
Abstract
Aims: Transcatheter aortic valve implantation for a failing surgical bioprosthesis (TAV-in-SAV) has become an alternative for patients at high risk for redo surgical aortic valve replacement (redo-SAVR). Comparisons between these approaches are non-existent. This study aimed to compare clinical and echocardiographic outcomes of patients undergoing TAV-in-SAV versus redo-SAVR after accounting for baseline differences by propensity score matching.
Methods and results: Patients from seven centres in Europe and Canada who had undergone either TAV-in-SAV (n=79) or redo-SAVR (n=126) were identified. Significant independent predictors used for propensity scoring were age, NYHA functional class, number of prior cardiac surgeries, urgent procedure, pulmonary hypertension, and COPD grade. Using a calliper range of ±0.05, a total of 78 well-matched patient pairs were found. All-cause mortality was similar between groups at 30 days (6.4% redo-SAVR vs. 3.9% TAV-in-SAV; p=0.49) and one year (13.1% redo-SAVR vs. 12.3% TAV-in-SAV; p=0.80). Both groups also showed similar incidences of stroke (0% redo-SAVR vs. 1.3% TAV-in-SAV; p=1.0) and new pacemaker implantation (10.3% redo-SAVR vs. 10.3% TAV-in-SAV; p=1.0). The incidence of acute kidney injury requiring dialysis was numerically lower in the TAV-in-SAV group (11.5% redo-SAVR vs. 3.8% TAV-in-SAV; p=0.13). The TAV-in-SAV group had a significantly shorter median total hospital stay (12 days redo-SAVR vs. 9 days TAV-in-SAV; p=0.001).
Conclusions: Patients with aortic bioprosthesis failure treated with either redo-SAVR or TAV-in-SAV have similar 30-day and one-year clinical outcomes.
Abbreviations
COPD: chronic obstructive pulmonary disease
eGFR: estimated glomerular filtration rate
EuroSCORE: European System for Cardiac Operative Risk Evaluation
LVEF: left ventricular ejection fraction
NYHA: New York Heart Association
PCI: percutaneous coronary intervention
PS: propensity score
SAVR: surgical aortic valve replacement
STS: Society of Thoracic Surgeons
TAV-in-SAV: transcatheter aortic valve in surgical aortic valve
THV: transcatheter heart valve
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation in a failing surgical aortic valve (TAV-in-SAV) was first reported in 2007 in an elderly patient with multiple comorbidities and at extreme risk for redo surgery1. Since then, numerous small TAV-in-SAV case series have demonstrated good results with this procedure, with 30-day risk of mortality ranging from 0% to 7.4%2-4.
The historical gold standard treatment for patients with a failing surgical bioprosthesis is redo-SAVR. Although the clinical outcomes of redo-SAVR approach those of the index procedure in low-risk patients, periprocedural mortality rates increase significantly in those at high risk, with in-hospital mortality ranging from 2.3 to 16.4%5-7. This is especially true in the elderly as the odds ratio of mortality for redo-SAVR is 1.49 (1.10-1.97) per decade6. TAV-in-SAV is a less invasive procedure that obviates the need for sternotomy and cardiopulmonary bypass, and eliminates the risk of injury to a substernal internal mammary arterial graft in patients with previous coronary artery bypass grafting (CABG).
Recently, a 459-patient TAV-in-SAV registry8 reported 30-day and one-year mortality rates of 7.6% and 16.8%, respectively. Of note, moderately elevated post-procedural mean gradients >20 mmHg were reported in 27% of patients. In March 2015, the FDA approved the Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA) for the treatment of a failing surgical bioprosthesis in high/extreme surgical risk patients. In October 2015, the Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) received the same approval.
Studies comparing matched patients undergoing redo-SAVR or TAV-in-SAV are currently inexistent. While observational case series suggest similar clinical outcomes between the two approaches, patient characteristics are often dissimilar, making any comparison difficult and possibly futile8,9. Adequately powered prospective randomised trials are unlikely given the sample size requirements in this very specific subgroup of patients10.
To investigate the comparative clinical effectiveness between TAV-in-SAV and redo-SAVR, we conducted a propensity score-matched analysis across seven international centres. Results from this study could validate the use of TAV-in-SAV as an alternative to redo-SAVR.
Methods
PATIENT SELECTION AND IDENTIFICATION OF THE STUDY POPULATION
Patients were eligible for study inclusion if they had undergone TAV-in-SAV or redo-SAVR for a failing aortic bioprosthesis (stenosis, regurgitation or both) between January 2007 and January 2015 in one of seven centres in Europe and Canada: Antwerp University Hospital, Antwerp, Belgium; Ferrarotto Alessi Hospital, Catania, Italy; German Heart Centre, Munich, Germany; Lille University Health Centre, Lille, France; Rigshospitalet, Copenhagen, Denmark; Royal Victoria Hospital, Montreal, Canada; and Universitätsklinikum Bonn, Bonn, Germany. All patients were identified by retrospective assessment of institutional databases. The selection of patients for TAV-in-SAV or redo-SAVR was performed at an institutional level, following consideration of the risk profile of each case and Heart Team assessment. For all patients, centres submitted a dedicated case report form detailing patient baseline characteristics, echocardiographic data, procedural information and scheduled clinical follow-up.
The study included all consecutive patients who were potential candidates for either a TAV-in-SAV or redo-SAVR. Patients were excluded if they underwent redo-SAVR for paravalvular leak, valve thrombosis or endocarditis. Concomitant coronary revascularisation performed by either percutaneous or surgical techniques was permitted. Local ethics committees approved the retrospective collection of data, and all subjects gave written informed consent for their intervention.
DATA COLLECTION AND STUDY ENDPOINTS
Baseline data were complete for all patients, except logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) and STS scores which were unavailable in 35% of redo-SAVR patients. The primary endpoint was 30-day mortality. Secondary endpoints included one-year mortality, stroke, myocardial infarction, new pacemaker implantation, acute kidney injury requiring dialysis, post-procedural mean gradient, and length of hospital stay. Endpoints were defined according to the updated Valve Academic Research Consortium (VARC) criteria11. Pulmonary hypertension was defined as systolic pulmonary artery pressure above 50 mmHg as estimated by tricuspid regurgitation jet on Doppler echocardiography. Procedure urgency was defined as being performed in a hospitalised patient rather than electively.
STATISTICAL ANALYSIS
Baseline clinical and surgical characteristics were compared using Fisher’s exact test for categorical variables and the Student’s t-test for continuous variables. Propensity scores were derived by including age and sex with pre-treatment variables that were independently associated with treatment selection at p<0.10 in a multivariable model of all variables. Propensity scores among patients undergoing either TAV-in-SAV or redo-SAVR were then matched using a calliper range of ±0.05 to obtain matched pairs of patients. Replacements were permitted (maximum of four uses) in the redo-SAVR group.
In addition to matching, we also compared redo-SAVR and TAV-in-SAV using the inverse probability of treatment weights technique. Patients with propensity scores below the 2.5th percentile in the TAV-in-SAV group and above the 97.5th percentile in the redo-SAVR group were removed. Next, a weight was attributed to each remaining patient according to the inverse of their propensity score, and comparisons were performed on this weighted and trimmed data set.
Survival at 30 days and one year was plotted using Kaplan-Meier curves and between group differences were calculated using the log-rank test. A p-value <0.05 was considered significant. Statistical analyses were performed with SPSS, Version 23 (IBM Corp., Armonk, NY, USA).
Results
BASELINE CHARACTERISTICS
A total of 205 consecutive patients meeting inclusion and exclusion criteria underwent redo-SAVR (n=126) or TAV-in-SAV (n=79) for a failing aortic bioprosthesis during the study period. Patients undergoing TAV-in-SAV were older, more likely to have had multiple prior cardiac surgeries, be in NYHA functional Class III or IV, have higher logistic EuroSCORE and STS scores, and display more comorbidities (Table 1).
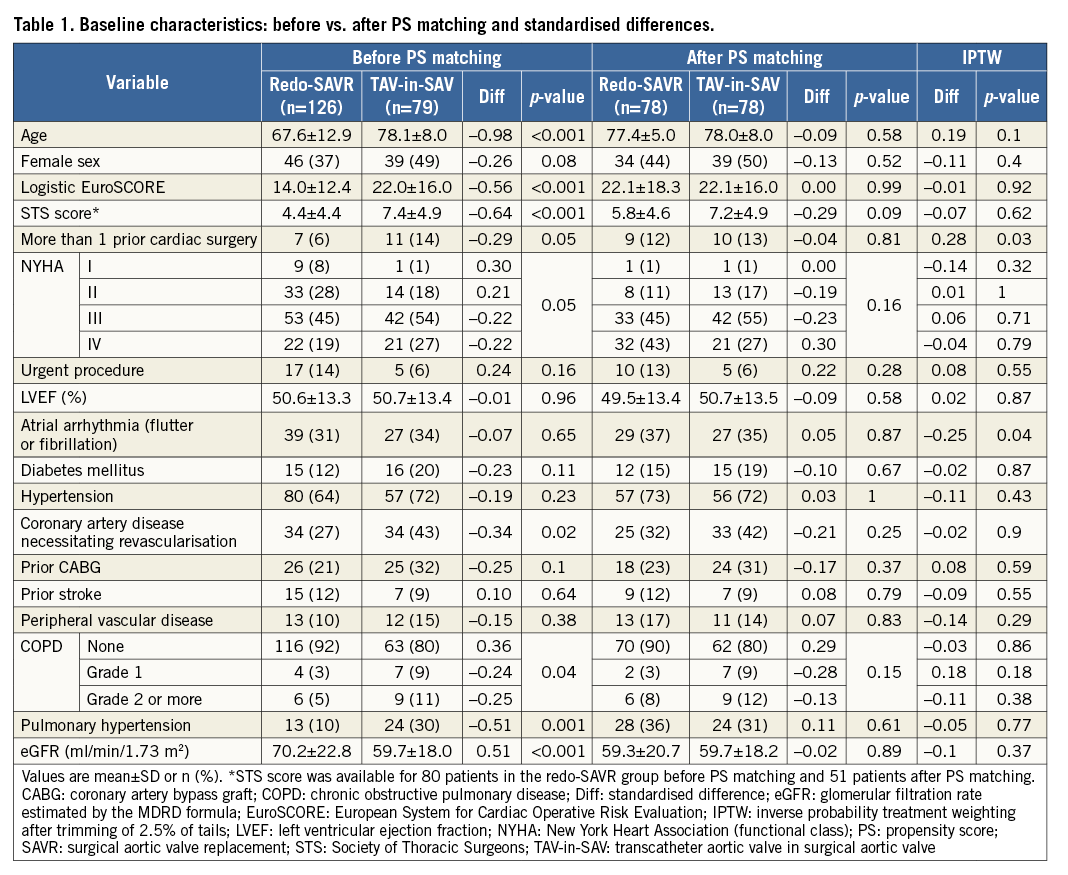
Standardised differences between groups after trimming and weighting were less than 0.20 (i.e., small) for all variables except atrial fibrillation and number of previous surgeries (Table 1).
PROPENSITY SCORE MATCHING
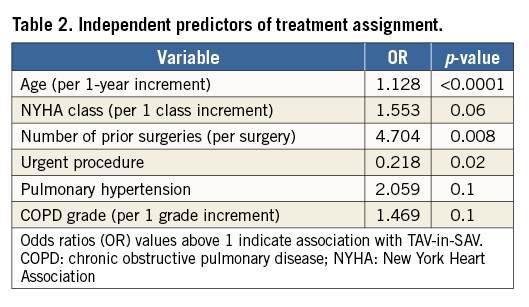
Pre-treatment variables found to be independent predictors of treatment selection and used for propensity scoring were age, NYHA functional class, number of prior cardiac surgeries, urgent procedure, pulmonary hypertension, and chronic obstructive pulmonary disease (COPD) grade (Table 2). These variables were all associated with assignment to TAV-in-SAV, with the exception of urgent procedure, which was associated with redo-SAVR.
Propensity score matching generated 78 pairs of patients. Baseline characteristics were similar between groups after propensity matching (Table 1). In each pair, redo-SAVR occurred at a median of 421 days before TAV-in-SAV. However, in 28 of 78 pairs, TAV-in-SAV predated redo-SAVR.
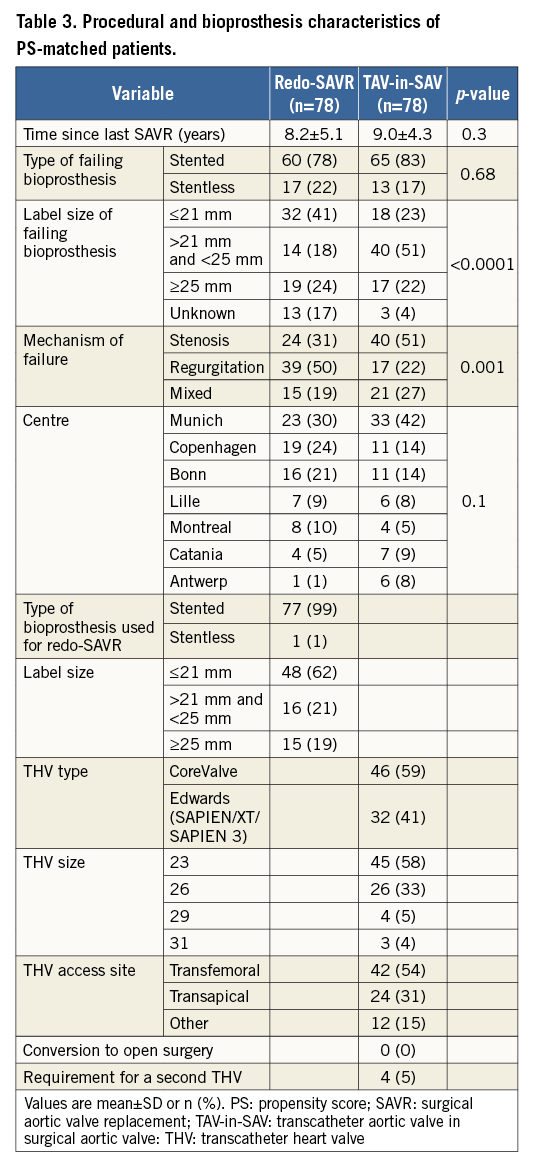
The surgical and bioprosthesis characteristics of matched patients are displayed in Table 3. The mean delay between index procedure and either redo-SAVR or TAV-in-SAV was 8.2 and 9 years, respectively (p=0.30). This delay was less than three years in 16.4% of the total population, with regurgitation being the most common mode of failure in this “accelerated failure” subgroup. While pure regurgitation was the mode of failure in half the patients in the redo-SAVR group (50.0%), pure stenosis was the most common mode of failure in TAV-in-SAV patients (51.3%). In total, 25 patients in the redo-SAVR group (32%) had coronary artery disease (CAD) necessitating revascularisation. Of these, 21 underwent concomitant CABG at the time of surgery; the other four underwent PCI as conduits for bypass were unavailable. In the TAV-in-SAV group, 33 patients (42%) had CAD necessitating revascularisation (p=0.25 vs. redo-SAVR group). PCI was performed before TAV-in-SAV in 32 patients, and at the time of procedure in one patient. A Carpentier-Edwards (Edwards Lifesciences) was the failing bioprosthesis in 19 patients of the redo-SAVR group (24.4%) and 34 patients of the TAV-in-SAV group (43.6%). Other valves were found less frequently. Types and sizes of surgical bioprostheses or transcatheter heart valves (THV) used in both groups are also displayed in Table 3. Among patients undergoing TAV-in-SAV, self-expanding and balloon-expandable THV were used in similar proportions, and transfemoral access was chosen in 53.8% of patients. Conversion to open surgery did not occur in any of the TAV-in-SAV patients, and requirement for a second THV during the procedure occurred in four (5.1%) cases.
CLINICAL OUTCOMES
Table 4 describes clinical outcomes at 30 days and one year for both groups before and after propensity score matching. Time-to-event curves comparing both treatment modalities after matching are depicted in Figure 1. Thirty-day follow-up was complete in 99.4%, and 88.5% at one year. Thirty-day Kaplan-Meier mortality was 2.5% higher in the redo-SAVR group compared to the TAV-in-SAV group, but this difference did not reach statistical significance (6.4 vs. 3.9%, respectively; p=0.49). One-year mortality was similar between groups (13.1 redo-SAVR vs. 12.3% TAV-in-SAV; p=0.80). Similar results were found when using inverse probability of treatment weights (Table 5).
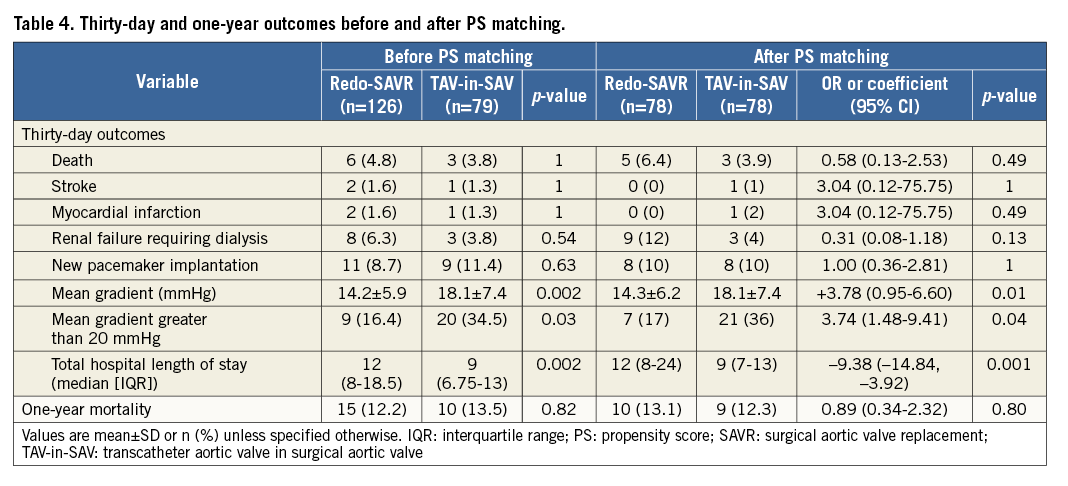
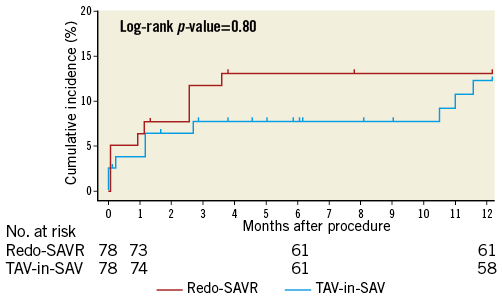
Figure 1. Cumulative incidence of all-cause mortality.
Cumulative incidence (%) of all-cause one-year mortality in redo-SAVR (red line) and TAV-in-SAV (blue line). There was no difference in one-year mortality between groups.
VALVE TYPES
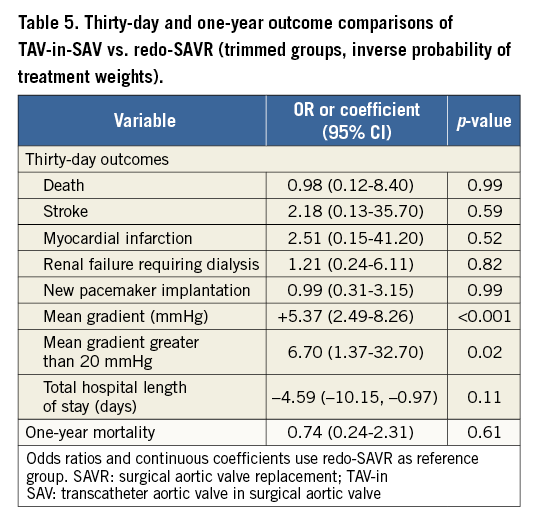
Kaplan-Meier curves of one-year mortality according to failing surgical bioprosthesis type are shown in Figure 2. Mortality was significantly higher in patients undergoing TAV-in-SAV for a failing stentless bioprosthesis as compared to redo-SAVR (32.7 vs. 0%, p=0.01). For patients with a failing stented bioprosthesis, mortality rates were lower with TAV-in-SAV compared to redo-SAVR, but this failed to reach statistical significance (8.3 vs. 17.2%, p=0.12). Mortality rates were similar between groups when stratified by failure mechanism and failing prosthesis size. There was no difference in 30-day mortality between patients who underwent TAV-in-SAV with the Edwards THV compared to those who underwent TAV-in-SAV with the Medtronic CoreValve THV (3.1 vs. 4.3%, respectively; p=0.77).
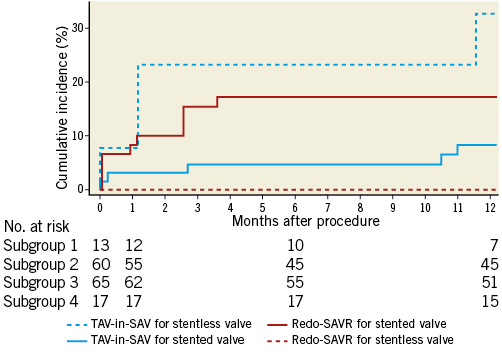
Figure 2. Cumulative incidence of all-cause mortality stratified by failing bioprosthesis type. Cumulative incidence (%) of all-cause one-year mortality in TAV-in-SAV for failing stentless valve (group 1, dashed blue line), redo-SAVR for failing stented valve (group 2, solid red line), TAV-in-SAV for failing stented valve (group 3, solid blue line), and redo-SAVR for failing stentless valve (group 4, dashed red line). There was a statistically significant difference in one-year mortality between groups 1 and 4.
SECONDARY OUTCOME MEASURES
Propensity-matched groups did not differ with respect to the following clinical outcomes: stroke, myocardial infarction and new pacemaker implantation. Patients with a failing stentless bioprosthesis had a higher rate of new pacemaker implantation compared to those with a stented bioprosthesis, regardless of treatment modality (23.3 vs. 6.4%; p=0.01). There was three times more renal failure requiring dialysis in the redo-SAVR group compared to the TAV-in-SAV group; however, this difference did not reach statistical significance (11.5% vs. 3.8%, respectively; p=0.13). Median total hospital length of stay was three days shorter in the TAV-in-SAV than in the redo-SAVR group (9 vs. 12 days, p=0.001) (Table 4). Similar results were found when using inverse probability of treatment weights (Table 5).
ECHOCARDIOGRAPHIC OUTCOMES
At 30 days, redo-SAVR was associated with a lower mean gradient compared to TAV-in-SAV (14.3 mmHg vs. 18.1 mmHg, p=0.01). This difference was due to a significantly higher mean gradient in the Edwards TAV-in-SAV group (21.3±7.2 mmHg) compared to patients in the CoreValve TAV-in-SAV group (15.4±6.7 mmHg) and the redo-SAVR group (14.3±6.2 mmHg) (Figure 3A). This difference between devices was found regardless of failing device inner diameter (Figure 3B). Furthermore, post-procedural transaortic gradients >20 mmHg were more than twice as likely in the Edwards TAV-in-SAV group (51.9%) compared to the CoreValve TAV-in-SAV and redo-SAVR groups (22.6 and 17.1%, respectively). Figure 3C displays mean post-procedural gradients stratified according to the type of failing bioprosthesis. Independent predictors of higher post-procedural gradients were a failing stented bioprosthesis (increase of 2.5 mmHg; p=0.02), treatment with an Edwards THV (increase of 4.7 mmHg compared to redo-SAVR and 4.3 mmHg compared to CoreValve; both p<0.01), and lower EuroSCORE (increase of 0.31 mmHg per 5% decrease in EuroSCORE; p=0.02).
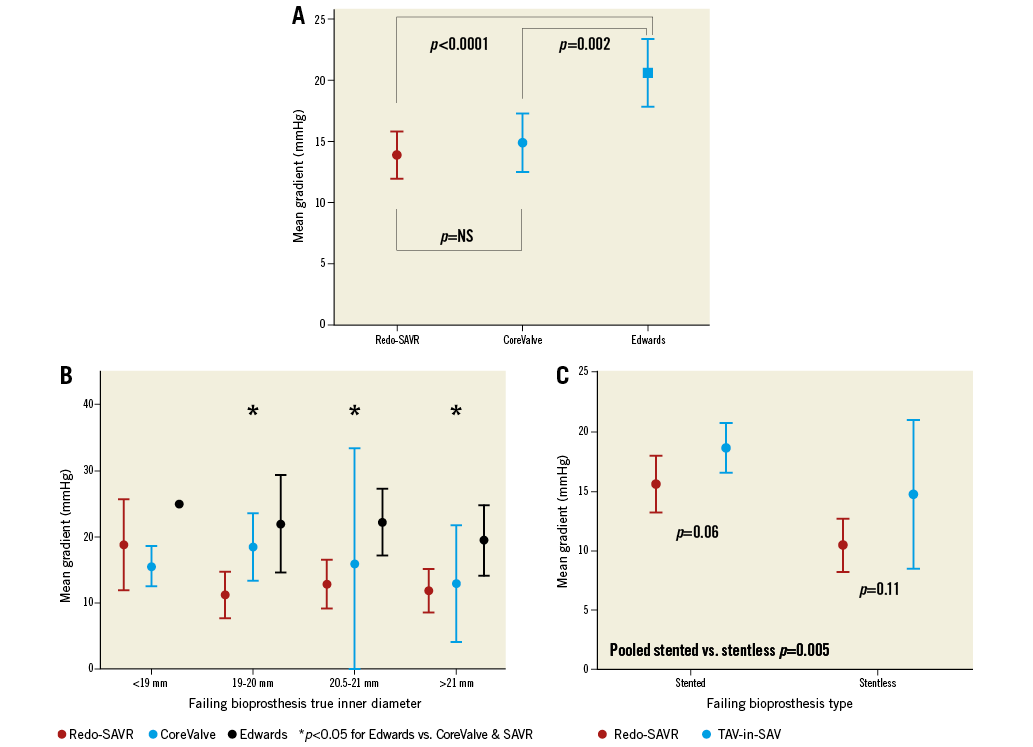
Figure 3. In-hospital mean gradient by treatment type. A) Post-procedural mean aortic gradient for patients in the redo-SAVR group (red circle), CoreValve TAV-in-SAV group (blue circle), and Edwards TAV-in-SAV group (blue square). Error bars represent 95% confidence interval. B) In-hospital mean gradient stratified by failing bioprosthesis true inner diameter and replacement device. P-values are for comparisons between Edwards (black) and redo-SAVR grouped with CoreValve (red and blue) for each size. Error bars represent 95% confidence interval. Only one case was performed with the Edwards device for the smallest size (<19 mm). C) In-hospital mean gradient stratified by failing bioprosthesis type. Post-procedural mean aortic gradient for patients with a failing stented bioprosthesis (left) and a failing stentless bioprosthesis (right). P-values are for comparisons between redo-SAVR (red) and TAV-in-SAV (blue). Error bars represent 95% confidence interval. The difference in gradients for pooled stented vs. stentless bioprostheses was statistically significant (p=0.005).
Discussion
The major findings of this propensity-matched comparison of redo-SAVR and TAV-in-SAV for failing aortic bioprostheses are as follows: 1) redo-SAVR and TAV-in-SAV for a failing bioprosthesis showed similar rates of 30-day and one-year mortality; 2) total hospital length of stay was shorter with TAV-in-SAV than redo-SAVR; 3) mean post-procedural gradients were higher with Edwards TAV-in-SAV compared to redo-SAVR and CoreValve TAV-in-SAV; and 4) failing stentless bioprostheses were associated with a higher rate of new pacemaker implantation and a lower mean post-procedural gradient, regardless of treatment modality.
Mortality rates of 3.9% at 30 days for TAV-in-SAV are nearly two times lower than those reported in other studies3,12. The Valve-in-Valve registry reported a 30-day mortality rate of 7.6% in patients with a similar risk profile8. This may be due to site selection bias, as we included only high-volume centres in the present study. While smaller valve sizes and valve stenosis were shown to be associated with higher mortality rates in the Valve-in-Valve registry, this was not found to be the case in our study.
With respect to the surgical group, the 6.4% and 13.1% mortality rates at 30 days and one year, respectively, are comparable to those reported in the surgical literature. Jones et al reported a 30-day mortality rate of 6.4% in an all-comers series6. Eitz et al reported 30-day and one-year mortality rates of 16.4% and 23%, respectively, in a cohort of 71 octogenarians5. However, comparisons to this last series may be inappropriate because patients with emergent indications such as endocarditis and thrombosis were excluded from our study.
The higher mortality rates with TAV-in-SAV in patients with a failing stentless bioprosthesis should be treated with circumspection as they are based on only four events in a small subgroup. Only one death occurred in the first 30 days, indicating that mortality may be due to patient characteristics more than procedural failure.
The main advantage of stentless bioprostheses over stented valves seems to be the superior haemodynamic profile achieved after the procedure with lower mean gradients. This is due to the increased space in the aortic root of patients with stentless valves.
Redo-SAVR also allows increased space in the aortic root compared to TAV-in-SAV. However, the CoreValve bioprosthesis, with its supra-annular leaflet position, yielded similar post-procedural gradients to redo-SAVR, while the Edwards THV (intra-annular leaflets) produced higher gradients, regardless of failing device size13. The CoreValve device may be better suited to tackle TAV-in-SAV but, in our study, higher mean gradients did not seem to translate into midterm clinical events.
There was no difference between TAV-in-SAV and redo-SAVR with respect to other clinical outcomes. The rate of renal failure requiring dialysis was numerically higher in the redo-SAVR group, but statistical significance was not found, possibly because of lack of power. The similar outcomes regarding pacemakers (something which is different from the literature concerning procedures performed on the native aortic valve) may be due to the more extensive debridement involving the ventricular septum in redo-SAVR (thereby increasing the pacemaker rate in that group) and the relative “shielding” of the septum by the failing prosthesis stent for TAV-in-SAV (thereby decreasing the pacemaker rate in that group). The relatively high rate of new pacemaker implantation in the stentless bioprosthesis group undergoing TAV-in-SAV may be related to baseline conduction disturbances, the absence of “shielding”, and deeper than usual implantation in these challenging cases.
As reported in previous comparisons between TAVR and SAVR, less invasive approaches are associated with shorter durations of hospital stay14. Total duration of hospital stay was similar to those reported in the PARTNER A trial.
Limitations
The present study has limitations inherent to all retrospective analyses. First, reasons for treatment allocation cannot be fully resolved, despite attempts for propensity matching. The large residual standardised differences after PS matching are a possible marker of residual confounding. Certain baseline characteristics, such as failing bioprosthesis size and mechanism of failure, were dissimilar. Both of these are proven to be related to better outcomes in TAV-in-SAV. On the other hand, STS scores may appear to favour redo-SAVR. In this context, the direction and magnitude of bias cannot be determined. Matching for bioprosthesis would have been ideal, but impractical because the number of matched pairs would decrease. Smaller valves were treated more often by redo-SAVR because there are fewer transcatheter devices available for small surgical valves (for example, for a 19 mm Carpentier-Edwards SAV, only the Edwards SAPIEN 20 mm is recommended). Including more patients from more centres would not, in our view, modify the proportions of small surgical valves treated by redo-SAVR or TAV-in-SAV. With respect to revascularisation, groups differ because PCI was undertaken before the procedure in the TAV-in-SAV group, rather than during the procedure, as in redo-SAVR. Sites decided to perform PCI before TAV-in-SAV or CABG during redo-SAVR based on different criteria and did not report this in the same manner. Therefore, adjustment for this variable could not be performed. This retrospective trial used data that were not audited, and the collected haemodynamic data were not core laboratory adjudicated. STS scores were not available for a significant proportion of surgical patients. However, most of the elements that make up the STS score were not missing and fairly well balanced after matching. We preferred not to calculate missing STS scores with the data at hand, as these calculated “post hoc” values would be biased by the use of a calculator version that does not match the date of the procedure, giving artefactually low values15. In addition, the logistic EuroSCORE was almost identical between groups after matching. Follow-up was limited to one year, limiting the long-term assessment of haemodynamic findings. However, results up to one year are the current standard in studies focused on the elderly (mean age >77 years in each group) undergoing this type of procedure. Large randomised controlled trials would palliate most of these limitations. Realistically, as these are relatively infrequent procedures, we may never see an adequately powered randomised trial.
Conclusions
Patients with aortic bioprosthesis failure treated with either redo-SAVR or TAV-in-SAV have similar 30-day and one-year clinical outcomes.
| Impact on daily practice Patients with a failing aortic bioprosthesis at high risk for redo surgery have similar outcomes whether they undergo TAV-in-SAV or redo-SAVR. |
Conflict of interest statement
D. Mylotte, L. Søndergaard, J. Bosmans, T. Modine, J-M. Sinning, E. Grube, G. Nickenig, F. Mellert, S. Bleiziffer, R. Lange, G. Martucci, and N. Piazza report being proctor and/or consultant for Medtronic. O. De Backer, L. Søndergaard, and N. Piazza report being proctor and/or consultant for St. Jude Medical. L. Søndergaard, T. Modine, J-M. Sinning, E. Grube, G. Nickenig, and F. Mellert report being proctor and/or consultant for Boston Scientific. L. Søndergaard, M. Barbanti, J-M. Sinning, G. Nickenig, and F. Mellert report being proctor and/or consultant for Edwards Lifesciences. T. Modine reports being proctor and/or consultant for General Electric. The other authors have no conflicts of interest to declare.

