term data from the Valve-in-Valve International Data (VIVID) voluntary multinational registry, in which, among 1,006 patients undergoing VIV-TAVI procedures, the estimated survival at eight years was 38.0% with a median survival of 6.2 years (95% CI: 5.7-6.7 years)4. Furthermore, the current meta-analysis by Mahmoud et al suggests that the lower survival is driven mostly by comorbidities rather than by adverse cardiac events such as myocardial infarction (1.0% at one-year follow-up) or stroke (6.0% at three-year follow-up)3. Whether VIV-TAVI prosthesis thrombosis influenced the outcomes is questionable. VIV-TAVI prostheses have a higher risk of subclinical and clinical leaflet thrombosis compared to TAVI prostheses for native aortic valve stenosis5. The lack of information regarding this complication limits the current analysis; there still remain no robust data to address its prevalence in VIV-TAVI patients.
There is a large body of evidence demonstrating the key role of TAVI prosthesis performance indicators as well as the sizes and types of failed surgical bioprosthesis in terms of long-term survival. In the current meta-analysis, some of the indicators of valve performance, such as the outcome of significant post-TAVI aortic regurgitation (7.0%, 95% CI: 5-10%) and mean pressure gradient across the valve (16.16 mmHg, 95% CI: 15.30-17.02 mmHg), remained relatively low at 30 days. The proportion of patients with residual stenosis (defined as mean post-procedural gradient ≥20 mmHg), reported to be a common finding after VIV-TAVI and a predictor of symptom persistence and higher mortality5,6, is missing in the current manuscript. Pre-existing severe prosthesis-patient mismatch (PPM) and use of a small failed bioprosthesis (internal diameter ≤20 mm) are associated with a higher residual transvalvular gradient4,6 and incomplete or asymmetrical frame expansion of the VIV-TAVI prosthesis. With these, related excessive mechanical stress leads to an accelerated degeneration process of the leaflets. While evidence suggests that transcatheter and surgical valves have similar durability, data regarding long-term structural valve deterioration after VIV-TAVI procedures are very sparse.
The above-mentioned factors and the type of TAVI prosthesis used during VIV-TAVI procedures impact substantially on both the need for reintervention (mostly due to restenosis) and long-term mortality4,7. Supra-annular self-expanding valves (SEV) are associated with lower transvalvular gradients compared with balloon-expandable valves (BEV). One third of patients receiving BEV and a quarter of those receiving SEV during VIV-TAVI procedures have residual stenosis7. On the other hand, residual paravalvular leakage and the need for new pacemaker implantation are higher with SEV compared to BEV4,7. Despite this, long-term data from the VIV-TAVI subgroup of the VIVID registry demonstrated a three times higher need for reintervention after BEV implantation compared to SEV implantation, subhazard ratio 3.34 (95% CI: 1.26-8.85)4. Unfortunately, the current meta-analysis cannot add to the available evidence due to the lack of information about specific types/sizes of surgical bioprosthesis, pre-existing PPM and type of TAVI prosthesis implanted.
Another important and life-threatening complication of VIV-TAVI procedures is coronary ostial obstruction. It is threefold to fourfold more common after VIV-TAVI compared to native valve TAVI, with a previously reported frequency of 2.5% to 3.5%8. The risk of coronary obstruction is dependent on the characteristics of the pre-existing surgical bioprosthesis and the relationship of its leaflets with the coronary ostia (supra-annular valve positions, internal or no stent frames, bulky and long leaflets extending outward beyond the surgical device frame) as well as on anatomical features such as narrow sinuses of Valsalva, narrow sinotubular junctions and low-lying coronaries in a narrow aortic root. Again, neither incidence of coronary obstruction nor descriptive data regarding anatomical features of the aortic root are given in the current meta-analysis. Furthermore, information about the technical aspects of the procedure (bail-out stenting, surgical leaflet modification, supra-annular valve implantation, bioprosthetic valve fracture) that are closely linked to the clinical efficacy of VIV-TAVI is lacking.
However, the current meta-analysis, which represents the largest study to date exploring the outcomes of VIV-TAVI, supports the usage of VIV-TAVI procedures in patients with aortic bioprosthetic valve failure; on the other hand, many questions regarding the various technical issues need to be solved. The continuously growing evidence in this field highlights the need for improved operative techniques and patient-tailored prosthesis selection during SAVR, very accurate TAVI prosthesis selection, as well as innovative technical considerations for VIV-TAVI procedures.
Conflict of interest statement
J. Mehilli has received lecture fees from AstraZeneca, Edwards Lifesciences, Biotronik, Boston Scientific, Bristol-Myers Squibb, and Medtronic, and institutional research grant support from Boston Scientific. C. Giannini has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

