Abstract
Background: Limited information is available on outcomes in patients with bicuspid aortic valve (BAV) stenosis undergoing transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR), as pivotal randomised trials excluded patients with BAV pathology due to anatomic complexity.
Aims: The aim of the study was to compare early outcomes between TAVI and SAVR in patients with BAV stenosis.
Methods: We queried the Nationwide Readmission Database (NRD) between 2016 and 2018 to identify adults who underwent TAVI or SAVR for BAV stenosis. The study’s primary outcome was in-hospital mortality. Secondary outcomes were 30-day and six-month major adverse cardiovascular events (MACE). We matched both cohorts using propensity score matching, and applied logistic and Cox-proportional hazard regression to compute the odds ratio (OR), the hazard ratio (HR), and the 95% confidence interval (CI).
Results: Out of 17,068 patients with BAV stenosis, 1,629 (9.5%) patients underwent TAVI and 15,439 (90.5%) underwent SAVR. After propensity score matching (PSM), we found 1,393 matched pairs. Of the matched pairs, 848 had complete six-month follow-ups. In the PSM cohort, TAVI was associated with reduced in-hospital mortality (0.7% vs 1.8%, OR: 0.35, 95% CI: 0.13-0.93; p=0.035), and a similar rate of MACE at 30 days (1% vs 1.5%, OR: 0.65, 95% CI: 0.27-1.58; p=0.343) and at six months (4.2% vs 4.9%, HR 0.86, 95% CI: 0.44-1.69; p=0.674), compared with SAVR.
Conclusions: In the propensity score-matched cohort, TAVI was associated with reduced odds of in-hospital mortality and a similar risk of 30-day and six-month MACE, supporting the feasibility of TAVI in BAV patients without a need for concurrent aortic root repair.
Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart disease, with a national prevalence of 0.5% to 2% across the population of the United States1. BAV is commonly associated with various complications such as aortic valve stenosis, regurgitation, endocarditis, aortic aneurysm, and aortic dissection1. BAV stenosis typically presents at a younger age compared with tricuspid aortic stenosis. Among patients with BAV stenosis, 12% to 37% of patients develop moderate or severe aortic stenosis with or without aortic regurgitation along the disease course2. Currently, surgical aortic valve replacement (SAVR) is considered an optimal management strategy for BAV stenosis, as these cohorts are comparatively younger than their tricuspid counterparts, and surgical management may also involve aortic root repair3.
Transcatheter aortic valve implantation (TAVI) is non-inferior to SAVR with regard to short- and intermediate-term mortality when treating severe symptomatic aortic stenosis in patients with a tricuspid aortic valve, irrespective of the surgical risk45. The current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines state that “in patients with BAV and symptomatic, severe aortic stenosis, TAVI may be considered as an alternative to SAVR after consideration of patient-specific procedural risks, values, trade-offs, and preferences, and when the surgery is performed at a comprehensive valve center”4. The randomised controlled trials (RCTs) on TAVI have traditionally excluded patients with BAV morphology, given the concern for higher post-procedure complication rates due to their anatomical uniqueness. Few studies have investigated the role of TAVI in BAV stenosis and demonstrated favourable outcomes, especially with the utilisation of newer-generation devices67. There is a dearth of RCTs investigating the role of TAVI versus SAVR in BAV. It is imperative to study the outcomes in real-world patients with BAV stenosis undergoing TAVI versus SAVR. We compared early outcomes and resource utilisation between TAVI and SAVR among patients with BAV stenosis from real-world data.
Methods
Data source
We obtained the study population from the Nationwide Readmission Database (NRD) from 2016 to 2018. NRD is an all-payer database sponsored by the Healthcare Cost and Utilization Project, a healthcare body established by the Agency for Healthcare Research and Quality. NRD includes the data derived from US hospitals in 28 geographically dispersed states, designed to represent approximately 58.7% of all US hospitalisations. The NRD utilises a de-identified unique number for tracking each patient and determines readmissions across hospitals within a calendar year. The present study was deemed exempt from the Cleveland Clinic institutional review board as the database contained de-identified datasets with prior ethical committee approval. This study followed the reporting guidelines specified by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement8.
Study population
We queried the NRD using the International Classification of Diseases, Tenth Revision, Procedure Coding System (ICD-10-PCS) codes to identify all adults who underwent TAVI or SAVR (n=370,196). We summarised the ICD-10 codes used to identify the study sample in Supplementary Table 1, which were utilised in a previous study910. We excluded patients who underwent concomitant coronary artery bypass grafting (CABG), mitral, pulmonary and tricuspid valve surgeries, atrial septal defect repair, ventricular septal defect repair, and aortic root surgery to identify patients with isolated aortic valve replacement, reducing the possibility of a selection bias toward SAVR (n=246,158). Further, we excluded patients with isolated aortic valve regurgitation, age <18 years, missing length of stay, and those discharged in December to allow for a complete 30-day follow-up period. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes for bicuspid/congenital aortic valve were further applied to arrive at our final cohort of patients (n=17,068) with BAV stenosis who underwent isolated TAVI (n=1,629) or SAVR (n=15,439). The flow diagram for patient selection has been summarised in Figure 1.
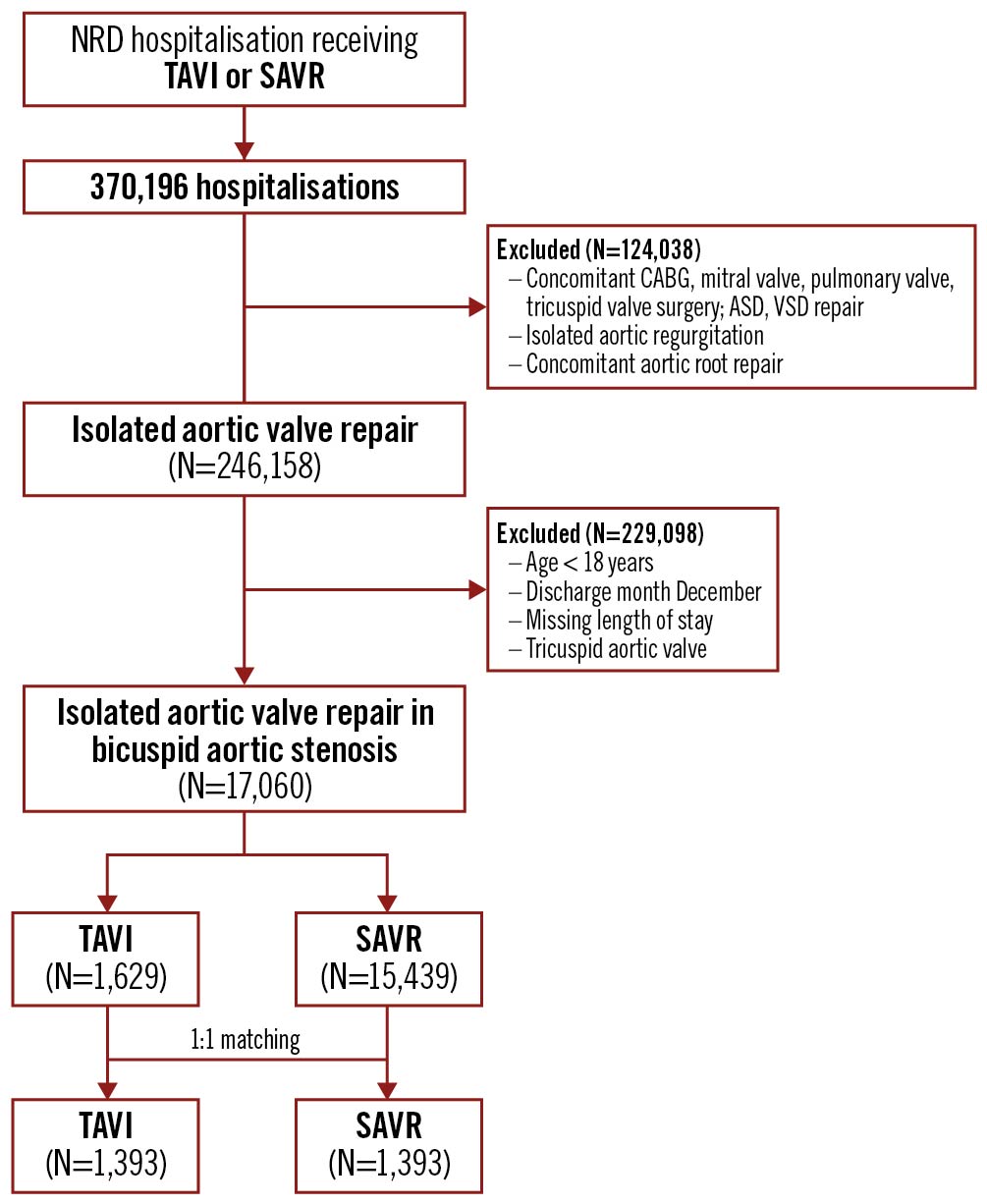
Figure 1. Patient selection flow diagram. ASD: atrial septal defect; CABG: coronary artery bypass graft; NRD: nationwide readmission database; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation; VSD: ventricular septal defect
Patient and hospital characteristics
We utilised NRD variables for patients’ age, gender, median household income for patients’ ZIP code, the primary payer, and type of admission (elective/non-elective)11. We used the Elixhauser comorbidity variables to include hypertension, diabetes, hyperlipidaemia, peripheral vascular disease, smoking, obesity, chronic heart failure, pulmonary circulation disorder, chronic pulmonary disease, chronic renal failure, chronic liver disease, cancer, and coagulopathy. ICD-10-CM codes were used to define the prior history of myocardial infarction (MI), stroke/transient ischaemic attack (TIA), atrial fibrillation, prior history of percutaneous coronary intervention (PCI), prior history of CABG, carotid artery disease, and history of defibrillator/pacemaker implantation. The ICD-10 codes used to identify these variables are summarised in Supplementary Table 1. We used NRD’s hospital variables to identify hospital size according to the number of beds, teaching status, hospital location, and procedural volume. The procedural volume at each hospital was calculated by adding all the weighted admissions with SAVR or TAVI, then classifying them into quintiles (first [smallest], second, third, fourth, and fifth [largest]). Hospitals that fall in the fifth quintile were considered high procedural volume hospitals, and hospitals that fall in the first to fourth quintile were considered low procedural volume hospitals.
Study outcomes
The study’s primary outcome was in-hospital mortality. Secondary outcomes were short-term (30-day) and medium-term (six-month) major adverse cardiovascular events (MACE). MACE was a composite of all-cause mortality during readmission, stroke readmission, and cardiovascular (CV) hospitalisation. Readmission outcomes were identified by applying ICD-10 codes to the primary diagnosis field of readmission. For 30-day/six-month outcomes, we defined time-to-event as the time from the discharge date of the index admission to the occurrence of an event. In case of multiple events, only the first hospitalisation after the index admission was counted. Other in-hospital outcomes included were discharge disposition to home (compared with other facilities), acute stroke, major bleeding, vascular complications, acute kidney injury (AKI), cardiorespiratory complications, permanent pacemaker implantation, valvular complications, paravalvular leak, the need for cardiothoracic procedure, post-procedure length of stay (LOS), and cost. Major bleeding was defined as bleeding requiring blood transfusion. Post-procedure LOS was calculated by subtracting the time to procedure from the given LOS for that admission. The ICD-10 codes used to identify these outcomes are summarised in Supplementary Table 1. Short-term (30-day)/medium-term (six-month) outcomes included all-cause readmission, CV hospitalisation, all-cause mortality, stroke, permanent pacemaker implantation, and valvular complications. CV hospitalisation included hospitalisation due to myocardial infarction, heart failure, or arrhythmia. Arrhythmia events were defined as a composite of ventricular tachycardia, fibrillation, supraventricular tachycardia, atrial flutter, or complete heart block. We calculated hospital costs by multiplying hospital charges by the corresponding cost-to-charge ratio. All costs were adjusted for the inflation/consumer price index. Thirty-day and six-month mortality included death during readmission. Out-of-hospital death was not covered in the database. Definitions of other outcomes are described in the Supplementary Appendix.
Statistical analysis
We presented categorical variables as numbers and percentages and continuous variables as means with standard deviation or median with interquartile range (IQR). Categorical variables were compared between the two groups using the chi-square and Fisher exact tests. Continuous variables with normal distribution were compared using the Student’s t-test, and those not normally distributed were compared using Wilcoxon rank-sum test.
We generated two propensity score-matched cohorts for patients who underwent TAVI or SAVR: one for in-hospital, short-term outcomes, and the other for medium-term outcomes, in order to have complete 30-day and six-month follow-ups for all patients. A propensity score was generated using all covariates described in Table 1 including patient demographics, comorbidities, hospital characteristics, admission type, primary payer, and household income through multivariable logistic regression. Patients with similar propensity scores in the two groups were matched using a 1-to-1 scheme without replacement using a greedy method. Maximum propensity score differences (calliper width) of 0.1 were permitted between matched pair observations in various models to keep standardised differences to less than 10%. Patients without matched observations were excluded. The appropriateness of all models was assessed by receiver operating characteristic curve/C-statistic, which was 0.86 as shown in Supplementary Figure 1, Supplementary Figure 2. The standardised difference was used to assess the balance of variables between two matched cohorts and was depicted graphically (Supplementary Figure 3, Supplementary Figure 4). In calculating 30-day and six-month outcomes, we excluded patients who died in hospital.
We applied univariate logistic regression for in-hospital and 30-day outcomes to compute the odds ratio (OR) with 95% confidence interval (CI) in the matched cohort. We applied univariate Cox-proportional hazard regression for six-month outcomes to compute the hazard ratio (HR) with 95% CI. The proportionality assumption was not violated for the Cox model for any outcomes, with the global test p-value being more than 0.05. For LOS and cost, we applied a generalised linear model with logit link and gamma distribution to compute the OR with 95% CI. Kaplan-Meier curves were constructed for six-month MACE and 30-day/six-month all-cause readmission.
Sensitivity analysis
In 1:1 matching, many patients were excluded from the analysis. Hence, we applied the inverse probability of treatment weighting (IPTW) method to check for the robustness of our results for primary outcome in-hospital mortality.
Unmeasured bias analysis
We conducted previously validated falsification endpoint and “E-value” analysis to evaluate the robustness of our findings121314. In the falsification method, we selected an alternative outcome that may not have been expected to be causally affected by the treatment being studied. Then, we assessed if study intervention (TAVI) affected alternative outcomes by using a similar method to assess for other study outcomes. If no treatment effect was seen for the alternative outcome, it implied balanced unmeasured covariates between the two groups. We chose the composite of pneumonia, gastrointestinal infection and urinary tract infection readmission as an alternative outcome and studied the effect of interventions. The E-value identified the minimum strength of association that unmeasured confounders may need to have had, with both treatment and outcome conditional on measured covariates, to fully explain the observed association. This estimated what the relative risk may have to be for any unmeasured confounder to overcome the observed association of study intervention with study outcomes.
All p-values were two-sided, with p<0.05 considered as statistically significant. All statistical analyses were conducted using appropriate weighting, stratifying, and clustering samples, using the svy package of Stata, version 16.1 (StataCorp).
Results
A total of 17,068 patients who underwent SAVR or TAVI for BAV stenosis between 2016 and 2018 were identified. Of these patients identified, 15,439 (90.5%) underwent SAVR compared with 1,629 patients (9.5%) who had TAVI (Table 1). After matching, 1,393 matched pairs were found with an entire 30-day follow-up and 848 matched pairs were found with an entire six-month follow-up.
Baseline characteristics
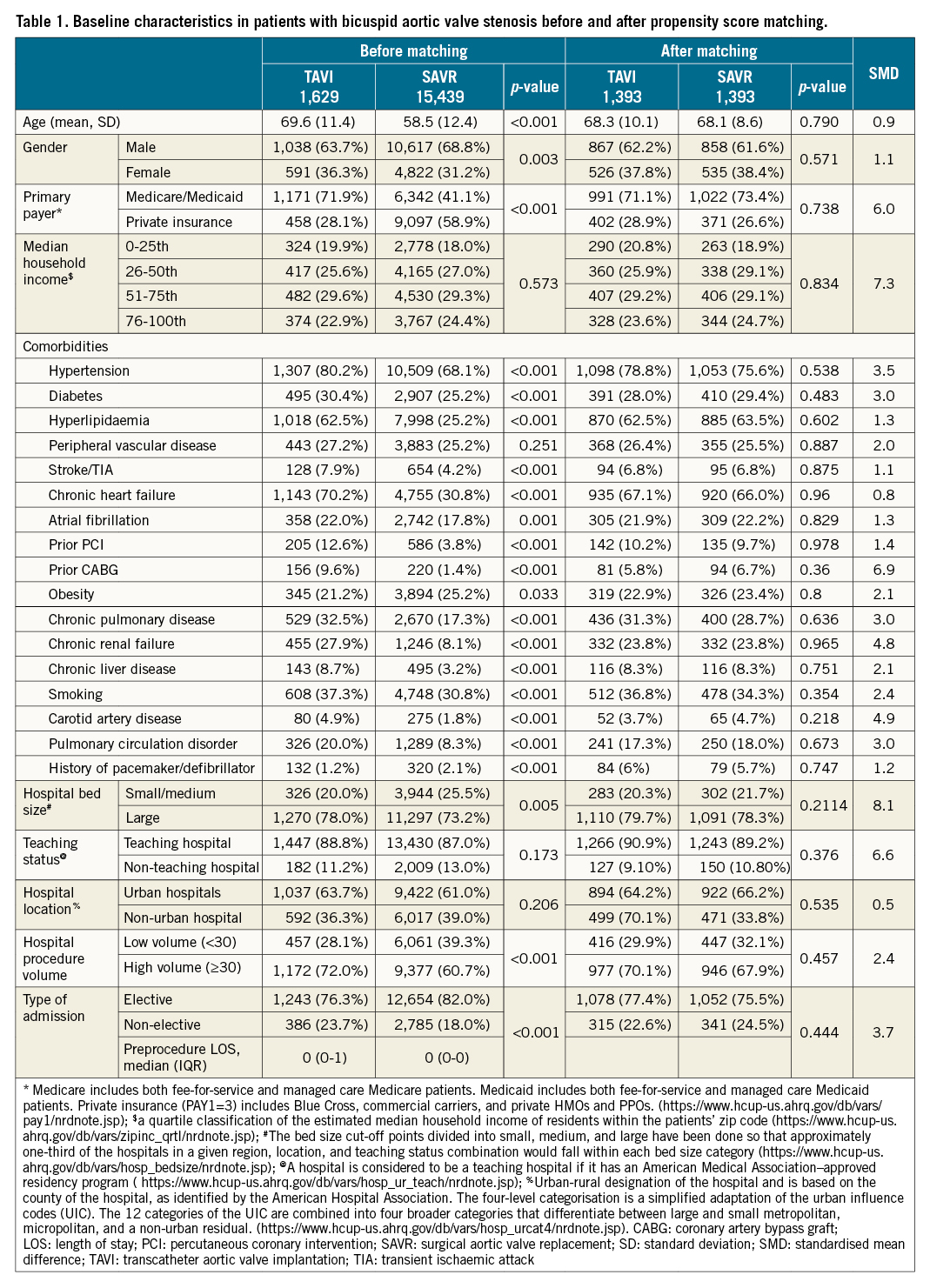
Table 1 shows baseline characteristics of TAVI and SAVR patients before and after matching. Before matching, the TAVI population was older (mean/SD age [years]: 69.6±11.4 vs 58.5±12.4; p<0.001) and had more female patients (31.2% vs 36.3%; p=0.003) than SAVR. TAVI was performed more commonly in patients with Medicare/Medicaid than SAVR. TAVI had a higher prevalence of all comorbidities, except obesity, which was higher in SAVR. TAVI was performed more frequently in high volume and large hospitals (based on bed size) than SAVR. More TAVI was performed as a non-elective procedure than SAVR (23.7% vs 18%; p<0.001). Median household income, frequency of peripheral vascular disease, and teaching hospital and urban hospital status were similar between TAVI and SAVR.
In-hospital outcomes
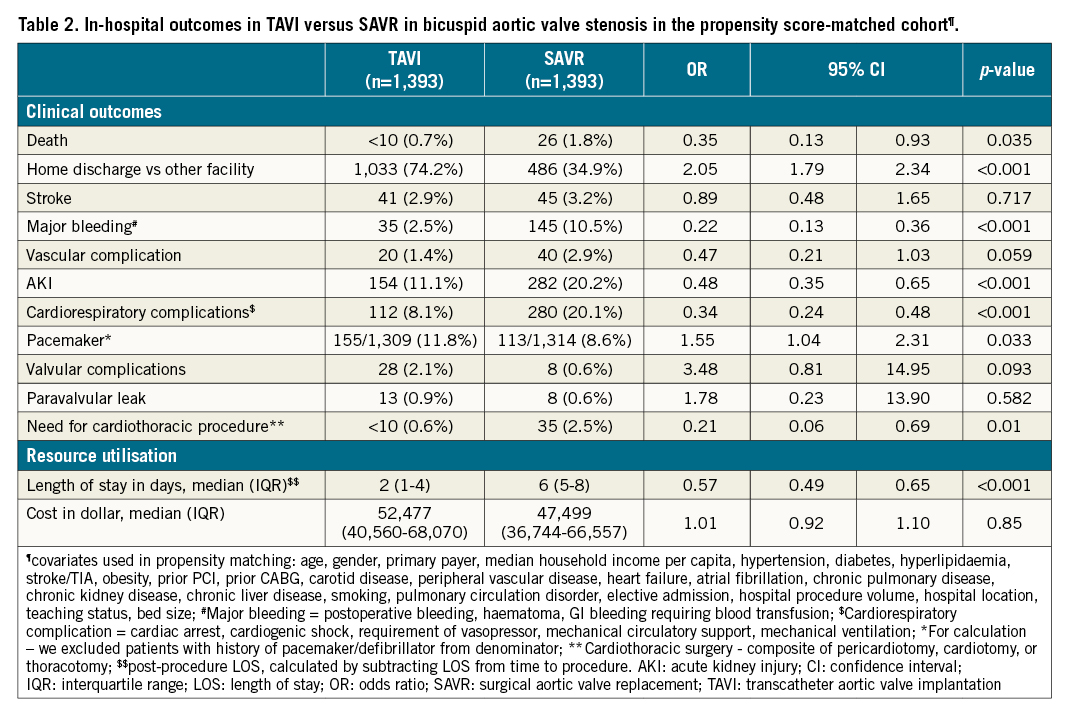
Patients undergoing TAVI compared with SAVR were associated with lower odds of in-hospital death (0.7% vs 1.8%, respectively, OR: 0.35, 95% CI: 0.13-0.93; p=0.035). TAVI patients were more likely to be discharged home (vs other facilities) than SAVR (TAVI: 74.2% vs SAVR: 34.9%, OR: 2.05, 95% CI: 1.79-2.34; p<0.001). Patients undergoing TAVI were associated with decreased odds of major bleeding (TAVI: 2.5% vs SAVR: 10.5%, OR: 0.22, 95% CI: 0.13-0.36; p<0.001), vascular complications (TAVI: 1.4% vs SAVR: 2.9%, OR: 0.47, 95% CI: 0.21-1.03; p=0.059), AKI (TAVI: 11.1% vs SAVR: 20.2%, OR: 0.48, 95% CI: 0.35-0.65; p<0.001), cardiorespiratory complications (TAVI: 8.1% vs SAVR: 20.1%, OR: 0.34, 95% CI: 0.24-0.48; p<0.001), and need for post-procedure cardiothoracic procedure (TAVI: 0.6% vs SAVR: 2.5%, OR: 0.21, 95% CI: 0.06-0.69; p=0.010). TAVI was associated with 43% shorter LOS compared with SAVR (TAVI: two days vs SAVR: six days, OR: 0.57, 95% CI: 0.49-0.65; p<0.001). However, TAVI was associated with higher odds of pacemaker implantation (TAVI: 11.8% vs SAVR: 8.6%, OR: 1.55, 95% CI: 1.04-2.31; p=0.033) compared with SAVR. There were no differences in odds of in-hospital stroke, valvular complication, and cost between TAVI and SAVR (Table 2).
Short-term (30 days), medium-term (six months) outcomes
There was no difference in 30-day/six-month MACE (30-day, TAVI: 1.0% vs SAVR: 1.5%, OR: 0.65, 95% CI: 0.27-1.58; p=0.343; six-month, TAVI: 4.2% vs SAVR: 4.9%, HR: 0.86, 95% CI: 0.44-1.69; p=0.674) between TAVI and SAVR (Table 3). There was no difference in risk of all-cause readmission (30-day, TAVI: 11.3% vs SAVR: 11.9%, OR:0.92, 95% CI: 0.63-1.36; p=0.684; six-month, TAVI: 15% vs SAVR: 15.1%, OR: 1.0, 95% CI: 0.69-1.45; p=0.998), CV hospitalisation, all-cause mortality, stroke, and valvular complication between the two groups at 30 days and six months. However, TAVI was associated with a higher risk of pacemaker implantation at 30 days (TAVI: 2.2% vs SAVR: 0.2%; p=0.006). Figure 2A-Figure 2C demonstrate the Kaplan-Meier curves for six-month MACE and 30-day/six-month all-cause readmission.
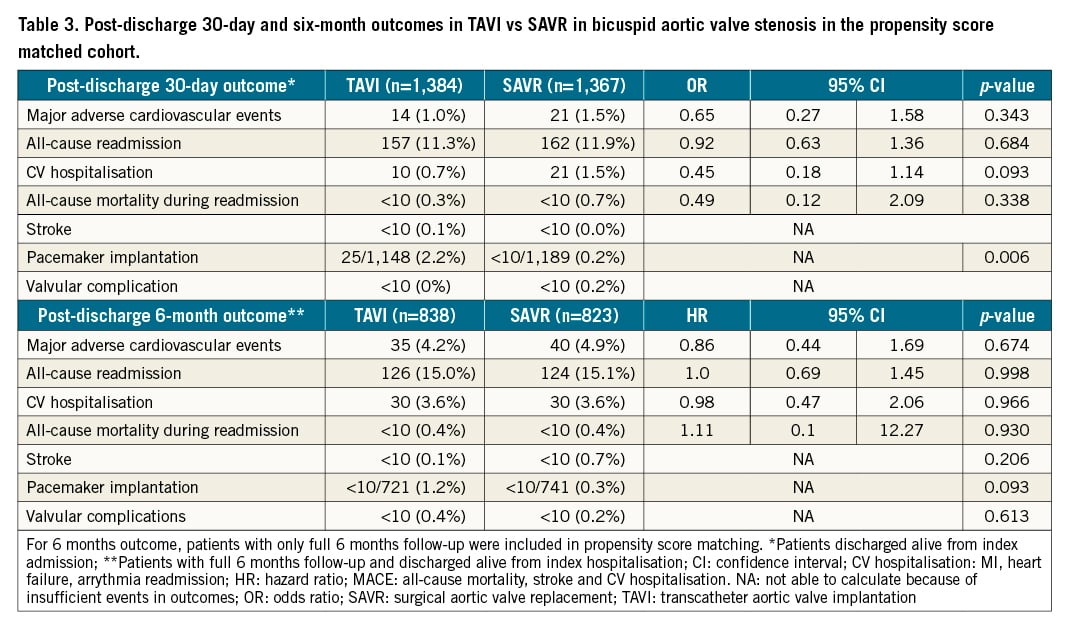
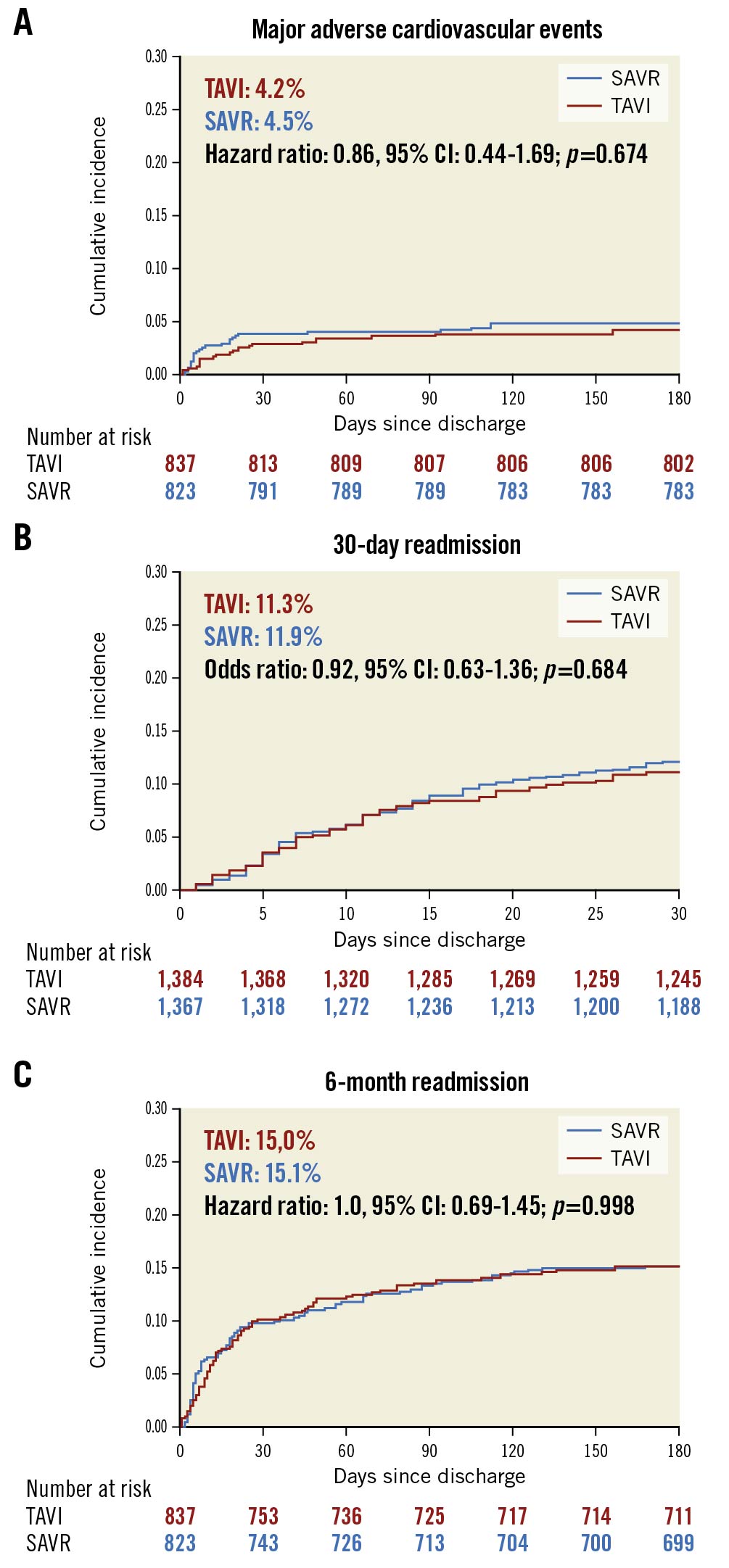
Figure 2. Kaplan-Meier graphs showing TAVI vs SAVR in BAV stenosis. A) Major adverse cardiovascular event. B) Thirty-day all-cause readmission. C) Six-month all-cause readmission. BAV: bicuspid aortic valve; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
Sensitivity analysis result
We assessed in-hospital mortality using the IPTW doubly robust method, and the result did not change (OR: 0.43, 95% CI: 0.23-0.94; p=0.026).
Unmeasured bias analysis
The falsification endpoint was similar between the two groups (30-day falsification, TAVI: <10, SAVR: <10; p=0.688; six-month falsification, TAVI: <10, SAVR: <10; p=0.783). In the “E-value” analysis, the observed OR of 0.35 for in-hospital mortality could be explained by an unmeasured confounder that was associated with both the treatment and the outcome by a relative risk of 5.16-fold each above the measured confounders, but weaker confounding could not do so.
Discussion
The largest nationally representative data were queried from 2016 to 2018 to report in-hospital and 30-day/six-month clinically important outcomes and resource utilisation among patients with BAV stenosis undergoing TAVI compared with SAVR. Our study highlighted that most patients with BAV stenosis underwent SAVR, and only a small portion of these patients underwent TAVI. Patients undergoing TAVI were older and had a higher percentage of associated comorbidities as expected. In the propensity score-matched cohort, TAVI was associated with a lesser risk of in-hospital death, major bleeding, vascular complication, AKI, cardiorespiratory complications, need for cardiothoracic procedure, and with shorter hospital stay. However, it was associated with higher odds of post-procedure pacemaker implantation, but there was no difference in valvular complications compared with SAVR. TAVI was associated with a similar risk of 30-day/six-month MACE, all-cause readmission, CV hospitalisation, stroke, and all-cause mortality but a higher risk of pacemaker implantation than SAVR (Central illustration).
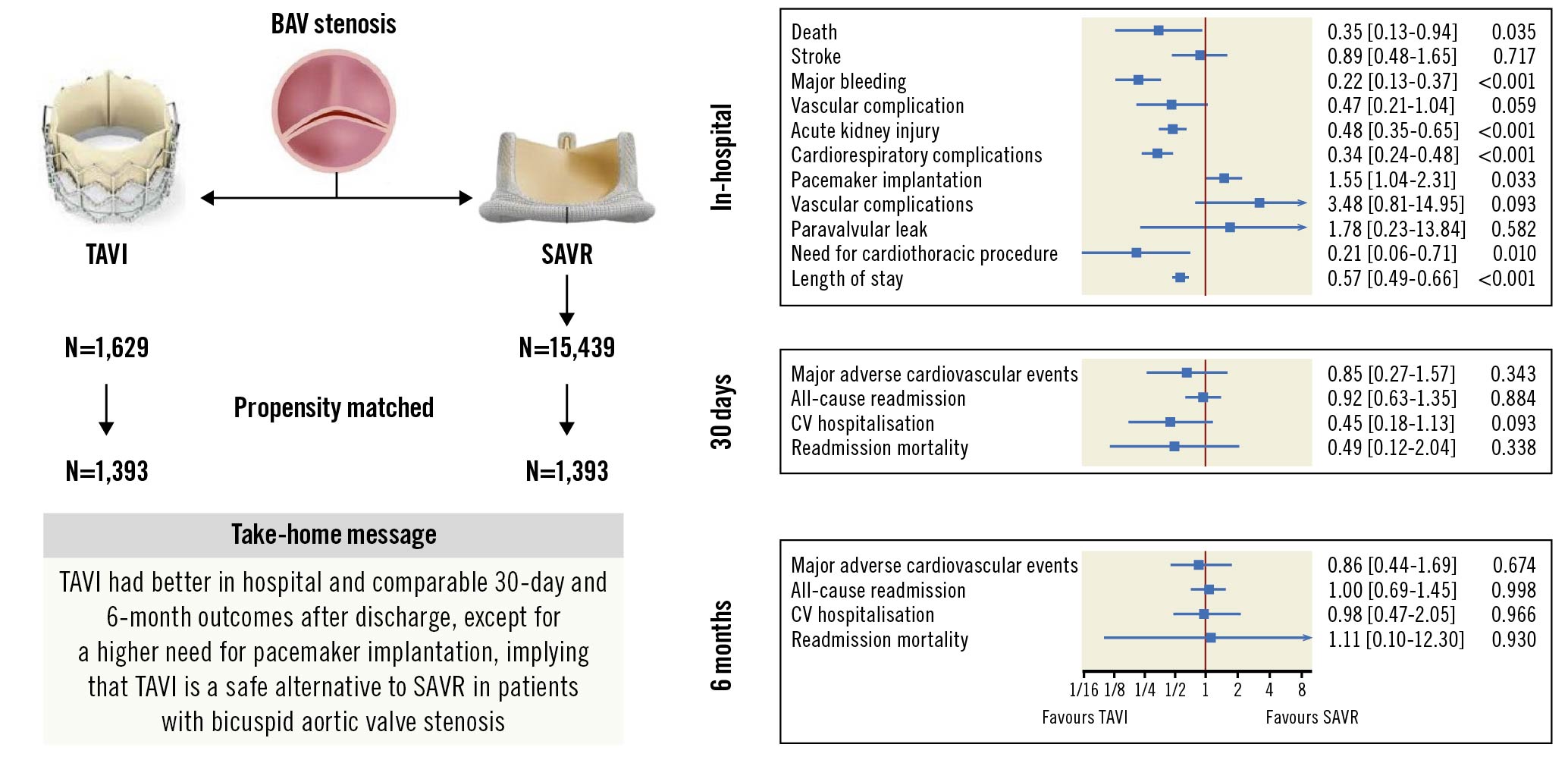
Central illustration. Transcatheter versus surgical aortic valve replacement in patients with bicuspid aortic valve. BAV: bicuspid aortic valve; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
Lower utilisation of TAVI for the treatment of BAV stenosis can be attributed to a current lack of randomised controlled trials comparing the two modes of treatment, and limited observational studies supporting the use of TAVI in BAV patients, due to their varied anatomical features15. BAV stenosis patients have a distinct aortic root anatomy, with associated aortopathy in nearly 25% of patients, and have a higher risk of aortic leaflet complications that increase the risk of procedural complications and might be associated with lower device success rates3. Additionally, BAV stenosis presents at a relatively younger age than tricuspid aortic stenosis, and the lack of long-term follow-up data for TAVI devices may have led to a lower use of TAVI in these patients16. Moreover, TAVI was not approved for low-risk patients until 2019, which could be the reason that the current study reported a lopsided patient distribution between the two treatment strategies, and reported older age and higher prevalence of comorbidities in TAVI compared with SAVR for BAV stenosis. The findings of our study are in accordance with previously published reports for tricuspid aortic valve910replacement. Paradoxically, our study reported a higher prevalence of obesity among BAV stenosis patients undergoing SAVR than those undergoing TAVI. Similar results were reported in previous studies in this specific cohort and in patients with aortic stenosis in general91017.
Regarding in-hospital outcomes, we reported decreased odds of in-hospital death in patients undergoing TAVI compared with SAVR. However, a study by Elbadawi et al using the national in-patient sample (NIS), reported no difference in in-hospital death9. The cohort in that study was treated between 2012 and 2016. We presume that improved operator skills, a better understanding of patient selection, and enhanced experience in managing these complex patient populations might be associated with better outcomes over the years. TAVI was associated with lower major bleeding, vascular complication, AKI, cardiorespiratory complication, need for cardiothoracic procedure, and shorter LOS with a similar risk of stroke and valvular complication compared with SAVR. This suggests that even in high-risk patients with complex aortic root anatomy, TAVI was associated with lower morbidity. As with TAVI for tricuspid aortic valves, TAVI for BAV is associated with higher odds of post-procedure pacemaker implantation. The increased odds of permanent pacemaker implantation, compared with SAVR, may be explained by factors such as over- or under-sizing the transcatheter valve in BAV stenosis patients, distinct anatomy, asymmetric leaflet calcification making a circular adaption of the transcatheter valve difficult, the inherent risk of conduction block with TAVI, and minimal experience in the treatment of bicuspid aortic valve1819.
We reported similar short-term (30-day) and medium-term (six-month) MACE, mortality, stroke, CV hospitalisation, and all-cause readmission between the two groups. We reported 1% cumulative incidence (of in-hospital and 30 days after discharge) of 30-day mortality comparable to a recently published single-arm prospective study in low-risk BAV patients (0.7%)20. The cumulative incidence of stroke at 30 days was 3%, comparable to the study by Forrest et al (4%)20. The observed similar outcomes between the two groups can be attributed to the minimally invasive nature of TAVI with few in-hospital complications, prescription of antithrombotic therapy, gradually increasing operator experience, and improved device design. However, TAVI compared with SAVR was associated with higher rates of permanent pacemaker implantation, probably for similar reasons as elaborated above for the increased odds of in-hospital pacemaker implantation. We reported a 14% cumulative incidence of pacemaker implantation at 30 days in TAVI, comparable to a study by Forrest et al (15%)20. The role of supra-annular sizing in tapering aortic root anatomy and annular sizing in tubular or flare aortic root configuration in patients with bicuspid aortic stenosis should be further studied as a preventive strategy for conduction blocks and permanent pacemaker implantation requirements in these patients21.
Our results showed that TAVI was associated with better in-hospital and comparable short-term and medium-term (six-month) outcomes compared with SAVR in BAV stenosis patients, with a slightly higher rate of pacemaker implantation, implying that TAVI might be a suitable alternative to SAVR if the long-term (5-10 years) outcomes with TAVI valves are satisfactory. We presume these findings were mostly related to patients with intermediate or higher surgical risk, as indicated by baseline characteristics and the fact that TAVI was not approved for low-risk patients during the study period. Moreover, based on the RCTs that showed better or comparable outcomes for TAVI in low-risk patients1522, we hypothesise that this will also be true in patients with BAV stenosis. The low prevalence of BAV stenosis patients requiring surgery will make it challenging to conduct adequately powered RCTs in the near future.
Study limitations
The present study has several limitations. The NRD used in the present analysis is an administrative database and can be subject to coding errors. However, considering the procedural codes used to identify our cohort, we anticipate minimal to nil error in coding. ICD-10 provides codes not specifically for BAV, but for congenital aortic valve pathology, which can include both unicuspid and BAV. However, since most of these patients have BAV, it would be reasonable to take the given codes for BAV pathology. The Society of Thoracic Surgeons (STS) risk score, symptomatic status of included patients, ejection fraction, echocardiographic findings, intraoperative post-procedural findings, phenotype of BAV, aortic annulus diameter, ascending aorta diameter, and prosthetic valve size are not captured in the database and can be an important determinant of outcomes in these patients. Moreover, NRD lacks information on balloon-expandable (BE) or self-expanding (SE) TAVI valves. However, a recently published network meta-analysis showed that both BE and SE valves were associated with a similar risk of all-cause mortality, cardiovascular mortality, stroke, and myocardial infarction but a higher risk of paravalvular leak and pacemaker implantation compared with SAVR in aortic valve stenosis23. We did not know the type of device used or the prescription and compliance to antithrombotic therapy post-procedure. Finally, we had no information on the quality of life or symptomatic improvement post-procedure in our study, due to the limitation of the database.
Despite the unavailability of information on prosthetic valve characteristics, BAV morphology or echocardiography parameters, the strength of the study lies in a large sample and multi-institutional cohort. Additionally, we applied a falsification endpoint and “E-value” method to assess the impact of unmeasured confounders10. The “E-value” estimate demonstrated that unmeasured confounders need higher association with both treatment and outcome over measured covariates, and successful falsification endpoint analysis indicated a low probability of having unbalanced residual confounders that can negate the measured effect. Hence, there is a low probability that results could be different if other variables were available.
Conclusions
In a nationwide, multicentre, propensity score-matched cohort study, TAVI was associated with reduced in-hospital mortality and morbidity, with similar early (30-day/six-month) MACE outcomes among BAV stenosis patients compared with SAVR. However, a need for permanent pacemaker implantation remains elevated with TAVI for these patients without any difference in valvular complications. The study supports the feasibility of TAVI in BAV patients who do not require concurrent aortic root repair, as an alternative to SAVR. However, the study results should be interpreted cautiously as anatomical information on the native and prosthetic valves was not available. RCTs will provide better clarity for the role of TAVI in patients with BAV stenosis.
Impact on daily practice
The RCTs on TAVI have traditionally excluded patients with BAV morphology due to anatomical complexities and concerns of higher complications with BAV morphology. TAVI was associated with lower in-hospital mortality, morbidities, and shorter LOS. There are no differences in short-term and medium-term MACE, mortality, stroke, CV hospitalisation, and readmission. The study supports the feasibility of TAVI as an alternative to SAVR in BAV patients who do not require concurrent aortic root repair. However, TAVI was associated with a higher pacemaker implantation rate than SAVR in patients with BAV stenosis. Randomised clinical trials in BAV patients with longer follow-up are required to confirm these findings.
Funding
This study was funded by the Ram and Sanjita Kalra Aavishqaar Fund (makeadent.org).
Conflict of interest statement
A. Kalra is the Chief Executive Officer and Creative Director of makeadent.org. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

