
All tissue valves will fail, eventually1. Fortunately, redo surgery can be an excellent option and can be performed with an “acceptable” risk in most patients. However, the morbidity associated with redo thoracotomy is unappealing to most patients, and the risk of mortality can become prohibitive in the setting of advanced age or comorbidities.
In this issue of EuroIntervention, Spaziano et al compare transcatheter heart valve (THV) implantation within failed surgical aortic valves (TAV-in-SAV) in 79 patients from seven European and Canadian centres2.
Outcomes were similar at 30 days and one year in propensity-matched patients undergoing reoperative surgical aortic valve replacement (redo-SAVR). More specifically, 30-day mortality was numerically, but not significantly, lower with TAV-in-SAV than with redo-SAVR (3.9% vs. 6.4%, p=0.49).
This sort of comparative analysis adds substantially to prior larger multicentre registries which have examined the outcomes associated with TAV-in-SAV in isolation. In turn, these registries may help in interpreting the findings of this smaller propensity analysis. The Valve-in-Valve International Data (VIVID) registry, SAPIEN XT PARTNER 2 valve-in-valve study, and the CoreValve US Expanded Use study have reported outcomes in 459, 365, and 227 patients, respectively (Table 1)3-5.
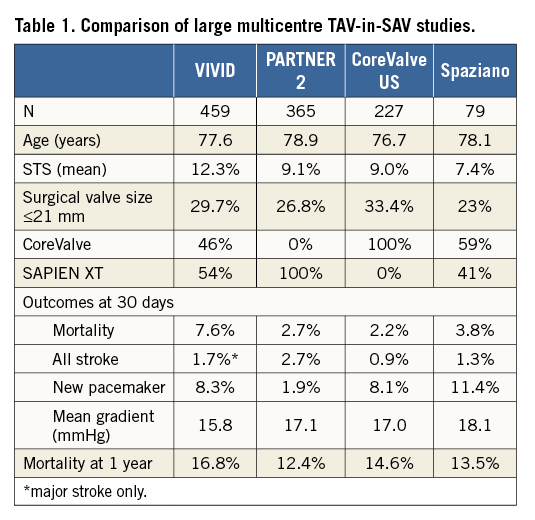
In these three large studies, the Society of Thoracic Surgeons (STS) predicted risk of mortality with redo-SAVR ranged from 9% to 12.3%. The actual mortality ranged from 7.6% in the earlier international VIVID registry down to 2.2% and 2.7% in the more recent, rigorous and prospective North American studies. In the most recent continued access PARTNER 2 registry (269 patients, STS 8.8%), mortality fell dramatically to 0.7%. By way of comparison, the STS estimate of mortality in a 60-year-old male or female without symptoms or comorbidities undergoing redo-SAVR would be 1% or 1.6%, respectively (http://riskcalc.sts.org/). Were mortality risk the only concern, then one might argue that TAV-in-SAV might be a reasonable option even in low surgical risk patients. However, there are issues beyond 30-day mortality to consider.
Durability is one such concern. Although we have documentation of durability in excess of five years, and anecdotally beyond 10 years, with THVs in the setting of native aortic stenosis, we have minimal documentation of late durability with TAV-in-SAV6. Because THVs are constrained within the surgical bioprosthesis, they are routinely underexpanded. Suboptimal expansion results in suboptimal leaflet coaptation, which is known to lead to more rapid leaflet degeneration. Reassuringly, these three large valve-in-valve registries, with a total of 951 patients and follow-up to one year and beyond, have not documented worrisome signals of structural valve degeneration3-5. Although this is reassuring for elderly patients with comorbidities, it may be less reassuring for younger, lower-risk patients with the potential for longevity. It seems likely that durability will not equal that seen with native valve implants or, importantly, with redo-SAVR. When TAV-in-SAV implants do fail, further transcatheter options or redo-SAVR with THV explantation options may be limited.
When considering a TAV-in-SAV procedure, the haemodynamic performance of the constrained THVs is often excellent, but at times can be suboptimal. Importantly, transaortic mean gradients at 30-day follow-up in the SAPIEN XT PARTNER 2 and in the CoreValve US study were virtually identical at 17.0 and 17.1 mmHg, respectively4,5. These gradients are higher than seen in the setting of THV implantation in native aortic stenosis. But do these higher gradients matter? Irrespective of gradients, the great majority of patients, regardless of implant type, experience marked symptomatic and functional improvement.
Theoretically, the CoreValve™ device (Medtronic, Dublin, Ireland) may offer some advantages, as the tissue leaflets are positioned higher in the frame than with most other balloon-expandable, mechanically expanded, or self-expanding THVs. This may allow more optimal leaflet function in a supra-annular position. Sub-analyses in the VIVID and PARTNER 2 registries document higher gradients with intra-annular balloon-expandable valves that may be clinically relevant in smaller surgical bioprostheses. A surgical valve label size <21 mm or an internal diameter of ≤20 mm has been suggested as a point at which these differences may become clinically relevant, although this remains controversial3,4,7.
Arguably, with newer, repositionable THV designs, high implantation with supra-annular valve function may be even more reliably accomplished. Recent studies have documented that high implantation of SAPIEN-type valves, improved valve delivery systems, and the availability of smaller 20 mm diameter balloon-expandable valves can result in markedly improved THV function and lower gradients7. Moreover, although it was originally thought that surgical valves have fixed internal dimensions, recent experience has shown that this is not necessarily the case. High-pressure dilation can stretch or fracture surgical valve rings and markedly increase the internal dimensions of most current surgical bioprostheses, allowing implantation and more complete expansion of even larger THVs8,9.
When choosing between surgery and transcatheter procedures for failed bioprostheses, consideration needs to be given to both the morbidity and mortality associated with these two strategies.
Additional issues include the late implications for optimal haemodynamic function, coronary access, the potential advantages of concomitant cardiac procedures, durability and, in younger and lower-risk patients, the future implications when the second bioprosthetic valve fails.
Although speculative, it seems likely that, with ongoing advances in transcatheter valve implantation, THV implantation will become the default strategy for most patients with failed surgical and transcatheter valves. It is unlikely that we will ever have definitive randomised trials, but the evidence is mounting.
Conflict of interest statement
J. Webb is a consultant to Edwards Lifesciences and Abbott Vascular. D. Wood is a consultant to Edwards Lifesciences. D. Murdoch has no conflicts of interest to declare.

