Abstract
Transcatheter aortic valve implantation (TAVI) within failed bioprosthetic surgical aortic valves (valve-in-valve TAVI) has become an established procedure, currently approved for patients deemed at high risk for repeat aortic valve intervention. Although less invasive than surgical reoperation, challenges of valve-in-valve treatment include higher rates of malposition, prosthesis-patient mismatch and coronary obstruction. Thus, optimal patient selection and preprocedural planning is of the utmost importance to minimise the risk of these complications. In this review article we provide a fully illustrated overview of the most significant periprocedural operative considerations for valve-in-valve TAVI.
Introduction
In the last decade, bioprosthetic valves have been increasingly implanted instead of mechanical valves. These devices are prone to degeneration and failure. A concern is the potential for an upcoming pandemic of bioprosthesis failure, particularly as younger patients are treated with bioprosthetic valves1. Given that some of these patients may not be surgical candidates, valve reintervention may require a less invasive approach. The volume of valve-in-valve (ViV) transcatheter aortic valve implantation (TAVI) is expected to grow significantly. Current devices and techniques have been successful in the treatment of most degenerated bioprosthetic valves. The main limitations of ViV TAVI are directly related to the lack of space in the aortic root (i.e., residual elevated gradients, severe prosthesis-patient mismatch [PPM]) and mechanical complications related to the deflection of surgical bioprosthetic valve leaflets (i.e., coronary obstruction). This review provides a contemporary overview of the preprocedural evaluation, procedural technique and clinical outcomes in ViV TAVI.
TYPES OF SURGICAL BIOPROSTHETIC AORTIC VALVE
There are numerous bioprosthetic heart valve designs with different proprietary anticalcification treatments. These devices differ in their tissue characteristics, frame designs and implantation methods. They commonly have an unique fluoroscopic appearance, which is essential for optimal ViV TAVI deployment. Surgical bioprosthetic valves are commonly stratified according to the type of tissue (porcine versus bovine pericardial) or according to the frame (stented, stentless, or sutureless valves). Initially, most surgical bioprosthetic aortic valves were implanted at the plane of the annulus (intra-annular), but they were limited by reduced effective orifice area. The current surgical bioprosthetic aortic valves are implanted above the annulus (supra-annular), allowing a larger effective orifice and decreasing the risk of severe PPM. In addition, tissue leaflets are conventionally mounted to the internal aspect of the stent posts; however, several surgical bioprosthetic valves are designed with externally mounted leaflets, e.g., Mitroflow (LivaNova PLC/Sorin Group, Saluggia, Italy) and Trifecta™ (Abbott, Minneapolis, MN, USA). While these valves may have a better haemodynamic profile and reduce the risk of severe PPM, the risk of coronary obstruction following ViV TAVI is higher in certain anatomic conditions. In the future, improvements in surgical valve design, such as the expandable hinge in the INSPIRIS surgical aortic valve (SAV) (Edwards Lifesciences, Irvine, CA, USA), may facilitate ViV TAVI by being able to accommodate a larger transcatheter heart valve (THV) to reduce the risk of PPM.
MECHANISMS OF FAILURE IN SURGICAL BIOPROSTHETIC AORTIC VALVES
There are several common aetiologies for surgical bioprosthetic heart valve failure, according to the Valve Academic Research Consortium (VARC)-3 criteria2 (Table 1).
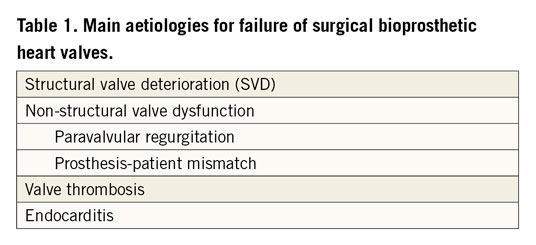
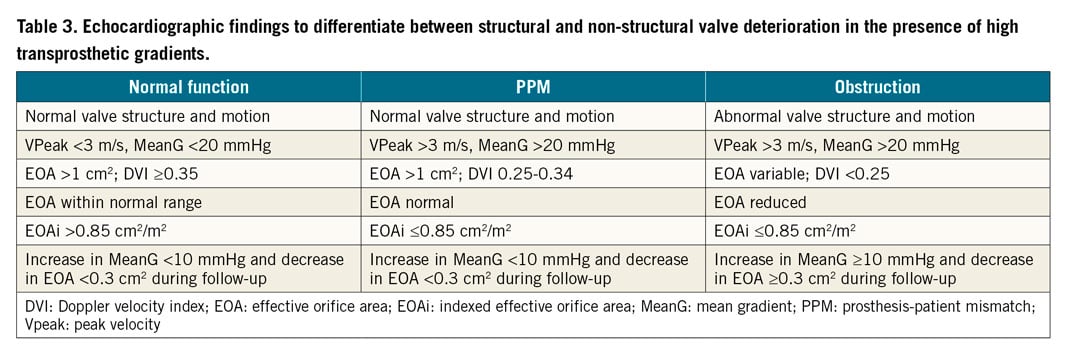
In general, mechanical valves are prone to thrombosis, while bioprosthetic valves are prone to valve deterioration. Structural valve deterioration (SVD) is defined as intrinsic permanent changes to the prosthetic valve, including wear and tear, leaflet disruption, flail leaflet, leaflet fibrosis and/or calcification, and strut fracture, manifested as stenosis and/or regurgitation. The proposed definition of SVD from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) is shown in Table 2. In general, bovine pericardial valves tend to fail more by stenosis, while porcine valves tend to fail more commonly by regurgitation. ViV TAVI is typically considered in patients with SVD rather than non-structural valve dysfunction, although patients with severe PPM with suitable anatomy may be considered for ViV TAVI with adjunctive procedures (e.g., balloon valve fracture). Figure 1 depicts different mechanisms of SVD based on transoesophageal echocardiography (TEE) evaluation. Echocardiographic findings to differentiate between structural and non-structural valve deterioration in the presence of high transprosthetic gradients are reported in Table 3.
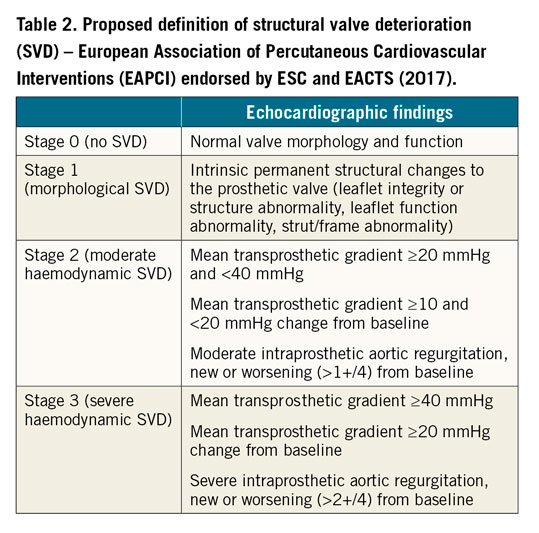
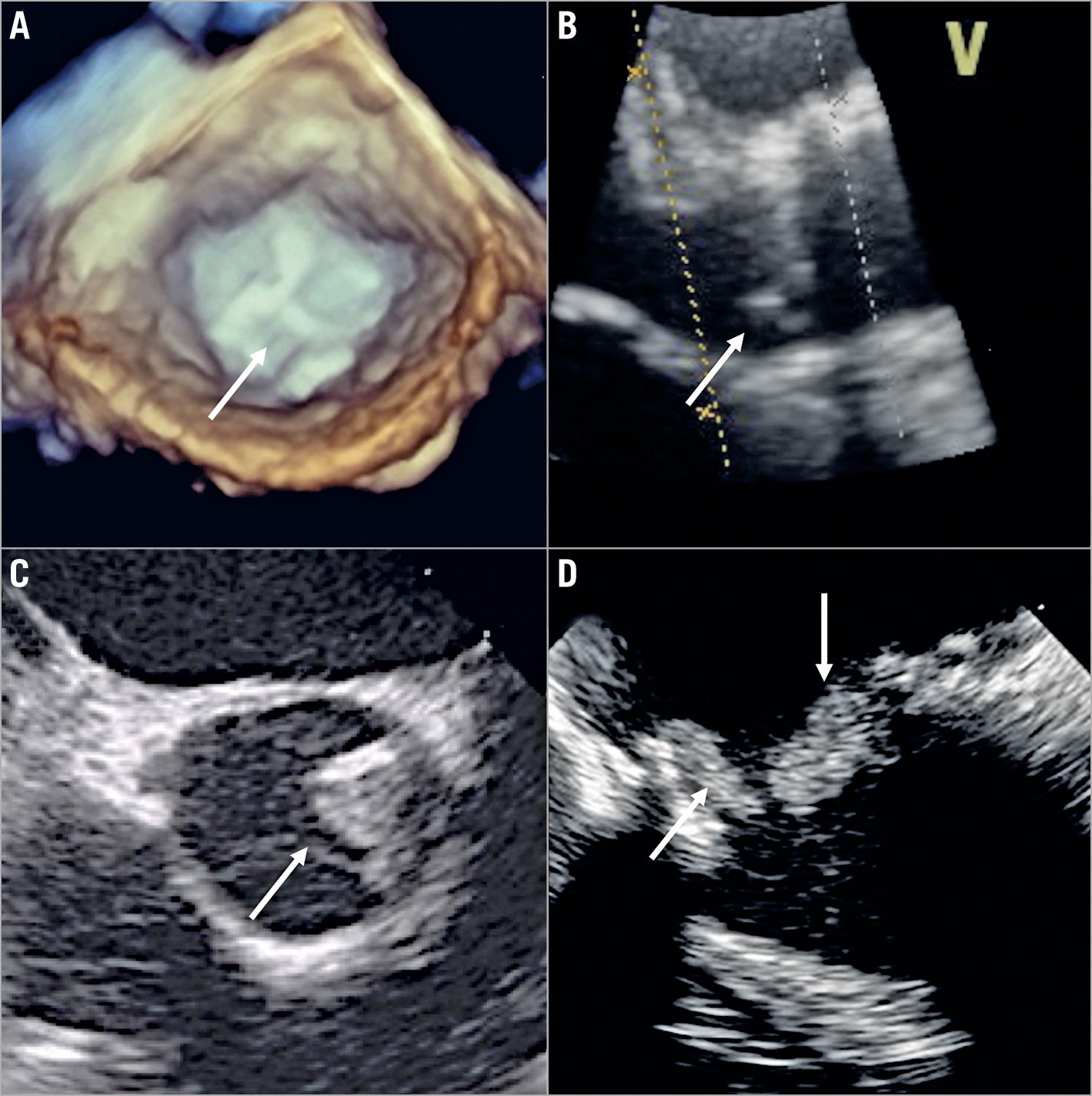
Figure 1. Different mechanisms of aortic bioprosthesis valve dysfunction on transoesophageal echocardiography (TEE) exam. A) Three-dimensional TEE image (short-axis view) of a stented bioprosthesis, showing thickened and calcified leaflets (arrow) causing severe stenosis. B) Two-dimensional TEE image (long-axis view) of a stentless bioprosthesis, showing prolapse of the non-coronary cusp (arrow) leading to severe aortic regurgitation. C) Two-dimensional TEE image (short-axis view) of a stentless bioprosthesis, showing a thrombus (arrow) at the level of the non-coronary cusp. D) Two-dimensional TEE image of a stented bioprosthesis, showing endocarditis vegetations (arrows) at the ventricular side.
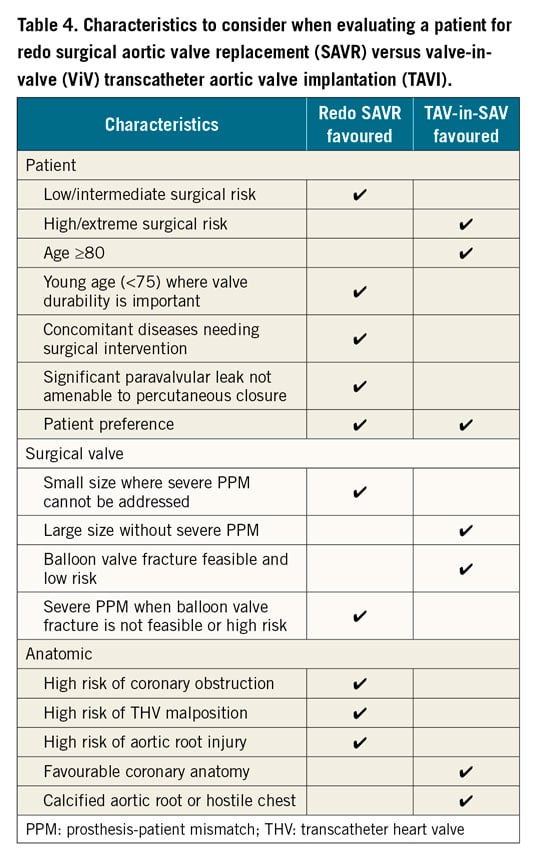
INDICATIONS FOR REDO SURGICAL AORTIC VALVE REPLACEMENT VERSUS ViV TAVI
There are no randomised trials offering clear indications on the best treatment choice. Current indications for ViV TAVI in a failing surgical bioprosthetic aortic valve include patients with SVD who are deemed high or extreme risk for reoperative surgical aortic valve replacement (SAVR). Intermediate or low surgical risk patients should be considered for redo SAVR unless anatomically unfavourable. Younger patients, where valve durability would be important, should also be considered for redo SAVR given the unknown long-term durability of ViV TAVI. Redo SAVR should also be considered in patients who have high-risk anatomy for coronary obstruction in ViV TAVI, where bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) is not feasible or when commissural alignment of the THV is necessary to avoid one of its commissural posts obstructing the split bioprosthetic valve leaflet. In patients with non-structural valve dysfunction, such as severe PPM or paravalvular leak (PVL), redo SAVR should be considered if balloon valve fracture (BVF) cannot be safely performed in the stented SAV or percutaneous treatment of the PVL is not feasible. A summary of criteria suggesting reoperative SAVR versus ViV TAVI is provided in Table 4.
PREPROCEDURAL PLANNING AND PROCEDURAL ASPECTS OF ViV TAVI
EVALUATING TYPES AND DIMENSIONS OF SURGICAL VALVES
Knowledge of the surgical bioprosthesis is critical to determine the feasibility of ViV TAVI and for procedural planning. An overview of commonly implanted SAVs is shown in Supplementary Figure 1-Supplementary Figure 3. The manufacturer, model and size of the SAV can be obtained either from the original operative report or from the implant card. Imaging modalities, such as fluoroscopy and computed tomography (CT), can also identify the above information.
The valve-in-valve (aortic) mobile application, developed by Vinayak Bapat, is an invaluable planning tool to educate operators on the anatomy of SAV, assess ViV TAVI feasibility and provide guidance on the ViV TAVI procedure3. Examples of how to use the valve-in-valve app are shown in Supplementary Figure 4.
As previously mentioned, SAVs can be divided into stented, stentless and sutureless tissue valves. The most common types of bioprosthetic SAV implanted worldwide are stented valves. Each model comes with a manufacturer labelled size which does not represent the true internal diameter (ID) of the prosthesis4. Stent ID, measuring the diameter of the stent frame alone, differs from the true ID depending on the type of stented SAV and how the tissue leaflets are mounted onto the stent frame (Figure 2, Figure 3). Supplementary Table 1 shows the main preprocedural features to consider at CT scan evaluation. In particular, a step-by-step approach to measure internal diameters of bioprosthetic SAVs is reported in Supplementary Figure 5.
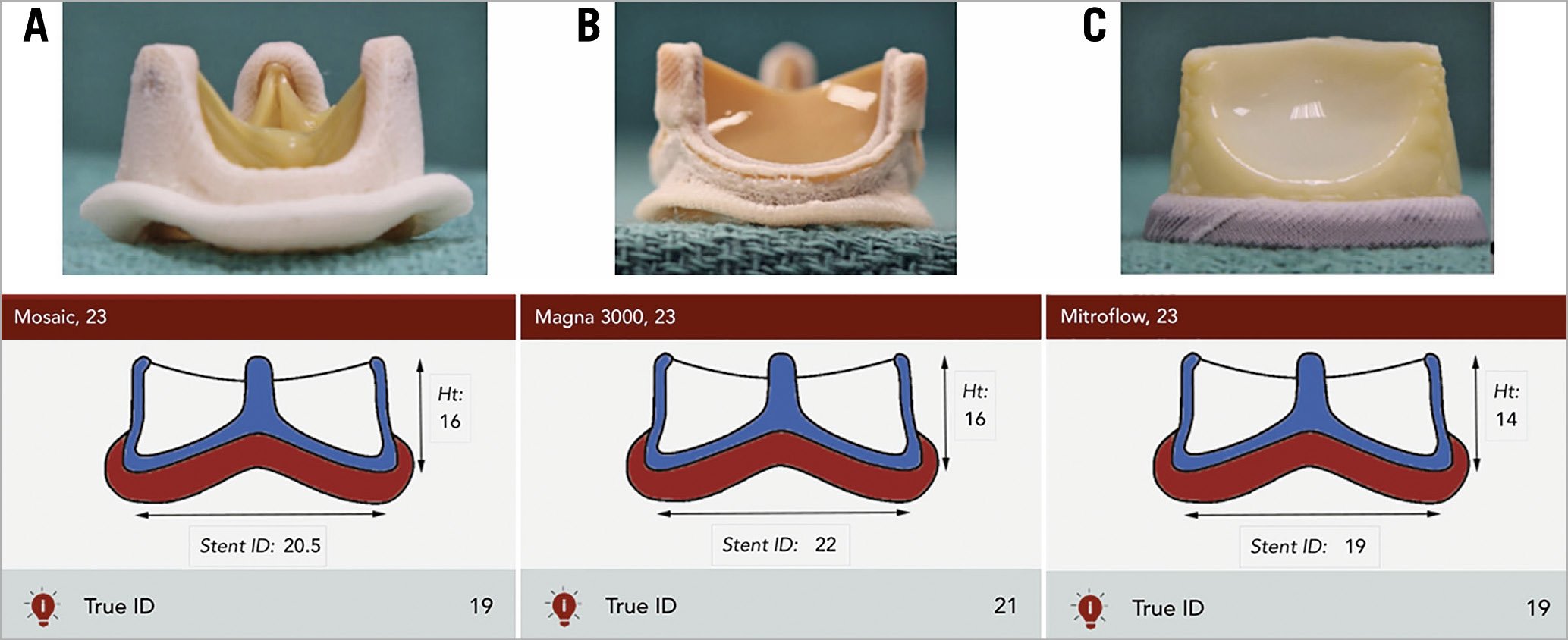
Figure 2. Examples of stented surgical aortic valves. A) 23 mm Mosaic® porcine valve (Medtronic). B) 23 mm Magna 3000 pericardial valve (Edwards Lifesciences). C) 23 mm Mitroflow externally mounted pericardial valve (LivaNova PLC), where the manufacturer labelled size, stent and true internal diameters are different.
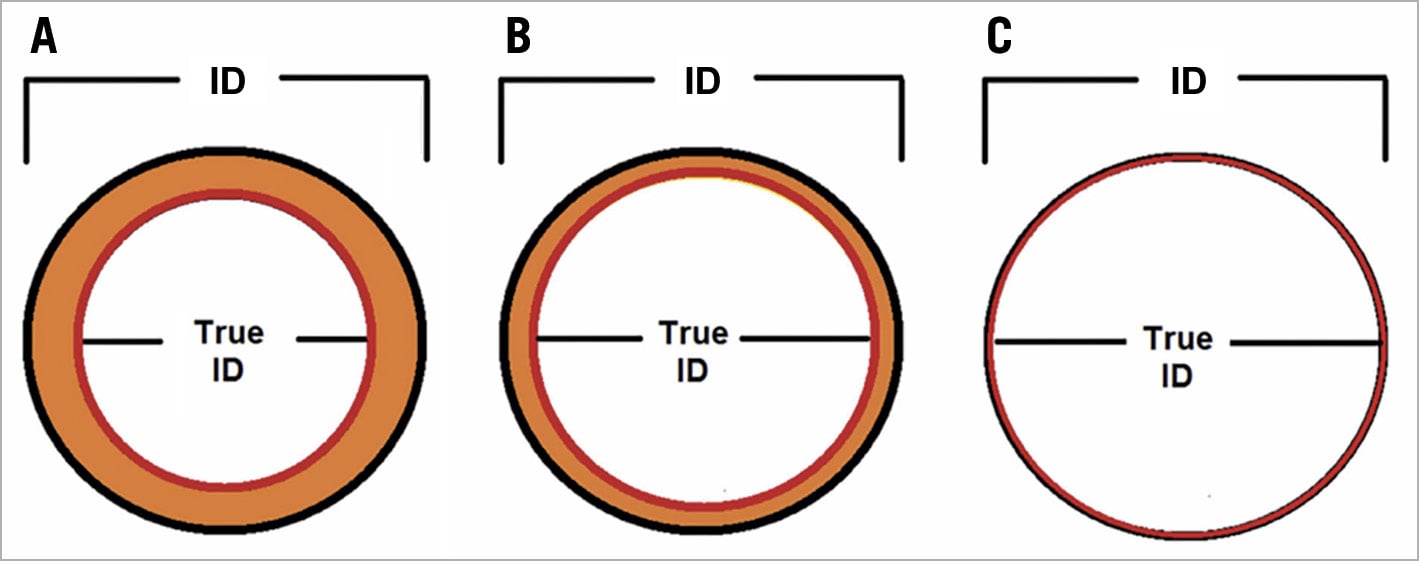
Figure 3. Effect of leaflet type and mounting on the stent and true internal diameter (ID) of stented surgical aortic valves. A) Porcine valves: true internal diameter (ID) is at least 2 mm less than the stent ID. B) Pericardial valves with leaflets sutured inside the stent frame: true ID is at least 1 mm less than the stent ID. C) Pericardial valves with leaflets sutured outside the stent frame: true ID is the same as the stent ID4.
EVALUATING CORONARY OBSTRUCTION RISK IN ViV TAVI
Coronary obstruction risk is known to be higher in ViV TAVI than in native TAVI5. This is due to the extension of the SAV leaflets beyond the aortic root above the sinotubular junction (STJ). After ViV TAVI, the SAV leaflets are displaced to create a cylinder effect causing sinus sequestration and sealing off flow to the coronaries. To assess the risk of coronary obstruction in ViV TAVI, a multidetector computed tomography (MDCT) scan should be performed and the valve-to-coronary (VTC) and valve-to-STJ (VTSTJ) distances should be measured. Distances <3 mm would be considered at high risk of coronary obstruction5. The BASILICA procedure can serve as an adjunctive technique in TAV-in-SAV to reduce coronary obstruction risk, by splitting the interfering SAV leaflet prior to TAVI6. A Valve-in-Valve International Data (VIVID) classification of aortic root anatomy in TAV-in-SAV has been proposed, together with a decision-making algorithm to guide procedural planning and need for BASILICA (Supplementary Figure 6, Figure 4),7. Bear in mind that there is a learning curve associated with the BASILICA procedure and reported stroke rates even in experienced centres were not negligible6. Commissural misalignment of the TAV facing the split portion of the SAV leaflet after BASILICA may still cause coronary obstruction. A more conventional technique of coronary stenting (e.g., chimney technique) may be more reproducible and less complex. An example of the CT-based step-by-step approach for the risk of coronary obstruction in ViV TAVI is shown in Supplementary Figure 7-Supplementary Figure 9.
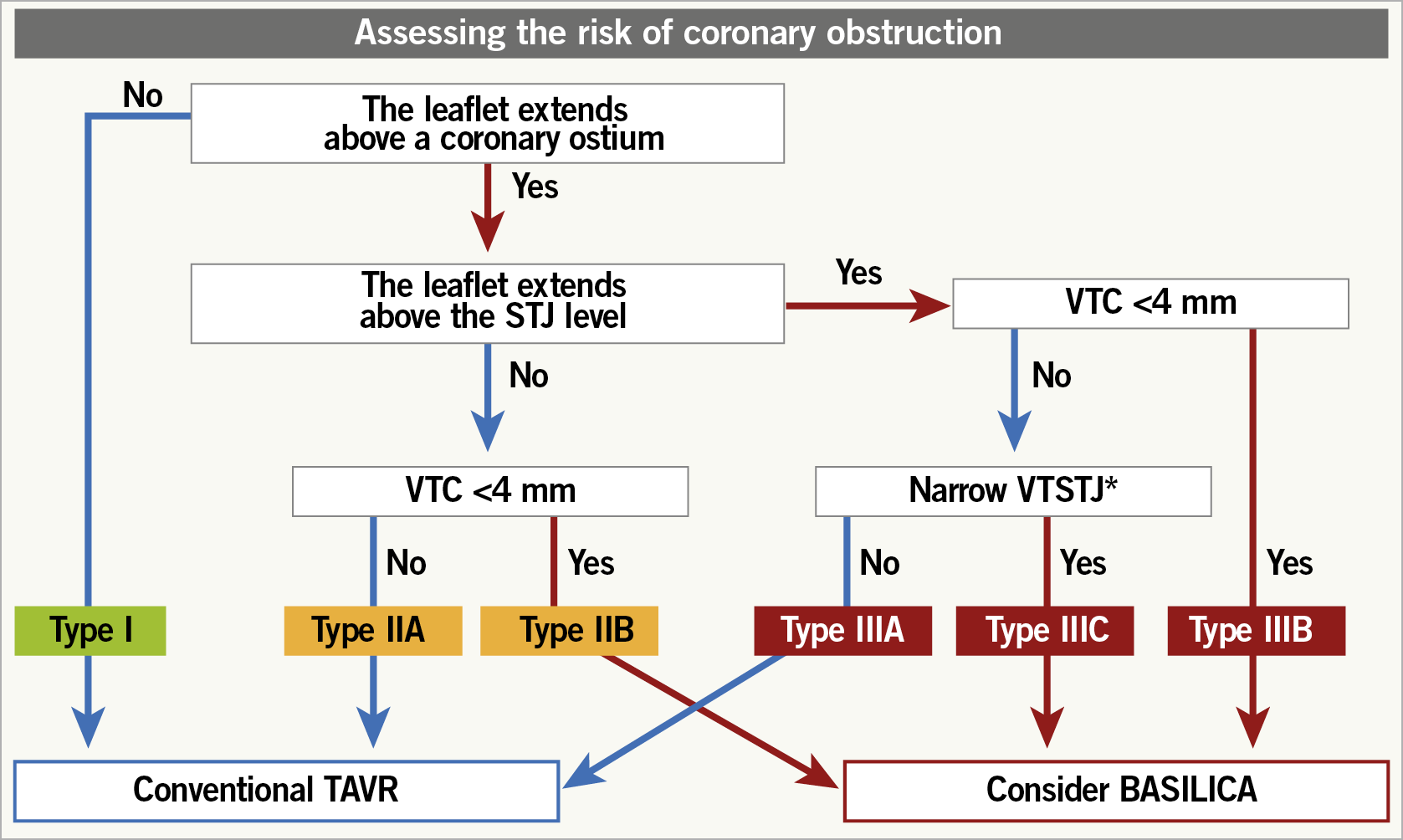
Figure 4. Proposed algorithm to determine aortic root anatomy and indication for BASILICA in ViV TAVI. * Either above, at, or up to 2 mm below the plane of the STJ. The threshold to define a narrow VTSTJ is unknown and is currently considered as <2.5-3.5 mm (<2.5 mm is a high-risk condition and 2.5-3.5 mm is an intermediate-risk condition)7. BASILICA: bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction; STJ: sinotubular junc-tion; TAVR: transcatheter aortic valve replace-ment; VTC: virtual transcatheter heart valve to coronary distance; VTSTJ: virtual transcatheter heart valve to sinotubular junction distance
TRANSCATHETER HEART VALVE SELECTION IN ViV TAVI
The type and size of TAV that can be implanted in a SAV can be found on the valve-in-valve app. A certain degree of oversizing is recommended to avoid TAV migration. Factors to consider when choosing a THV are listed in Table 5.
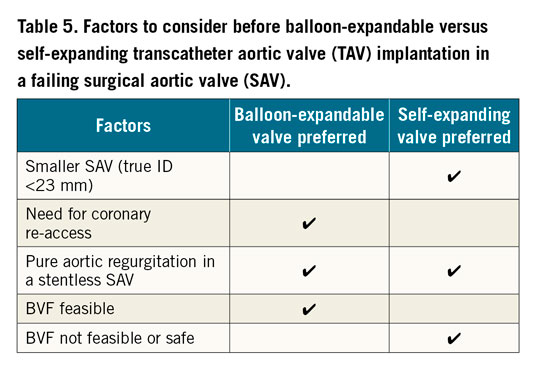
There is emerging evidence that improved haemodynamic performance of the THV can be achieved by aiming for a higher implant due to improved circularity of the THV and reduced pinwheeling of the leaflets8,9,10. Although balloon predilatation is rarely performed in ViV TAVI, post-dilatation may be beneficial to improve THV frame expansion and circularity to optimise THV leaflet function and haemodynamics. In smaller SAVs (manufacturer size ≤23 mm and in porcine valves ≤25 mm given the smaller stent/true ID), BVF, if deemed feasible and safe, may be considered to allow implantation of a larger THV or improve expansion of the planned THV in order to optimise haemodynamic performance.
In sutureless SAVs, the target implant depth depends on the specific sutureless valve and the THV size used given the ability to expand the sutureless valve frame. The valve-in-valve app provides guidance on preprocedural planning and implantation technique4. In stentless valves including homografts, TAVI positioning and deployment strategies should be similar to those in native TAVI, given the lack of fluoroscopic landmarks and the fact that aortic regurgitation is often the mechanism of SVD.
BALLOON VALVE FRACTURE OR REMODELLING IN ViV TAVI
In patients with severe PPM associated with smaller SAVs, ViV TAVI will not improve pre-existing PPM given that the THV is implanted within too small a SAV. In patients with severe PPM but no or mild SVD, ViV TAVI is unlikely to be beneficial because it does not modify the underlying PPM. BVF has been proposed as a technique to increase the true ID of the SAV to allow either a larger THV or a better expanded THV to be implanted in order to optimise haemodynamic performance, while potentially reducing the severity of the pre-existing PPM11. The technique involves using a non-compliant balloon to inflate within the SAV at a high pressure to fracture the stented SAV frame, to improve expansion of the implanted TAV or to allow a larger TAV to be implanted without the risk of frame underexpansion.
Not all stented SAVs can undergo BVF (Supplementary Table 2),12. Sutureless and stentless SAVs cannot undergo BVF; however, in sutureless valves you can potentially obtain balloon valve remodelling (BVR) by overexpansion. BVF should not be performed in patients with bio-Bentall aortic root replacement with a stented SAV due to the risk of aortic root rupture. This is due to the surgical suture line located at the ventriculo-aortic junction. A non-compliant balloon may disrupt that suture line and cause a potentially fatal tear in the aortic root. BVF may be performed before or after TAVI. The potential advantages and disadvantages of BVF before or after TAVI are listed in Table 6.
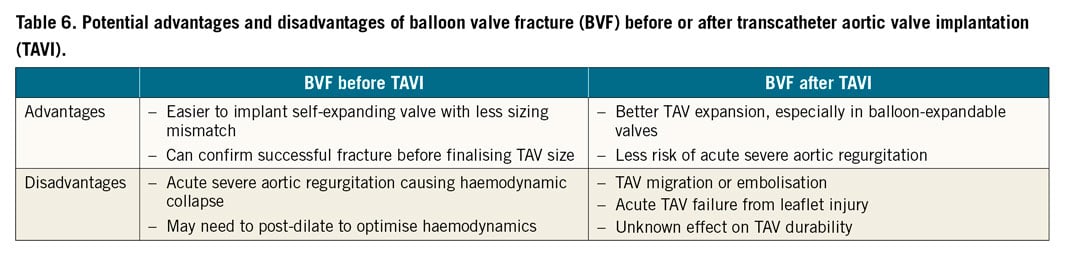
In terms of balloon selection and sizing, a non-compliant balloon should be selected (e.g., Atlas™ Gold, True™ balloon [both Becton, Dickinson and Company, Franklin Lakes, NJ, USA]). The ideal balloon size should always be more than the true ID and be at least equal to the stent ID if not the manufacturer label size. When doing BVF after TAVI, the balloon should be positioned, when feasible, to reduce contact with the THV leaflets in order to avoid injury. Using a 60 mL syringe plus an indeflator assembly connected with a high-pressure 3-way stopcock, under rapid ventricular pacing, the syringe is quickly emptied to inflate the balloon, then switched to cranking the indeflator to achieve high-pressure inflation13. The balloon waist release at fluoroscopy is associated with a sudden drop in inflation pressure. The balloon is then deflated and removed carefully to avoid the risk of THV migration.
Complications from BVF include acute severe aortic regurgitation from SAV causing haemodynamic collapse, THV migration, coronary obstruction, aortic root injury and THV failure due to balloon injury to the leaflets.
STROKE RISK AND CEREBRAL EMBOLIC PROTECTION
Degenerated surgical bioprostheses may be heavily calcified and/or friable, and prone to leaflet tearing. Despite this, no significant difference in stroke rate has been demonstrated between TAV-in-SAV procedures and TAVI for native aortic valve stenosis14. Therefore, a cerebral embolic protection device (CEPD) should be used based on the operative risk factors (e.g., complex valve-in-valve procedures with expected increased catheter manipulation, multiple valve repositioning manoeuvres, need for predilatation and post-dilatation/BVF, and BASILICA procedure).
ANTITHROMBOTIC REGIMEN
Patients who are candidates for ViV procedures usually have multiple comorbidities, leading to a higher risk of both thrombotic and bleeding events, which require optimal antithrombotic management. In the absence of randomised data, the optimal antithrombotic regimen after ViV procedures should be based on the patient’s specific anatomical (e.g., higher risk of leaflet thrombosis and sinus sequestration) and clinical (e.g., other indications for anticoagulant therapy such as atrial fibrillation) characteristics. Regardless of the periprocedural and early antithrombotic regimen, a careful and close clinical and echocardiographic monitoring for early recognition of signs of valve thrombosis and new onset of atrial fibrillation is also of the utmost importance in ViV patients.
CORONARY ACCESS AFTER ViV TAVI
Factors affecting the feasibility of coronary access after ViV TAVI are largely similar to those related to the risk of acute coronary obstruction during the index procedure7 (Figure 5). With the implantation of the transcatheter valve, the leaflets of the degenerated surgical prosthesis will be tilted up, thereby creating a cylindric covered stent through which a catheter will not be able to penetrate towards the coronary ostium. The height of this barrier (i.e., “neoskirt”)15,16,17,18 is determined by the length of the degenerated SAV leaflets. To anticipate the unfeasibility of coronary access after ViV TAVI, the following factors need to be evaluated:-
1. Location of the coronary ostium in relation to the neoskirt
This depends on both coronary ostium height and type of surgical bioprosthesis (and height of SAV implantation). Coronary cannulation will be easier if the ostium is above the neoskirt (type I), particularly if the THV type used for ViV TAVI has an open-cell design19,20,21 (Figure 6A).
2. Sinotubular junction dimensions
If the coronary ostium is located below the neoskirt, coronary cannulation is theoretically feasible only in the presence of a large STJ (type IIa and IIIa). Nevertheless, even in the presence of a large STJ junction, although the deployment of the THV might not cause a complete impairment of coronary flow (i.e., acute coronary obstruction), the VTSTJ might be not wide enough to allow selective coronary cannulation (Figure 6B). In this situation, percutaneous coronary intervention will be difficult, if not impossible.
3. Type of transcatheter heart valve
Irrespective of aortic root anatomy, use of a low frame THV is advantageous as compared to a taller frame device with regard to coronary cannulation. This is particularly true in the case of type IIa and IIIa anatomies. Importantly, available supra-annular devices have higher asymmetric commissures (Figure 7A). If a commissure lands in front of the coronary ostium because of THV misalignment (Figure 7B), coronary cannulation will be non-coaxial and thus particularly difficult if the ostium is located below the neoskirt. The latter issue might be mitigated by the possibility of aligning the THV to the surgical bioprosthesis (i.e., to the native aortic valve). While it has been shown that Evolut™ (Medtronic, Minneapolis, MN, USA) and ACURATE (Boston Scientific, Marlborough, MA, USA) THVs might be partially “orientable”, other available THVs cannot be aligned22.
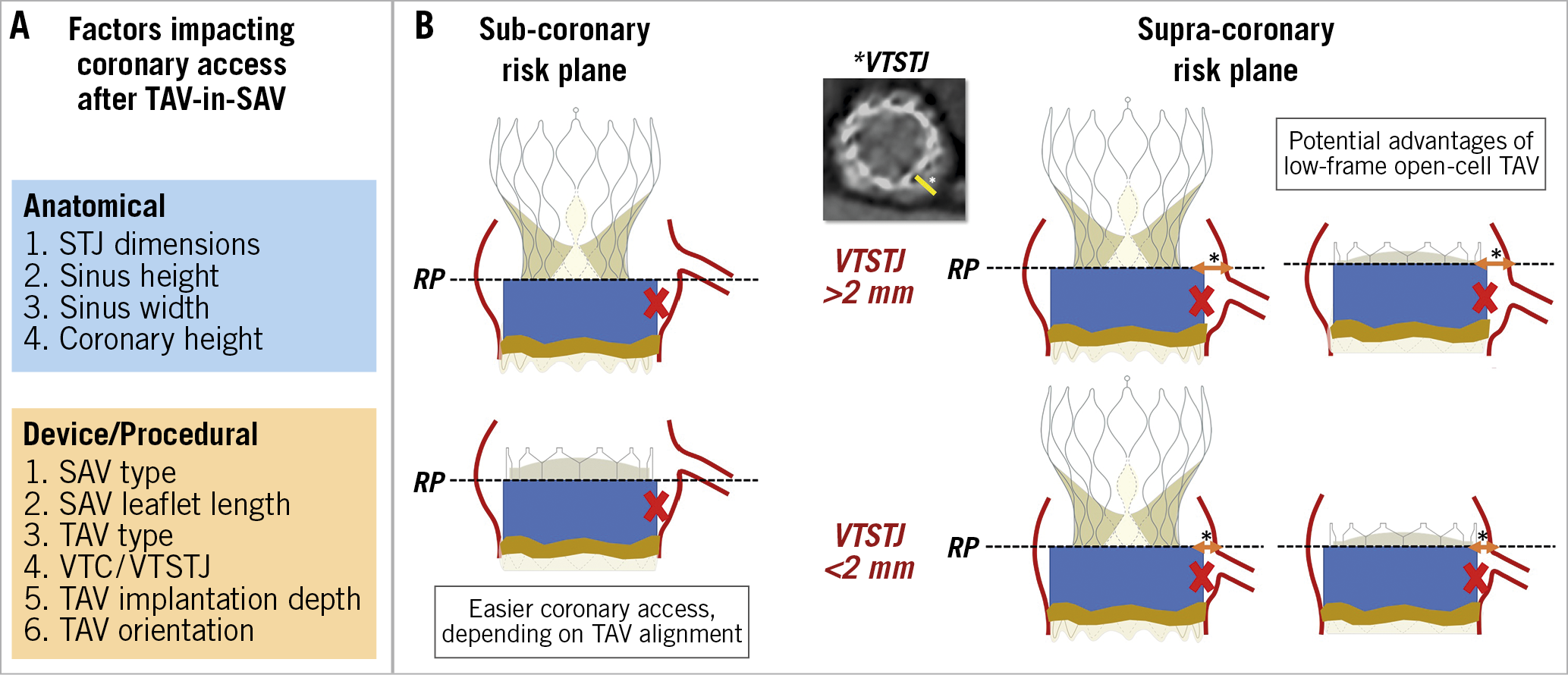
Figure 5. Factors impacting coronary access after TAV-in-SAV in prostheses with a sub-coronary or supra-coronary risk plane. A) Factors impacting coronary access after TAV-in-SAV. B) Sub-coronary risk plane and supra-coronary risk plane. RP: risk plane; SAV: surgical aortic valve; TAV: transcatheter aortic valve; VTC: virtual transcatheter heart valve to coronary distance; VTSTJ: virtual transcatheter heart valve to sinotubular junction distance
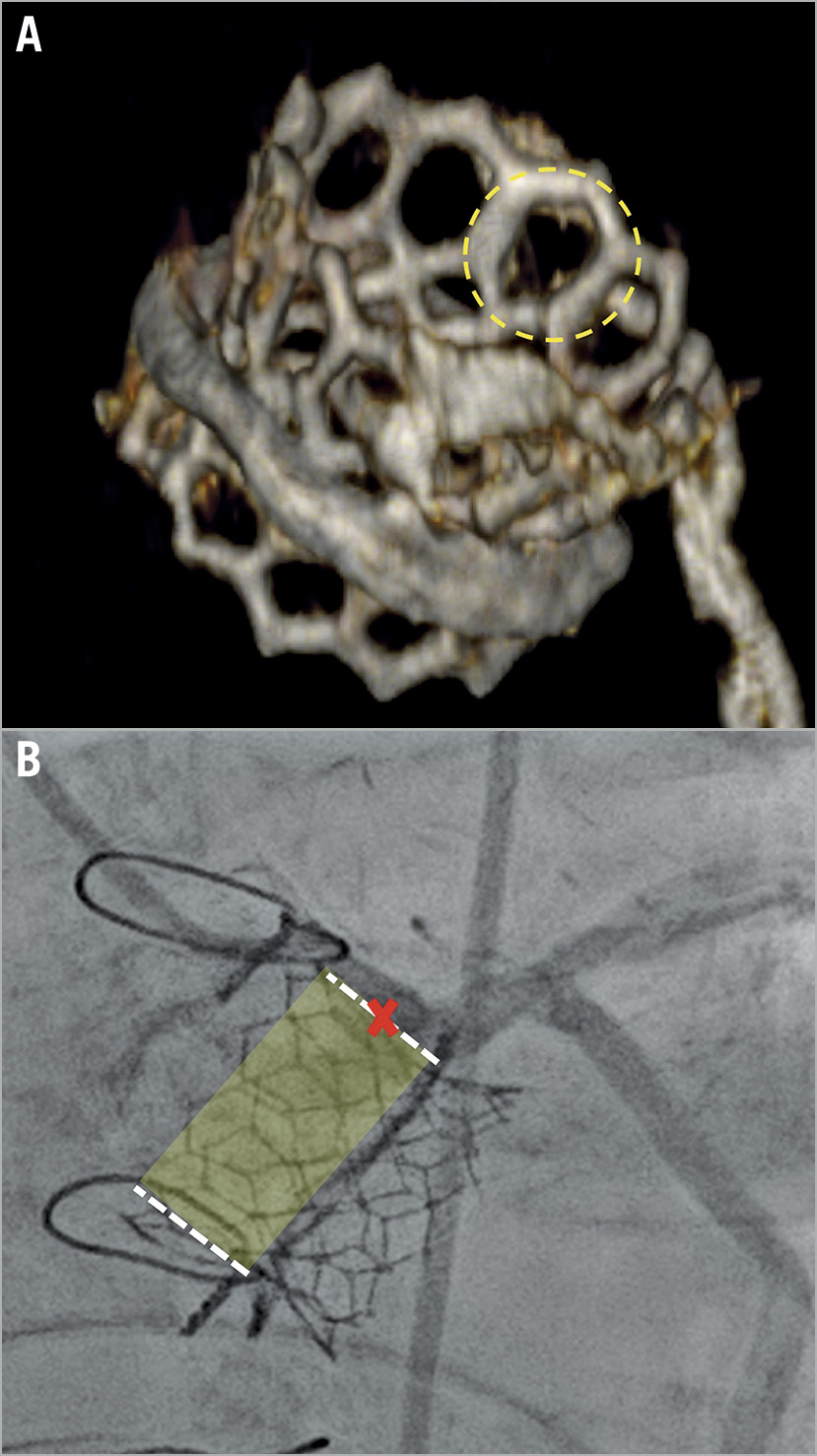
Figure 6. Two cases of ViV TAVI. A) Valve-in-valve TAVI with a SAPIEN 3 valve (Edwards Lifesciences). The dotted circle identifies an open cell in the upper part of the stent frame. B) A case of ViV TAVI with a small VTSTJ in which the coronary artery could not be selectively engaged despite the implantation of a low-frame prosthesis with large cell size.
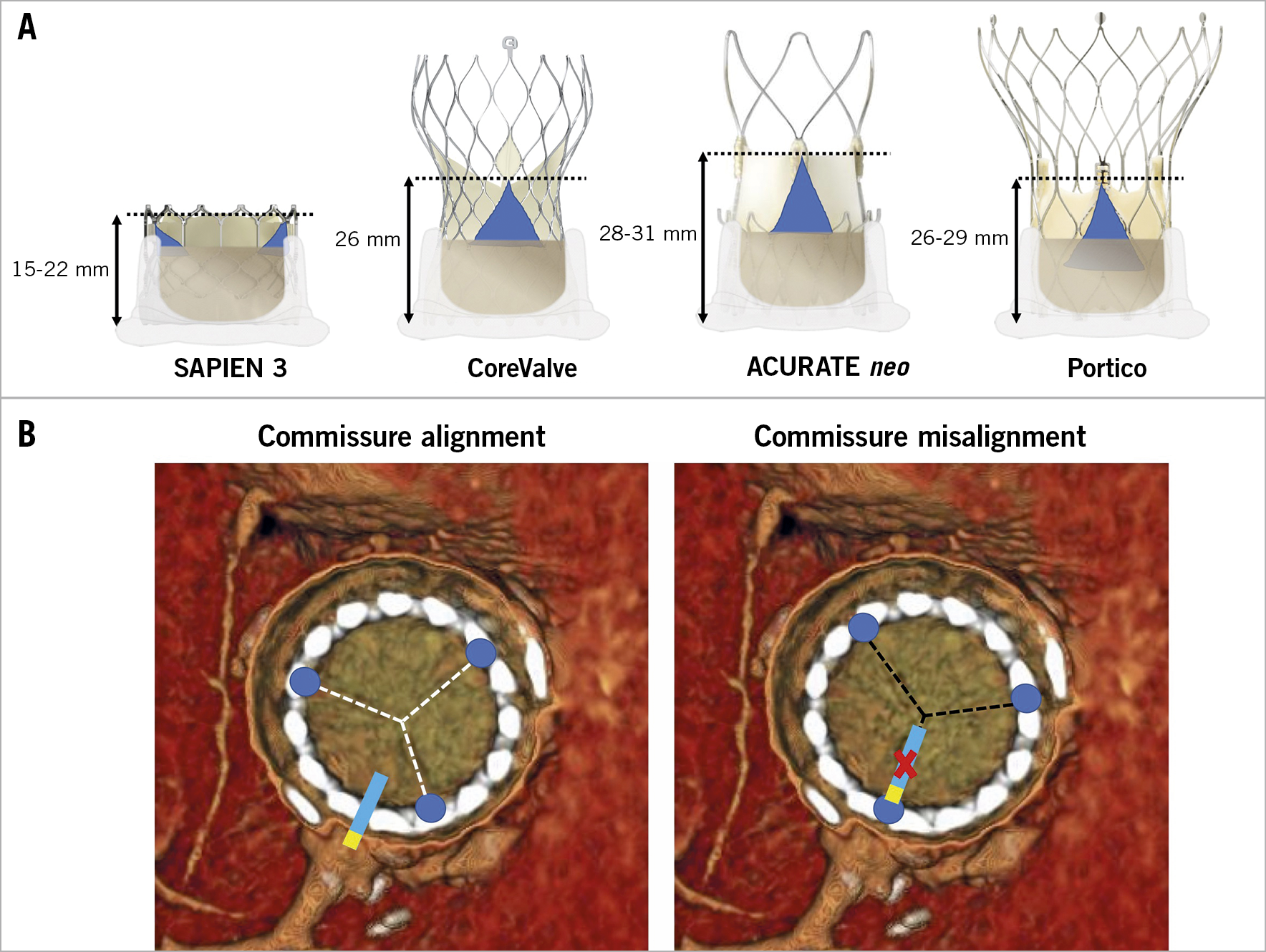
Figure 7. Impact on coronary access of misaligned high THV commissural posts. A) Commissure height of different transcatheter heart valves. B) Examples of neo-commissure alignment versus misalignment with the native coronary ostia.
CLINICAL OUTCOMES AFTER ViV TAVI
Clinical outcomes after ViV TAVI are different from outcomes after conventional TAVR in several respects23,24 (Table 7).
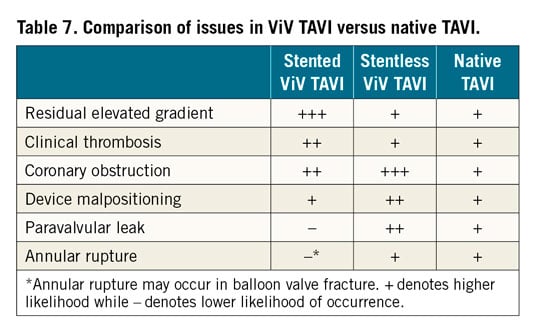
A matched comparison between conventional TAVI and ViV TAVI showed lower mortality in the ViV group, that persisted after adjusting for differences in baseline characteristics23. The frame of stented bioprosthetic valves enables protection to surrounding structures when a valve is implanted inside. As a result, the risk of mechanical complications such as annular injury and damage to the aortomitral curtain is lower in ViV TAVI. Similarly, conduction defects are lower, with the rate of pacemaker implantation after ViV TAVI consistently below 10%. The frame of the stented bioprosthetic valves also enables good support for sealing after ViV TAVI and, as a result, the risk of PVL is very low, if the previously implanted valve did not have PVL. On the other hand, some adverse events are more common in ViV TAVI. These include residual elevated gradients (especially in small and stenotic surgical valves), clinical thrombosis, THV malpositioning (especially in regurgitant stentless valves and those with poor fluoroscopic markers), and coronary obstruction.
Clinical outcomes after aortic ViV TAVI are significantly related to the characteristics of the surgical valve. Small and stenotic surgical valves are associated with inferior clinical outcomes. Data from the VIVID Registry, stratifying patients according to the valve size, showed that those with small surgical valves (label size ≤21 mm) had worse one-year mortality after ViV TAVI than those with intermediate and large surgical valves (25.2% vs 18.2% and 6.7%)25.
More contemporary smaller analyses with lower-risk patient populations have shown lower one-year mortality compared to the VIVID Registry23,26. The mechanism of failure consistently continues to be linked to the risk of elevated post-procedural gradients.
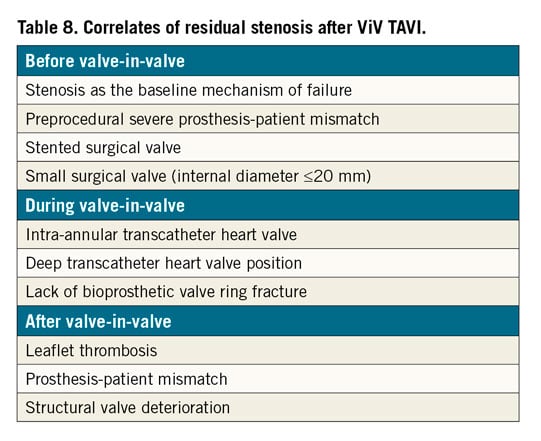
With greater operator experience and improved devices in comparison to those used a decade ago, the rate of most adverse events after ViV TAVI is decreasing. Nevertheless, post-ViV TAVI elevated gradients remain a significant issue27. Elevated gradients are relatively common after ViV TAVI and considered the Achilles’ heel of these procedures. Correlates for residual stenosis after ViV TAVI can be divided into preprocedural, procedural and post-procedural characteristics (Table 8). Long-term (8-year) outcomes after ViV TAVI were recently published by the VIVID Registry investigators28, with the main correlates for mortality and for reintervention including small true ID, pre-existing severe PPM and the use of balloon-expandable valves.
Supplementary Figure 10 shows different examples of challenging ViV TAVI cases.
Conclusions
In this review we have tried to encompass all the most relevant and updated achievements in the field of valve-in-valve TAVI. Specifically, we assessed different preprocedural planning aspects such as patient selection, coronary obstruction risk assessment, and THV selection (Central illustration). Moreover, we analysed relevant procedural features such as prosthesis implantation technique, the role of balloon valve ring fracture, and coronary access.
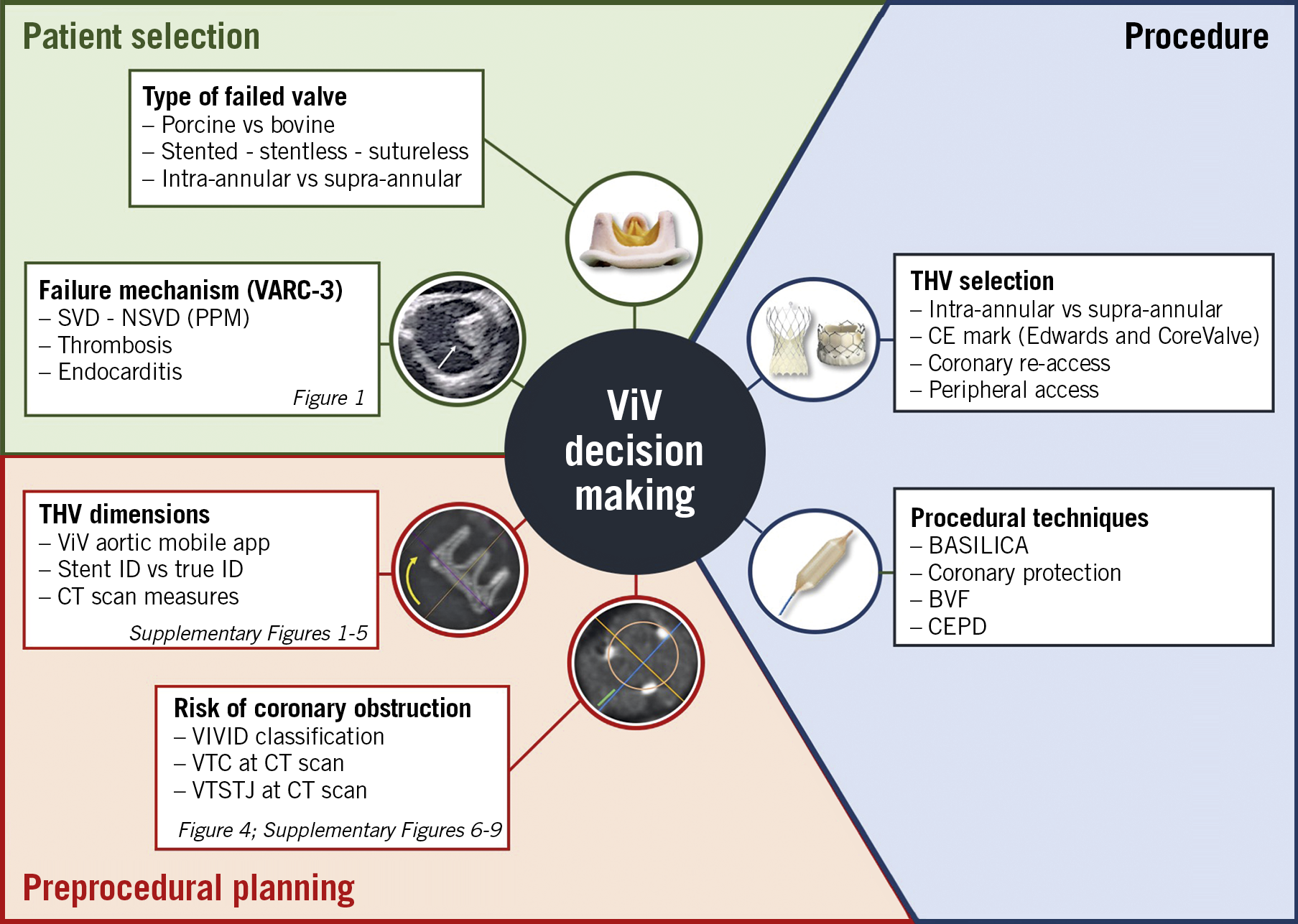
Central illustration. Overview of patient selection, preprocedural evaluation and procedural aspects of valve-in-valve TAVI. BASILICA: bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction; BVF: balloon valve fracture; CEPD: cerebral embolic protection device; CT: computed tomography; ID: internal diameter; NSVD: non-structural valve deterioration; SVD: structural valve deterioration; ViV: valve-in-valve; VIVID: Valve-in-Valve International Data; VTC: virtual transcatheter heart valve to coronary distance; VTSTJ: virtual transcatheter heart valve to sinotubular junction distance
Conflict of interest statement
G. Tarantini has received lecture fees from Medtronic, Edwards Lifesciences, Abbott and Boston Scientific. D. Dvir is a consultant to Edwards Lifesciences, Medtronic, Abbott, and PC Cardia. G. Tang is a physician proctor for Medtronic and a consultant for Medtronic, Abbott Structural Heart and W.L. Gore & Associates.
Supplementary data
To read the full content of this article, please download the PDF.

