Abstract
Background: Limited data are available regarding clinical outcomes of valve-in-valve (ViV) transcatheter aortic valve implantation (TAVI) following the United States Food and Drug Administration approval of ViV TAVI in 2015.
Aims: The aim of this study was to evaluate in-hospital, 30-day, and 6-month outcomes of ViV TAVI versus repeat surgical aortic valve replacement (SAVR) in patients with a failed aortic bioprosthetic valve.
Methods: This retrospective cohort study identified patients who underwent ViV TAVI or repeat SAVR utilising the Nationwide Readmission Database from 2016 to 2018. Primary outcomes were all-cause readmission (at 30 days and 6 months) and in-hospital death. Secondary outcomes were in-hospital stroke, pacemaker implantation, 30-day/6-month major adverse cardiac events (MACE), and mortality during readmission. Propensity score-matching (inverse probability of treatment weighting) analyses were implemented.
Results: Out of 6,769 procedures performed, 3,724 (55%) patients underwent ViV TAVI, and 3,045 (45%) underwent repeat SAVR. ViV TAVI was associated with lower in-hospital all-cause mortality (odds ratio [OR] 0.42, 95% confidence interval [CI]: 0.20-0.90, p=0.026) and a higher rate of 30-day (hazard ratio [HR] 1.46, 95% CI: 1.13-1.90, p=0.004) and 6-month all-cause readmission (HR 1.54, 95% CI: 1.14-2.10, p=0.006) compared with repeat SAVR. All secondary outcomes were comparable between the two groups.
Conclusions: ViV TAVI was associated with lower in-hospital mortality but higher 30-day and 6-month all-cause readmission. However, there was no difference in risk of in-hospital stroke, post-procedure pacemaker implantation, MACE, and mortality during 30-day and 6-month readmission compared with repeat SAVR, suggesting that ViV TAVI can be performed safely in carefully selected patients.
Introduction
The most significant limitation of bioprosthetic valves is structural deterioration over time, including regurgitation and restenosis1. With increased implantation of bioprosthetic valves, the management of prosthesis-related complications, including failed bioprosthetic valves, is evolving2. Traditional therapy for patients presenting with a failed aortic bioprosthetic valve was repeat surgical aortic valve replacement (SAVR), as SAVR is a class I recommendation from both European and American guidelines34. Isolated repeat SAVR for degenerated bioprosthetic valves accounts for ~7% of all aortic valve procedures5. However, repeat surgical procedure has been shown to have increased 30-day mortality, postoperative stroke, and pacemaker implantation because of advanced age, risk profile, and increased adhesions from the previous procedure compared with primary SAVR6.
Transcatheter aortic valve implantation (TAVI) is now the recommended modality for treatment of severe native aortic stenosis in high-risk surgical patients as well as being non-inferior to surgery in low- and intermediate-risk patients46. Valve-in-valve (ViV) TAVI is used as a therapeutic option for failed aortic bioprosthetic valves, particularly in patients with high or prohibitive surgical risk47. Small retrospective studies have shown shorter post-procedural stay, lower bleeding risk, and less acute kidney injury with ViV TAVI compared with repeat SAVR89. A meta-analysis comparing ViV TAVI with repeat SAVR showed better outcomes at 30 days without any differences at one year10. One study published using the Nationwide Readmission Database (NRD) from 2012-2016 showed improved short-term outcomes with ViV TAVI; however, this study does not represent contemporary practice11. There are limited data available evaluating trends and outcomes of ViV TAVI compared with repeat SAVR with a failed bioprosthetic valve following the Food and Drug Administration (FDA) approval for ViV TAVI. Our study aimed to investigate in-hospital, 30-day, and 6-month clinical outcomes following ViV TAVI versus repeat SAVR.
Methods
DATA SOURCE
The study utilised data from the NRD from 2016 to 20181213. We did not use the database before 2016 as it used International Classification of Diseases, Ninth Edition codes. Additionally, the FDA approved the CoreValve® (Medtronic, Minneapolis, MN, USA) and the SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) in 2015 for ViV TAVI. The NRD consists of all-payer databases for the Healthcare Cost and Utilization Project, established by the Agency for Healthcare Research and Quality. It is a nationally representative database comprising discharge records from 28 states, with approximately 35 million weighted discharges annually (excluding rehabilitation and long-term acute care facilities), regardless of the payer. The NRD represents approximately 58.2% of all U.S. hospitalisations. The study was exempt from the Cleveland Clinic institutional review board approval requirement as the database contained de-identified data sets with prior ethics committee approval.
STUDY POPULATION (Figure 1)
We queried the NRD 2016 to 2018 using the International Classification of Diseases, Tenth Revision (ICD-10; Z95.2)14 to identify all adults (≥18 years) with a history of prosthetic valves. We excluded hospitalisations with concomitant mitral, pulmonary, and tricuspid valvular diseases and a diagnosis of infective endocarditis15 to identify patients with only an aortic prosthetic heart valve. This exclusion strategy was not followed in the prior study published using NRD data11. We utilised ICD-10-PCS codes to identify patients who underwent TAVI or SAVR. After excluding patients aged <18 years, we created two study cohorts, one for in-hospital and 30-day outcomes after excluding discharges in December to allow for a complete 30-day follow-up period, and the second for 6-month outcomes after excluding discharges from July to December to have complete 6-month follow-up. Additionally, we excluded patients who underwent concomitant coronary artery bypass graft (CABG), mitral, tricuspid, or pulmonary valve, atrial or ventricular septum closure surgeries16 to arrive at the final cohort of patients with aortic prosthetic valves who underwent either TAVI or SAVR alone.
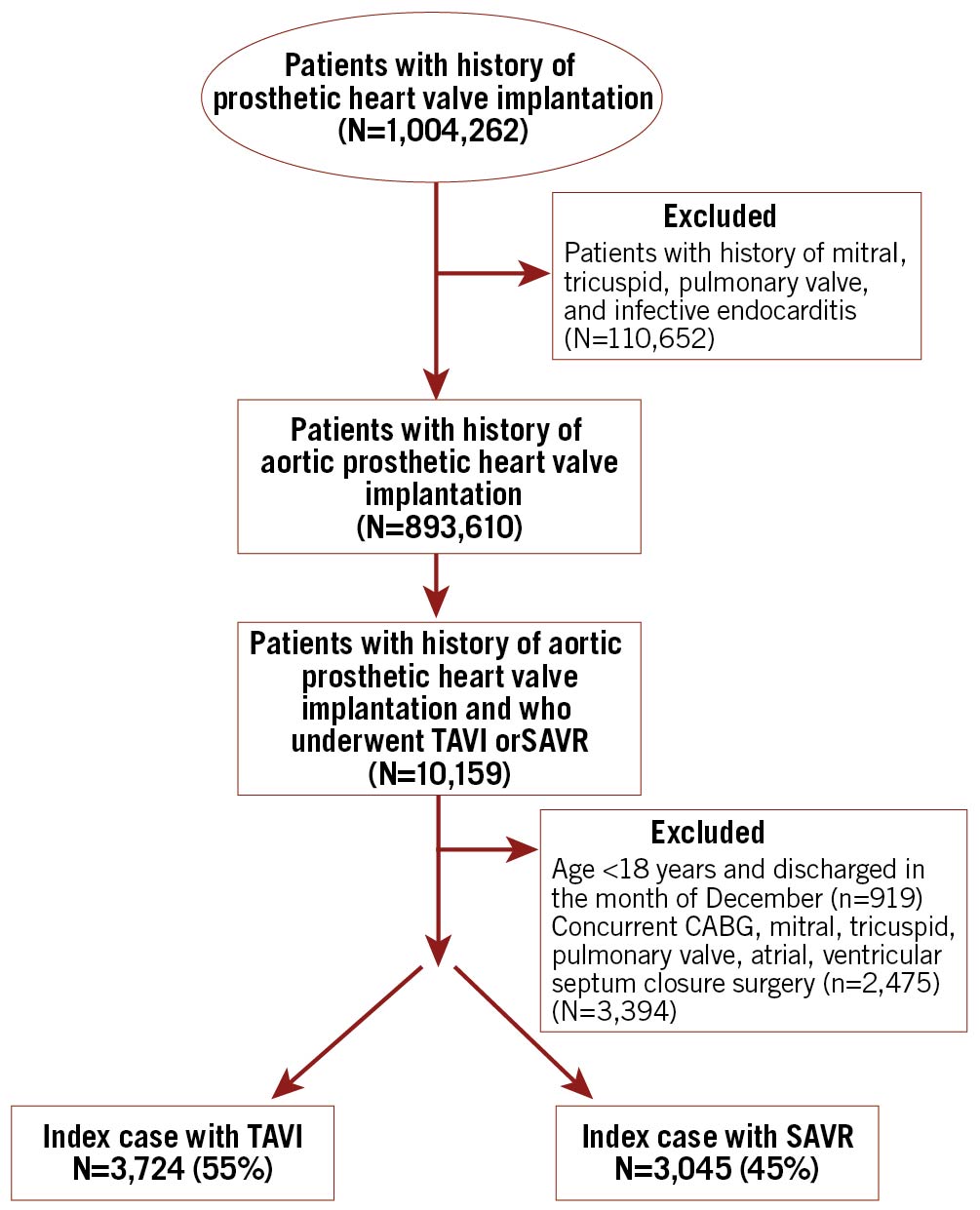
Figure 1. Flow chart of patient selection. SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
PATIENT AND HOSPITAL CHARACTERISTICS
We used NRD variables to identify each patient's age (in years), gender, median household income, primary payer (Medicare, Medicaid, private insurance), day of admission (weekend/weekday), and type of admission (elective/non-elective). We used ICD-10-CM codes to define the prior history of myocardial infarction, stroke/transient ischaemic attack (TIA), ischaemic cardiomyopathy, chronic heart failure, atrial fibrillation, prior history of percutaneous coronary intervention (PCI), prior history of CABG, and history of defibrillator/pacemaker. Elixhauser comorbidities were used to define hypertension, diabetes, hyperlipidaemia, valvular disease, peripheral vascular disease (PVD), smoker, alcohol abuse, obesity, chronic pulmonary disease, renal failure, liver disease, cancer, fluid and electrolyte imbalance, and coagulopathy. Under hospital characteristics, we included hospital size according to the number of beds (small/medium/large), teaching hospital, location of hospital (urban/non-urban), and ownership of hospital (private vs non-profit settings). Hospital volume was calculated by adding all the weighted admissions with TAVI or SAVR at each hospital, and was divided into low versus high procedure volume hospitals based on an annual procedure volume of 10.
STUDY OUTCOMES
The primary outcome for this study was all-cause readmission (30 days and 6 months) and in-hospital mortality. We defined readmission as hospital admission for any principal diagnosis from the discharge date of the index admission. In the case of multiple readmissions, only the first readmission from the index admission discharge date was counted. The secondary outcomes were major adverse cardiac events (MACE), mortality, major bleeding or vascular complications, non-cardiac infection, procedural complications, as well as resource utilisation which comprised length of stay (LOS) and total cost (in US dollars) during 30-day and 6-month readmission. In-hospital outcomes of interest were acute stroke, cardiorespiratory complications, vascular complications, major bleeding, pacemaker implantation, LOS, and cost. Mortality over 30 days and 6 months includes death during hospitalisation, while out-of-hospital death is not registered in the database. In-hospital LOS was defined as post-procedure, after excluding days from admission to the procedure. The definitions of all composite outcomes are given in Supplementary Appendix 1. We showed a trend of utilisation of procedures and their 30-day readmission over quarters. We showed predictors of 30-day, 6-month all-cause readmission in ViV TAVI and repeat SAVR. A subgroup analysis was also performed for 30-day all-cause readmission, stratified by gender, type of primary expected payer, presence or absence of renal failure, presence or absence of heart failure, bed size of the hospital (small/medium/large), and hospital by procedural volume to look for sources of heterogeneity. The definitions and ICD codes of outcome variables are given in Supplementary Table 1. ICD-10 codes used to select the final cohort and outcomes have been published previously141516.
STATISTICAL ANALYSIS
After assessing the distribution, continuous variables are presented as means with standard deviation or median with interquartile range, while categorical variables are presented as a frequency in percentages. Baseline patient and hospital characteristics were compared between ViV TAVI and repeat SAVR using the chi-square test for categorical variables and the Student’s t-test or Wilcoxon rank-sum test.
We created two propensity models (model 1 was for in-hospital and 30-day outcomes, and model 2 was for 6-month outcomes), as the sample sizes of the two cohorts were different. Propensity scores were used to match patients with ViV TAVI to repeat SAVR. A non-parsimonious multivariable logistic regression model was developed to estimate the propensity score for receiving ViV TAVI compared with repeat SAVR using clinically meaningful variables as planned (Supplementary Appendix 2, Supplementary Appendix 3). Then, a double-robust method was used to generate treatment weights, and the inverse probability of treatment weighting was used to match TAVI with SAVR using generalised linear models17. On the matched cohort, multivariable regression analysis was done for robustness. Logistic regression analysis was used to calculate the odds ratio (OR) with a 95% confidence interval (CI) for in-hospital outcomes. Cox regression was used for 30-day and 6-month outcomes to calculate the hazard ratio (HR) with a 95% CI.. The global test of proportionality assumption was not violated (global test p=0.243). For the length of stay and cost, linear regression was done to derive the coefficient. The balance of variables before and after matching is described in Supplementary Figure 1 and Supplementary Figure 2. We used logistic regression for predictors of all-cause readmission. Details of the models are given in Supplementary Appendix 4.
A Kaplan-Meier graph was constructed for all-cause readmission. To calculate a trend p-value for proportions, we conducted logistic regression for binary outcomes with outcomes of interest as the dependent variable and the year as the independent variable, adjusted for age and gender. All p-values were two-sided with a conventional significance threshold of p<0.05.
UNMEASURED BIAS ANALYSIS AND VALIDITY OF STUDY
To evaluate the robustness of our findings, we conducted a falsification endpoint and an “E-value” analysis for 30-day and 6-month readmission and in-hospital mortality18. E-value identifies the minimum strength of association that unmeasured confounders would need to have with both treatment and outcome, conditional on measured covariates, to explain the observed association fully. This estimates what the relative risk would have to be for any unmeasured confounder to overcome the observed association of study intervention with study outcomes. In the falsification method, we selected an alternative outcome that may not be expected to be causally affected by the treatment being studied19. Then, we assessed whether study intervention (ViV TAVI) affects alternative outcomes by using a similar method to assess other study outcomes. If no treatment effect is seen for the alternative outcome, it supports but does not prove that there may be a causal treatment effect for the study outcomes. Thus, a successful falsification analysis can strengthen the causal claims between study intervention and outcome in the observational study. We chose a composite of gastrointestinal and urinary tract infection readmission as an alternative outcome and studied the effect of interventions.
All statistical analyses were conducted using appropriate weighting, stratifying, and clustering samples to obtain national estimates using the svy package of Stata, version 16.1 (StataCorp, College Station, TX, USA). The information on the weighting technique is described in Supplementary Appendix 5.
Results
From the period 2016 to 2018, we included a total of 6,769 patients in the study who underwent a procedure for a failed aortic bioprosthetic valve, of whom 3,724 (55%) underwent ViV TAVI compared with 3,045 patients (45%) who had repeat SAVR (Table 1). Of these, 1,908 (53.8%) patients with ViV TAVI and 1,640 (46.8%) patients with repeat SAVR had a complete 6-month follow-up.
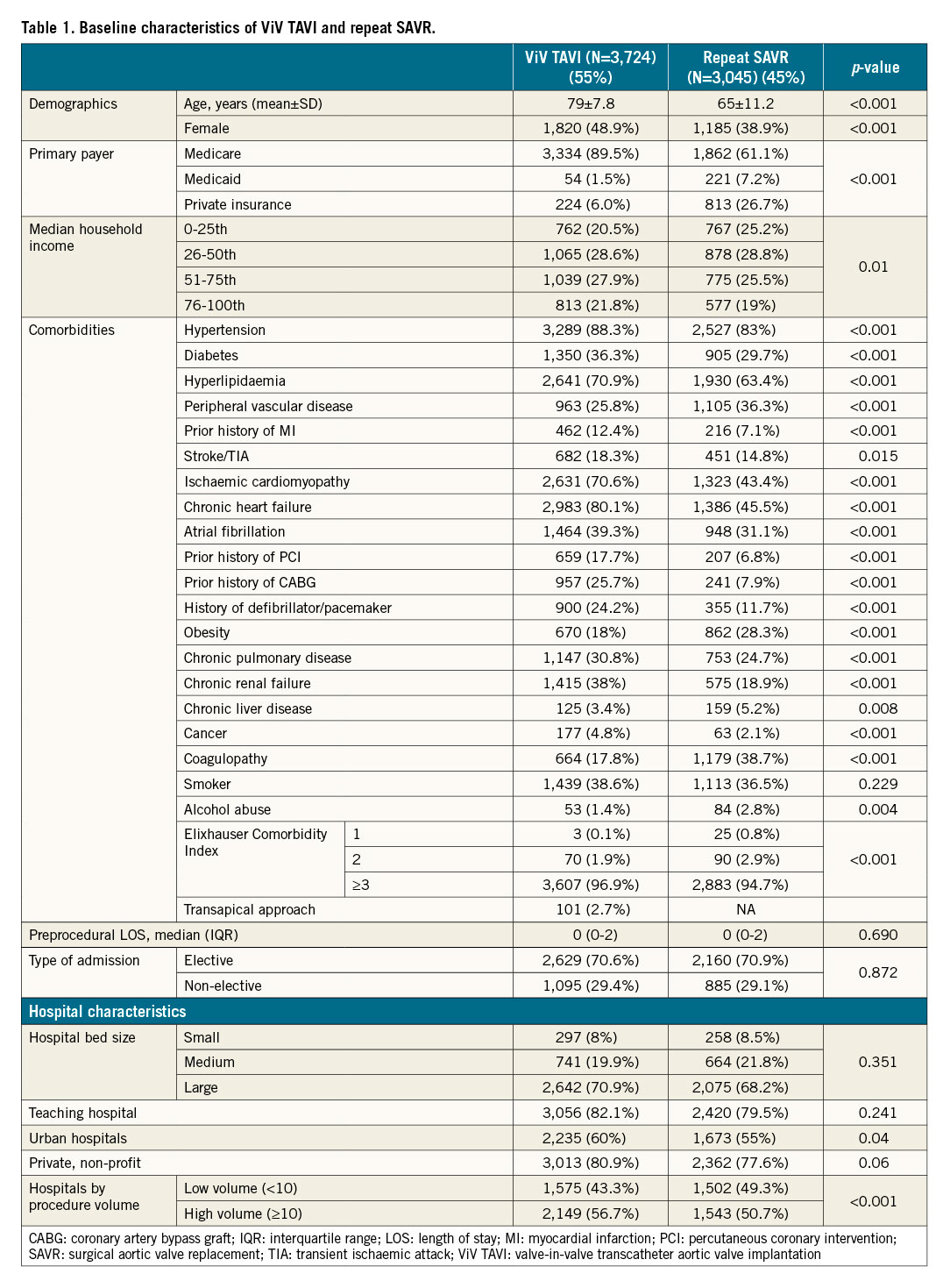
BASELINE CHARACTERISTICS (Table 1)
In females, ViV TAVI was performed more than repeat SAVR (48.9% vs 38.9%, p<0.001). The patients who underwent ViV TAVI were elderly (79±9.1 vs 65±13.4 years, p<0.001). The repeat SAVR cohort had a higher prevalence of peripheral vascular disease, obesity, liver disease, alcohol abuse, and coagulopathy. The ViV TAVI cohort had a higher prevalence of patients with hypertension, diabetes, hyperlipidaemia, prior history of MI, stroke/TIA, ischaemic cardiomyopathy, chronic heart failure, atrial fibrillation, prior history of PCI, prior history of CABG, history of defibrillator or pacemaker, chronic pulmonary disease, cancer, and chronic renal failure. ViV TAVI was performed more in hospitals with higher procedural volume compared with repeat SAVR. There was no difference in the type of admission, hospital bed size, teaching status of the hospital, or hospital ownership between the ViV TAVI group and the repeat SAVR group.
IN-HOSPITAL OUTCOMES (Table 2, Supplementary Table 2)
ViV TAVI was associated with lower in-hospital mortality (1.2% vs 3.4%, OR 0.42, 95% CI: 0.20-0.90, p=0.026), major bleeding (29.7% vs 67.7%, OR 0.10, 95% CI: 0.04-0.21, p<0.001), and cardiorespiratory complications (9.3% vs 26.5%, OR 0.32, 95% CI: 0.15-0.69, p=0.004). ViV TAVI was also associated with lower post-procedure median length of stay compared with repeat SAVR (4 days vs 10 days, adjusted coefficient = –4.75, 95% CI: –8.61 to –0.90, p=0.016). Differences in other in-hospital outcomes including acute stroke, vascular complications, pacemaker implantation, and cost were not statistically significant.
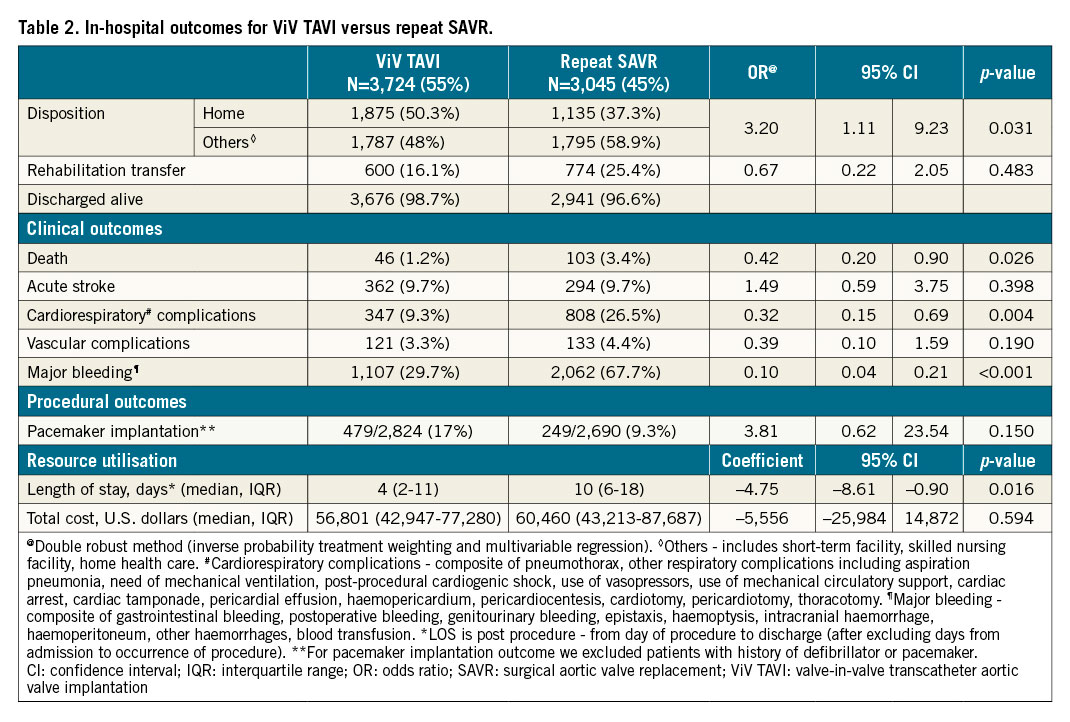
30-DAY AND 6-MONTH READMISSION OUTCOMES (Table 3, Supplementary Table 3)
Patients who underwent ViV TAVI were found to have increased hazards of 30-day and 6-month all-cause readmission compared with repeat SAVR patients (30-day: 16.1% vs 11.5%, HR 1.46, 95% CI: 1.13-1.90, p=0.004; 6-month: 33.8% vs 24.5%, HR 1.54, 95% CI: 1.14-2.10, p=0.006). The Kaplan-Meier graphs of 30-day and 6-month readmission are shown in Figure 2A and Figure 2B, respectively. In ViV TAVI, 0.95% of patients died during 30-day readmission, and 1.6% of patients died during 6-month readmission while, in repeat SAVR, 0.27% of patients died during 30-day readmission, and 0.4% of patients died during 6-month readmission. There was no difference in the rates of MACE, mortality, procedural complications, and length of stay during 30-day and 6-month readmission between the two groups. However, ViV TAVI was associated with a higher risk of major bleeding/vascular complications and non-cardiac infection than repeat SAVR.
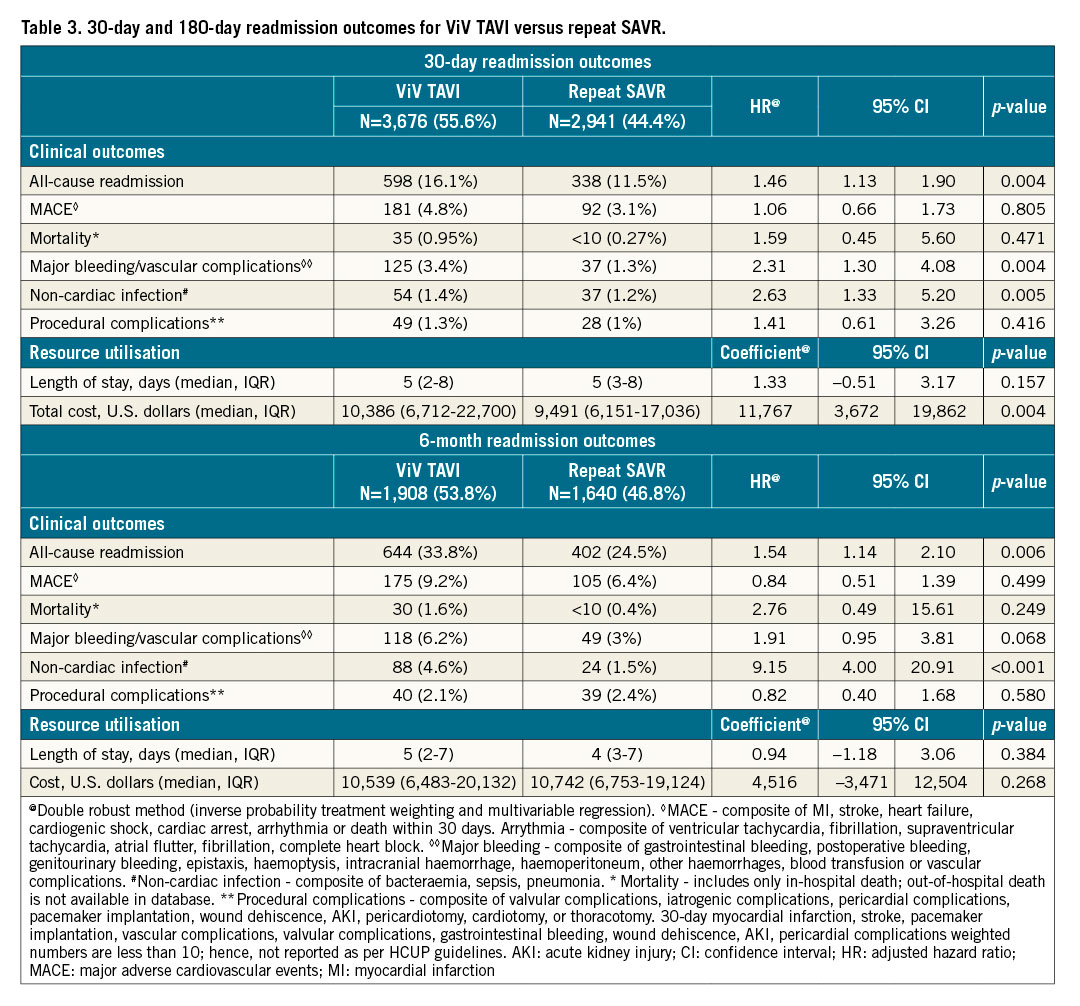
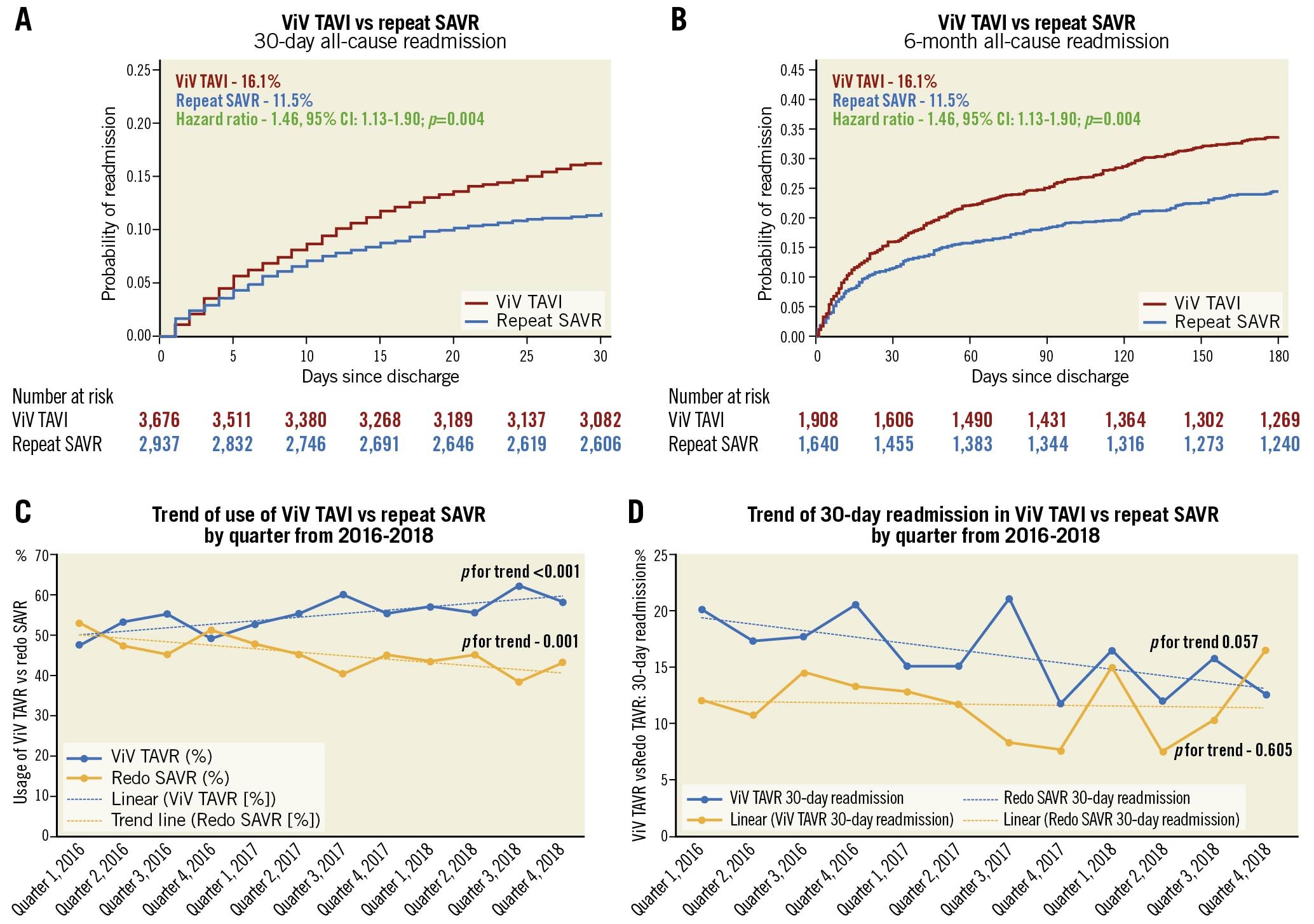
Figure 2. 30-day and 6-month readmission, trends of utilisation and 30-day readmission in ViV TAVI vs repeat SAVR. A) Kaplan-Meier graph of 30-day readmission between ViV TAVI and repeat SAVR. B) Kaplan-Meier graph of 6-month readmission between ViV TAVI and repeat SAVR. C) Trend of utilisation of ViV TAVI and repeat SAVR. D) Trend of 30-day readmission in ViV TAVI and repeat SAVR. SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation; ViV: valve-in-valve
REASONS FOR 30-DAY AND 6-MONTH ALL-CAUSE READMISSION (Supplementary Table 4, Supplementary Table 5)
In ViV TAVI, within 30 days, 219 (36.62%) readmissions were due to cardiac aetiologies, and 379 (64.88%) readmissions were due to non-cardiac aetiologies. Within 6 months, 304 (47.2%) readmissions were due to cardiac aetiologies, and 344 (52.8%) readmissions were due to non-cardiac aetiologies. In repeat SAVR, within 30 days, 115 (34.02%) readmissions were due to cardiac aetiologies, and 223 (65.98%) readmissions were due to non-cardiac aetiologies. Within 6 months, 231 (57%) readmissions were due to cardiac aetiologies, and 171 (42.5%) readmissions were due to non-cardiac aetiologies.
PREDICTORS OF 30-DAY AND 6-MONTH ALL-CAUSE READMISSION
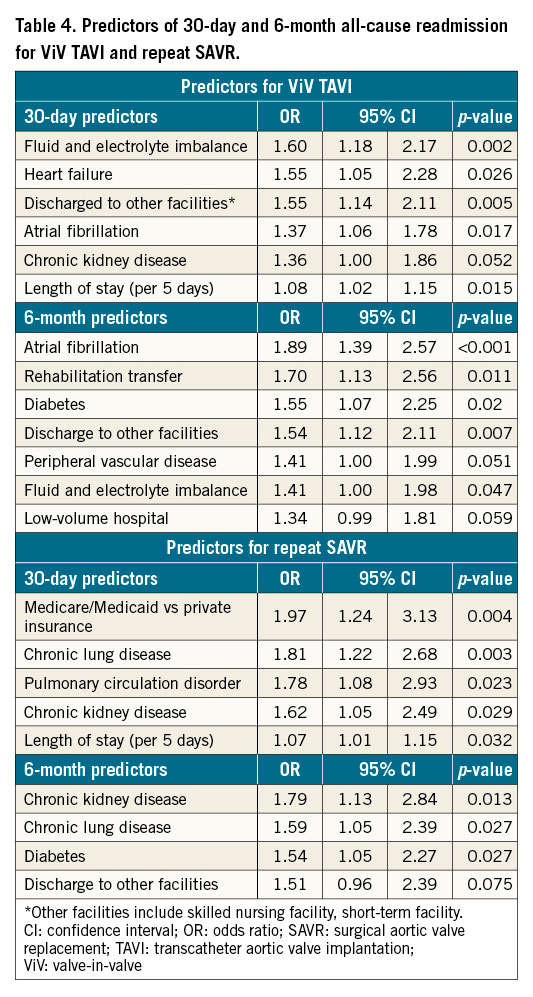
For ViV TAVI and repeat SAVR, ORs of significant predictors of all-cause readmission are described in Table 4, and ORs of all variables included in the multivariable model are given in Supplementary Table 6. For ViV TAVI, fluid and electrolyte imbalance, heart failure, atrial fibrillation, chronic kidney disease (CKD), PVD, diabetes, discharge to other facilities (compared with home discharge), and LOS were significant predictors of 30-day or 6-month readmission. For repeat SAVR, primary expected payer (Medicare/Medicaid), chronic lung disease, pulmonary circulation disorder, CKD, diabetes, discharge to other facilities, and LOS were significant predictors of 30-day or 6-month readmission.
SUBGROUP ANALYSIS (Supplementary Table 7)
We conducted a subgroup analysis to investigate the source of heterogeneity in the difference in 30-day readmission. None of the proposed subgroups showed an interaction except the primary expected payer (p for interaction=0.013).
TREND OF THE UTILISATION OF PROCEDURES AND READMISSION (Figure 2C, Figure 2D, Supplementary Table 8)
From 2016 to 2018, utilisation of ViV TAVI increased by 6.6% per quarter (p for trend <0.001), and repeat SAVR decreased by 6.1% per quarter (p for trend=0.001). From 2016 to 2018, the rate of 30-day all-cause readmission remained unchanged in repeat SAVR (p for trend=0.605), but there was an inclination towards a decreasing trend of 30-day readmission in ViV TAVI (p for trend=0.057).
UNMEASURED BIAS ANALYSIS
In the “E-value” analysis, the observed OR of 0.42 for in-hospital mortality, HR of 1.46 for 30-day readmission, and HR of 1.54 for 6-month readmission could be explained by an unmeasured confounder that was associated with both the treatment and the outcome by OR of 4.19-fold, HR of 2.28-fold, and HR of 2.45-fold each, respectively, above the measured confounders, but weaker confounding could not do so. The rate of the falsification endpoint remained similar between the two interventions (HR 1.39, 95% CI: 0.28-6.88, p=0.688).
Discussion
In this most extensive, multicentric, real-world, propensity score-matched analysis comparing ViV TAVI and repeat SAVR for failed bioprosthetic valves, ViV TAVI was associated with 58% lower in-hospital mortality, but 46% and 54% higher all-cause readmission at 30 days and 6 months, respectively (Central illustration). ViV TAVI was associated with lower post-procedure cardiorespiratory complications and major bleeding compared with repeat SAVR. There was no difference in vascular complications, stroke, or post-procedure pacemaker implantation during index admission between the groups. Reduced overall in-hospital complications led to lesser resource utilisation (more home discharges and lower LOS) during index admission with ViV TAVI. There was no difference in MACE, mortality, or procedural complications, but a higher rate of major bleeding or vascular complications and non-cardiac infection (a composite of pneumonia, sepsis, and bacteraemia) during 30-day and 6-month readmission in ViV TAVI. From 2016 to 2018, the utilisation of ViV TAVI for failed bioprosthetic aortic valves increased, and repeat SAVR trended down. There was an inclination towards a decreasing trend of 30-day readmission in ViV TAVI but this remained unchanged in repeat SAVR from 2016 to 2018.
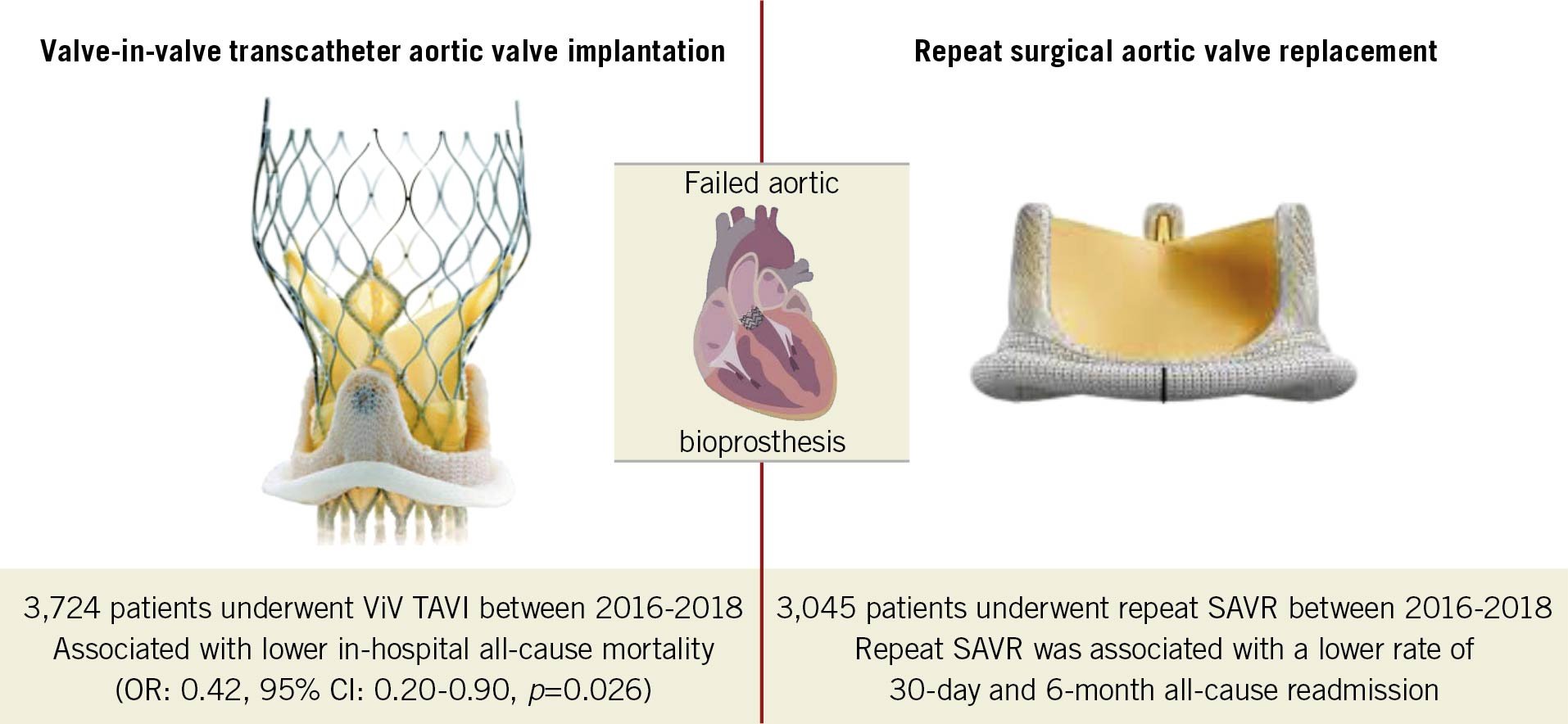
Central illustration. Valve-in-valve transcatheter aortic valve implantation versus repeat surgical aortic valve replacement in patients with a failed aortic bioprosthesis. SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation; ViV: valve-in-valve
Bioprosthetic valve utilisation has been increasing for the management of severe aortic stenosis in patients undergoing surgery2021. Compared with mechanical valves, bioprosthetic valves are associated with fewer thrombotic complications and the possible avoidance of long-term anticoagulation in addition to their superior haemodynamic profile20. Also, an ageing population is presenting for aortic valve replacement, leading to higher use of bioprosthetic valves than mechanical valves21. However, bioprosthetic valves have been associated with shorter durability as they are prone to degeneration, making them more vulnerable to reoperation20. There are limited data available on the long-term durability of the bioprosthetic valve22. Repeat SAVR has traditionally been performed in cases of failed bioprosthetic valves. ViV TAVI, an emerging technology, is recommended for patients at high or prohibitive surgical risk in the guidelines34, which explains the higher age, higher number of females, and higher baseline comorbidities in the ViV TAVI group compared with the repeat SAVR group. These comorbidities are independently associated with higher morbidity and mortality as they are part of the Society of Thoracic Surgeons (STS) PROM or EuroSCORE II risk calculation prior to the procedure.
Despite the patients in the ViV TAVI group having higher baseline comorbidities, ViV TAVI was associated with improved in-hospital outcomes, including all-cause mortality, cardiorespiratory complications, and bleeding compared with repeat SAVR in the current analysis. These combined led to less resource utilisation (i.e., more and earlier discharges to home). These results are consistent with previously published data showing improved short-term outcomes with ViV TAVI using the 2016 database11. In our analysis, newer-generation valves may have been utilised during the study period, which may have been associated with better outcomes23. Hence, the previous study may not represent contemporary outcomes reflecting the most current practices. Additionally, the previous study did not exclude patients with other valvular diseases, leading to the inclusion of patients who did not exclusively have a history of aortic bioprosthetic heart valve implantation11. Furthermore, a previously published meta-analysis and a propensity score-matched analysis also showed reduced LOS with ViV TAVI compared with repeat SAVR, similar to our study810. We demonstrated that ViV TAVI was associated with similar odds of pacemaker placement during the index hospitalisation, although evidence from previously published studies and meta-analysis suggested an increased risk of pacemaker implantation in patients undergoing ViV TAVI8910. Further, our study reported that 17% of the ViV TAVI population required permanent pacemaker implantation, slightly less than other studies reporting 18-25% pacemaker use910.
There was no major difference in the rates of MACE, mortality, or stroke between the two groups over 6-month follow-up. This is consistent with a previously published meta-analysis10. Contrary to previously published studies, we demonstrated higher all-cause readmission rates with ViV TAVI at short-term (30 days) and medium-term follow-up (6 months)811. To confirm these results, we conducted a falsification endpoint and “E-value” analysis to assess the impact of unmeasured confounders. “E-value” (unmeasured bias) analysis showed that an unmeasured confounder requires higher association with both treatment and outcome conditioned on measured covariates, and successful falsification endpoint analysis indicates less chance of having residual confounders to explain away the measured effect. Several plausible reasons could explain the higher readmission rate in the ViV TAVI group. The average age of patients included in the ViV TAVI group was significantly higher compared with previous studies811. Age is an independent predictor of poor prognosis, and the older population has been associated with slightly worse outcomes24. In addition, there were nearly 49% females included in the ViV TAVI group, which is higher than previously published studies. Female gender has been associated with higher short-term adverse outcomes and 30-day readmission rates8111325. Similarly, in the subgroup of females, a higher 30-day all-cause readmission rate was revealed. Finally, almost all the comorbidities (i.e., hypertension, diabetes, etc.) were significantly higher in the ViV TAVI group, suggesting a higher STS risk score than previously published studies81126.
We observed a significant rise in the procedural volume for ViV TAVI during the study period. This may lead to improved operator experience and eventually even better outcomes, as shown by a decreasing readmission trend in each quarter in this study. Additionally, the subgroup analysis in hospitals with higher procedural volume showed no difference in 30-day readmission between the two groups, again indicating improved operator experience at high-volume centres. Newer-generation valves with fluoroscopic markers, repositionability, or retrievability may also help to improve valve-related outcomes.
Limitations
This study has several limitations which must be considered when interpreting the results. First, NRD is an administrative database that carries an inherent selection bias and the potential for miscoding. However, the Agency for Healthcare Research and Quality (AHRQ) has quality control measures to secure best coding practices, to ensure that linkage to state-level data is verified and reliable, and to establish internal validation of diagnosis codes through multiple audits. Second, we did not have information on primary aortic valve manufacturer/sizing, anatomical characteristics of the replaced valve (leaflet length, internal stent diameter, supra-annular positioning of the implant, etc.), type of implanted valve in TAVI (CoreValve/Evolut/SAPIEN XT/SAPIEN 3), access site (percentage of non-femoral access), list of antiplatelet or antithrombotic agents, echocardiographic parameters (e.g., valve area, valve gradient, post-procedural effective orifice area, patient-prosthesis mismatch), and left ventricular ejection fraction. We may not have accounted for all the confounding factors even though we performed propensity-matched and unmeasured bias analyses. Smaller valve sizes (preferentially used in repeat SAVR) were associated with higher patient-prosthesis mismatch and worse outcomes27. Newer techniques such as bioprosthetic valve fracture may help to improve outcomes by reducing transvalvular gradients8. Third, we could not calculate the STS PROM risk score or EuroSCORE II, which can help to determine the risk of patients undergoing ViV TAVI versus repeat SAVR. Fourth, we could not assess outcomes such as acute kidney injury (AKI) and paravalvular leak (PVL) as AKI may occur before the procedure, and codes for PVL could be related to a failed bioprosthesis rather than post-procedure PVL. Despite these limitations, this analysis demonstrates clinical outcomes in an unselected patient population from the most current nationally available database, and the multi-institutional sample makes our results generalisable.
Conclusions
This analysis demonstrated that the use of ViV TAVI significantly increased during the study period, and was associated with lower in-hospital mortality and morbidity but higher 30-day and 6-month all-cause readmission compared with repeat SAVR in a propensity score-matched cohort. There were no differences in the post-discharge short-term or medium-term MACE, mortality during readmission, stroke, pacemaker implantation, or procedural complications between the two groups. However, there was an increased risk of major bleeding/vascular complications and non-cardiac infections in ViV TAVI compared with repeat SAVR. ViV TAVI can be performed safely in carefully selected patients. The choice to proceed with ViV TAVI versus repeat SAVR should be a shared decision based on available expertise, the individual patient, and valve characteristics until randomised clinical trials with longer follow-up garner more data concerning long-term clinical outcomes in this patient subset.
Impact on daily practice
Currently, repeat SAVR is a class I indication for patients presenting with a failed aortic bioprosthetic valve. ViV TAVI is used in patients with high or prohibitive surgical risk. Our study showed that utilisation of ViV TAVI increased significantly from 2016 to 2018. ViV TAVI was associated with a higher 30-day and 6-month readmission rate than repeat SAVR, but the trend of readmission was declining. ViV TAVI was associated with lower odds of in-hospital mortality and morbidity, and a similar incidence of post-procedural pacemaker implantation compared with repeat SAVR. ViV TAVI was associated with no difference in post-discharge MACE, stroke, mortality, or procedural complications but higher major bleeding/vascular complications during 30-day and 6-month readmission than repeat SAVR. Our results suggest that ViV TAVI can be performed safely in carefully selected patients, based on available expertise, the individual patient, and valve characteristics until further insights are available from randomised clinical trials.
Funding
makeadent.org’s Ram and Sanjita Kalra Aavishqaar Fund at Cleveland Clinic Akron General, Akron, OH, USA.
Conflict of interest statement
A. Kalra is the Chief Executive Officer and Creative Director of makeadent.org. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

