Abstract
Background: Transcatheter aortic valve implantation (TAVI) might be a feasible treatment option for more patients with bicuspid aortic valve (BAV) stenosis. However, long-term follow-up data in this population are scarce.
Aims: The aim of this study was to evaluate three-year outcomes after TAVI in patients with BAV.
Methods: A total of 246 consecutive patients who underwent TAVI at a single centre in China between March 2013 and February 2018 were enrolled in this study. Clinical outcomes, health status and echocardiography were followed and recorded for three years.
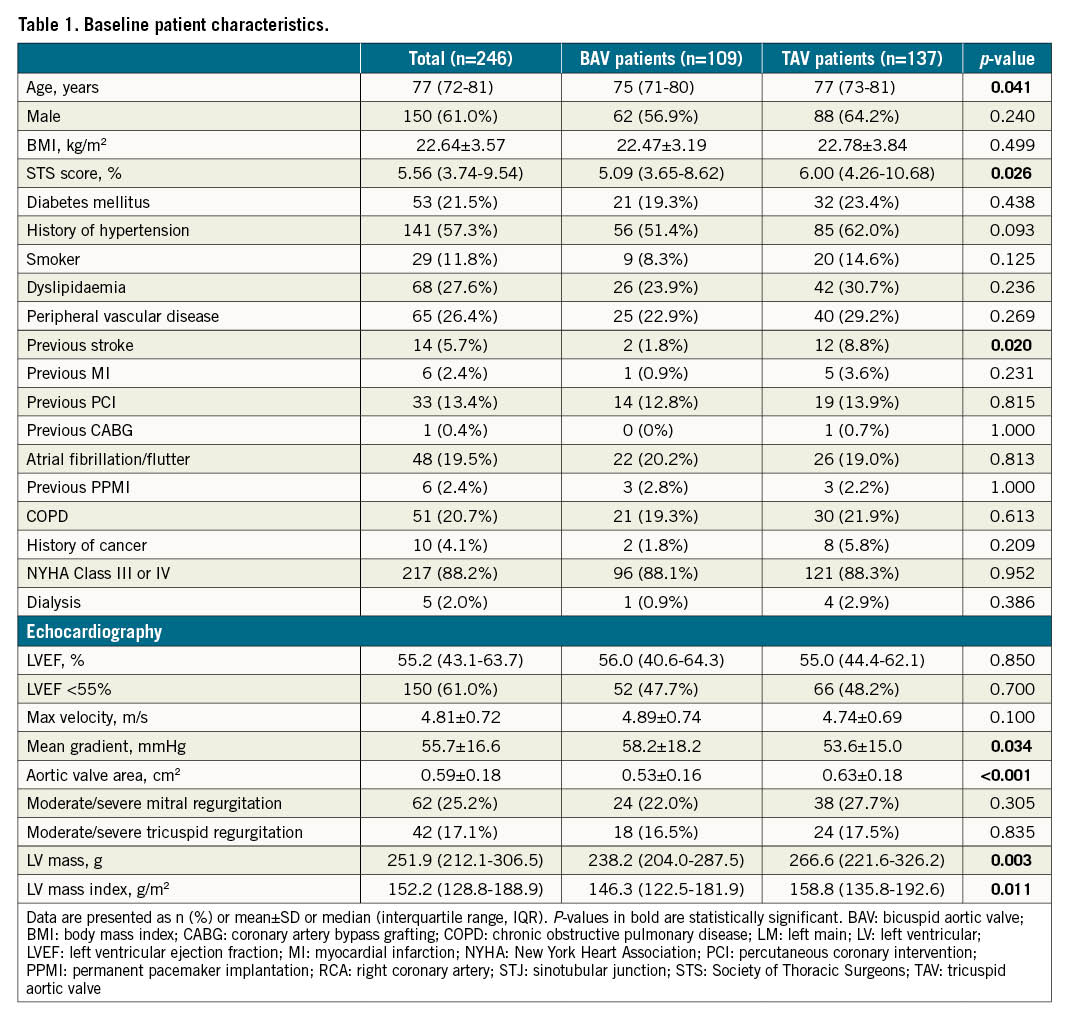
Results: Among 109 (44.3%) BAV patients, 61.5% were Type 0 and 36.7% were Type 1 BAV patients. BAV patients were younger (75 vs 77 years, p=0.041) and had a lower Society of Thoracic Surgeons (STS) score (5.09 vs 6.00, p=0.026) compared to tricuspid aortic valve (TAV) patients. There were no differences in three-year survival rates between bicuspid and tricuspid patients (87.1% vs 79.5%, log-rank p=0.126). Multivariate Cox regression analysis adjusting for confounding factors revealed a similar risk of all-cause mortality in the BAV population (hazard ratio [HR] 0.86, 95% confidence interval [CI]: 0.44-1.70, p=0.666). Except for the rate of permanent pacemaker implantation that was lower in BAV patients (11.9% vs 21.9%, p=0.041), the incidence of other clinical adverse events was comparable between the two groups. Both BAV and TAV patients showed an obvious improvement in valve haemodynamics, which was sustained for three years. In addition, similar left ventricular reverse remodelling was found during follow-up.
Conclusions: BAV patients showed similar satisfactory three-year clinical outcomes, persistent valve haemodynamics improvement, and obvious cardiac reverse remodelling after TAVI compared to TAV patients.
Introduction
Transcatheter aortic valve implantation (TAVI) was initially performed in inoperable patients or those at high risk for surgical aortic valve replacement (SAVR). The indication for TAVI gradually expanded to intermediate, even low-risk patients, and it was recommended for more patients with severe symptomatic aortic stenosis (AS) according to the updated guidelines123. An analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database revealed that TAVI has rapidly evolved over the last decade, currently accounting for over half of all aortic valve interventions4.
Bicuspid aortic valve (BAV) is the most common congenital valvular disease. In patients with BAV, aortic stenosis develops at an earlier age than in those with a tricuspid phenotype56. However, BAV patients have been systematically excluded from most randomised clinical trials because of unfavourable anatomical characteristics such as severe annular eccentricity, concomitant aortopathy, and severe calcification78910. As the indications for TAVI are extending to low-risk patients, more patients with bicuspid aortic valve stenosis will become candidates for TAVI.
To date, several studies have confirmed the short-term efficacy and safety of TAVI in BAV111213. However, long-term follow-up data in this population are scarce, which is particularly important when TAVI expands to younger patients with longer life expectancy. Therefore, this study was performed to compare the three-year outcomes of the TAVI procedure in bicuspid and tricuspid AS patients.
Methods
Study design and patient population
We retrospectively collected data from 257 consecutive patients who underwent TAVI at the Second Affiliated Hospital of Zhejiang University School of Medicine between March 2013 and February 2018. After excluding patients with quadricuspid aortic valves (n=2), pure aortic regurgitation (n=7), and those who underwent valve-in-valve procedures (n=2), the final study cohort enrolled 246 patients (Supplementary Figure 1).
A multidisciplinary Heart Team discussed and decided all TAVI procedures. If the anatomical conditions of the artery were acceptable, transfemoral TAVI was performed. All procedures were performed in our hybrid operating room under general anaesthesia or local anaesthesia with sedation. Most patients were implanted with self-expanding valves such as the VenusA-Valve (Venus Medtech), CoreValve (Medtronic), VitaFlow (Microport), and TaurusOne Valve (Peijia Medical). The remaining participants were implanted with the balloon-expandable SAPIEN XT (Edwards Lifesciences) or the mechanically expandable Lotus Valve (Boston Scientific).
The study was approved by the medical ethics committee and complied with the Declaration of Helsinki. All patients provided written informed consent for the procedure and the use of anonymous data for research.
Follow-up and data collection
The follow-up visits were performed 30 days after the procedure and then annually by our professional follow-up team. The majority of patients were followed up at our centre, while the rest were followed up via structured telephone interviews. All follow-up data were collected into our local database. Transthoracic echocardiography was required in the 30-day and annual follow-up. New York Heart Association (NYHA) status and clinical events were evaluated by a cardiologist. The complications were defined according to the Valve Academic Research Consortium-2 (VARC-2) consensus document14. Major adverse cardiovascular events (MACE) were defined as death, stroke, and myocardial infarction, as previously described15.
Imaging measurement
Patients underwent a standard screening including echocardiography and contrast-enhanced multidetector computed tomography (MDCT) before procedures. Preoperative MDCT data were available for all patients. Anatomical structures measured on MDCT were evaluated in 3mensio software (Pie Medical Imaging). The type of aortic valve was distinguished based on full-phase MDCT by two professional cardiologists (Q.F. Zhu and Y.X. He) and was reconfirmed by the two authors (D. Zhou and Y.C. Guo) following the description by Sievers et al16. The calcium volume of the device landing zone was quantified in 3mensio software, using the threshold of 850 Hounsfield units, as previously described171819. The implantation depth was measured by fluoroscopy in the non-coronary cusp direction, as previously described20. All echocardiograms were performed by experienced echocardiographers. Left ventricular (LV) mass was calculated using the Cube formula described in a previous study21.
Statistical analysis
Continuous variables were described as mean±standard deviation (SD) or median (interquartile range [IQR]) based on distributions. Normality tests were performed using the Shapiro-Wilk tests or Quartile-Quartile (Q-Q) plots. Continuous variables were compared between two groups using the unpaired Student's t-test or Mann-Whitney U-test according to their distributions. The comparison of echocardiographic data between baseline and different time points was performed using a paired samples t-test or Wilcoxon rank-sum test. Categorical variables were presented as percentages and were compared by the chi-square or Fisher’s exact test. The McNemar chi-square test was used for paired samples. Cumulative survival rates were calculated using the Kaplan-Meier (K-M) survival analysis, and the log-rank test was performed for comparison between two groups. Cox proportional hazard regression models were used to explore risk factors of three-year all-cause mortality. Variables with a p-value <0.10 in univariate Cox regression analysis were included in the multivariate model. In addition, logistic regression analysis was performed to identify potential confounding factors in bicuspid AS patients (Supplementary Table 1). Next, a multivariate Cox regression analysis adjusted for these confounding factors was carried out to identify whether bicuspid AS was a risk factor of three-year all-cause mortality. A two-tailed p-value of <0.05 was considered statistically significant. Bonferroni correction was applied when multiple comparisons were performed. Statistical analysis was performed using SPSS Statistics software version 23.0 (IBM Corp.).
Results
Patients and baseline characteristics
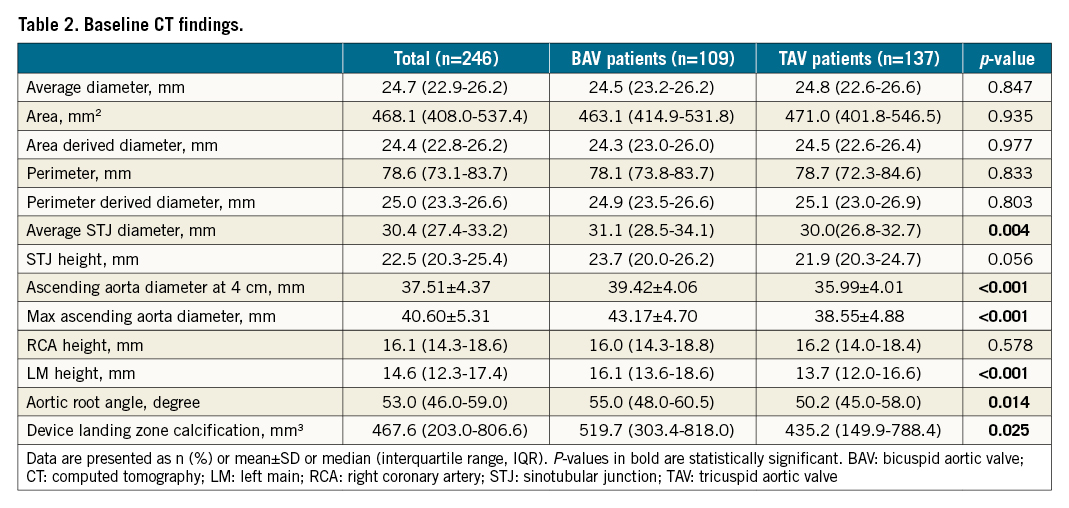
Among 246 consecutive patients who underwent TAVI between March 2013 and February 2018, 109 (44.3%) patients had BAV morphology, and the other 137 (55.7%) had TAV (Central illustration). Population features, baseline echocardiography characteristics and CT analyses are shown in Table 1 and Table 2. The median age was 77 years and 61% of the patients were male. The overall median STS score was 5.56 (interquartile range [IQR]: 3.74-9.54). Compared to tricuspid AS patients, BAV patients were younger (75 vs 77 years, p=0.041), had lower Society of Thoracic Surgeons scores (5.09 vs 6.00, p=0.026), and had a lower proportion of patients with stroke history (1.8% vs 8.8%, p=0.020). According to echocardiography, bicuspid AS patients had less LV hypertrophy, and a smaller aortic valve area but a similar mean gradient and velocity. A more horizontal aorta, more severe calcification, and larger aortic root anatomy, including sinotubular and ascending aorta, were found in BAV patients.
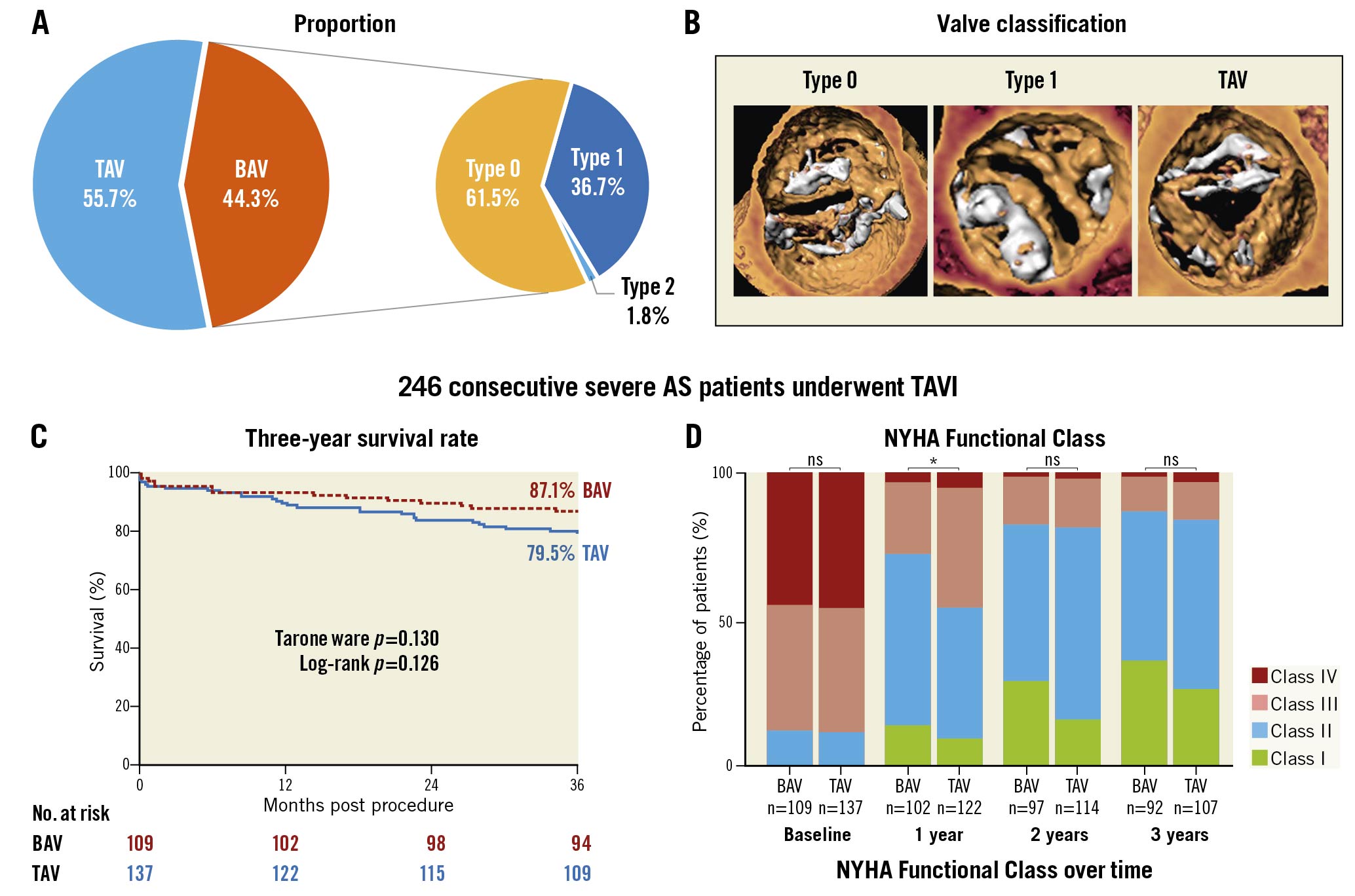
Central illustration. Population, three-year survival rate and NYHA Functional Class. A) Population proportion of BAV and TAV morphologies. B) Typical image of type 0, type 1 bicuspid aortic valve and tricuspid aortic valve. C) Kaplan-Meier estimates of the all-cause mortality. D) NYHA Functional Class at baseline and annual follow-up. BAV: bicuspid aortic valve; NYHA: New York Heart Association TAV: tricuspid aortic valve; TAVI: transcatheter aortic valve implantation
Procedural characteristics and outcomes
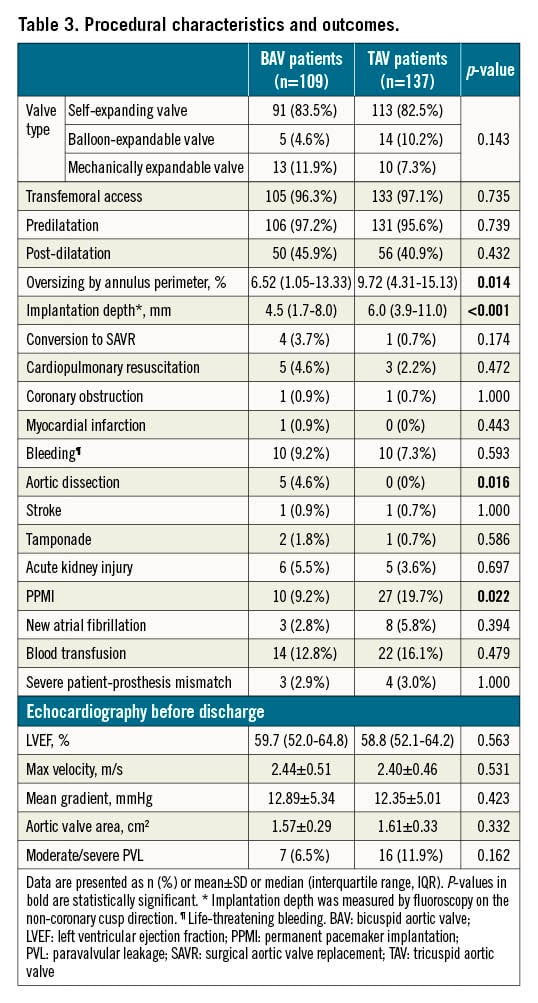
Self-expanding valves were used most frequently (82.9%), followed by mechanically expandable valves (9.3%). Most patients underwent TAVI via the transfemoral access (96.7%). Transsubclavian, transaortic or transcarotid TAVI was performed in the remaining patients. Predilatation was routinely performed in 96.3% of patients, and 43.1% of patients received post-dilatation. No differences in these procedural factors were found between BAV and TAV patients (Table 3). Most in-hospital outcomes were comparable between the two groups, except that the BAV population suffered more aortic dissection (4.6% vs 0%, p=0.016) and fewer permanent pacemaker implantations (PPMI) (9.2% vs 19.7%, p=0.022). Detailed information on the patients converted to open SAVR is provided in Supplementary Table 2. The 30-day all-cause mortality rate was similar between the two groups (4.6% vs 4.4%, p=1.000).
Long-term clinical outcome
The clinical data of 245 patients were available at three years (Supplementary Figure 1) (one BAV patient could not be contacted after the second-year follow-up). Long-term clinical outcomes are shown in Table 4. No significant differences in all-cause mortality (12.8% vs 20.4%, p=0.116) or cardiovascular mortality (5.5% vs 10.9%, p=0.129) were found between the BAV and TAV populations during three years of follow-up. The three-year survival rates were similar in bicuspid (87.1%, 95% confidence interval [CI]: 83.9-90.3) and tricuspid (79.5%, 95%CI: 76.0-83.0) AS patients (log-rank p=0.126) according to Kaplan-Meier estimates (Central illustration). Furthermore, the incidence of MACE, stroke, myocardial infarction and aortic valve reintervention was also comparable between the two groups. Nevertheless, the rate of permanent pacemaker implantation was consistently lower in bicuspid AS patients. Moreover, fewer BAV patients suffered NYHA Class III or IV symptoms at one-year follow-up, although the difference disappeared over the next two years (Central illustration). Additionally, some subgroup analyses were performed. The comparisons of BAV and TAV patients when considering only the patients treated with a self-expanding valve, balloon-expandable valve or mechanically expandable valve are shown in Supplementary Table 3 and Supplementary Table 4, respectively. In a subgroup analysis of BAV patients, no difference of three-year clinical outcomes was found between type 0 and type 1 patients (Supplementary Table 5).
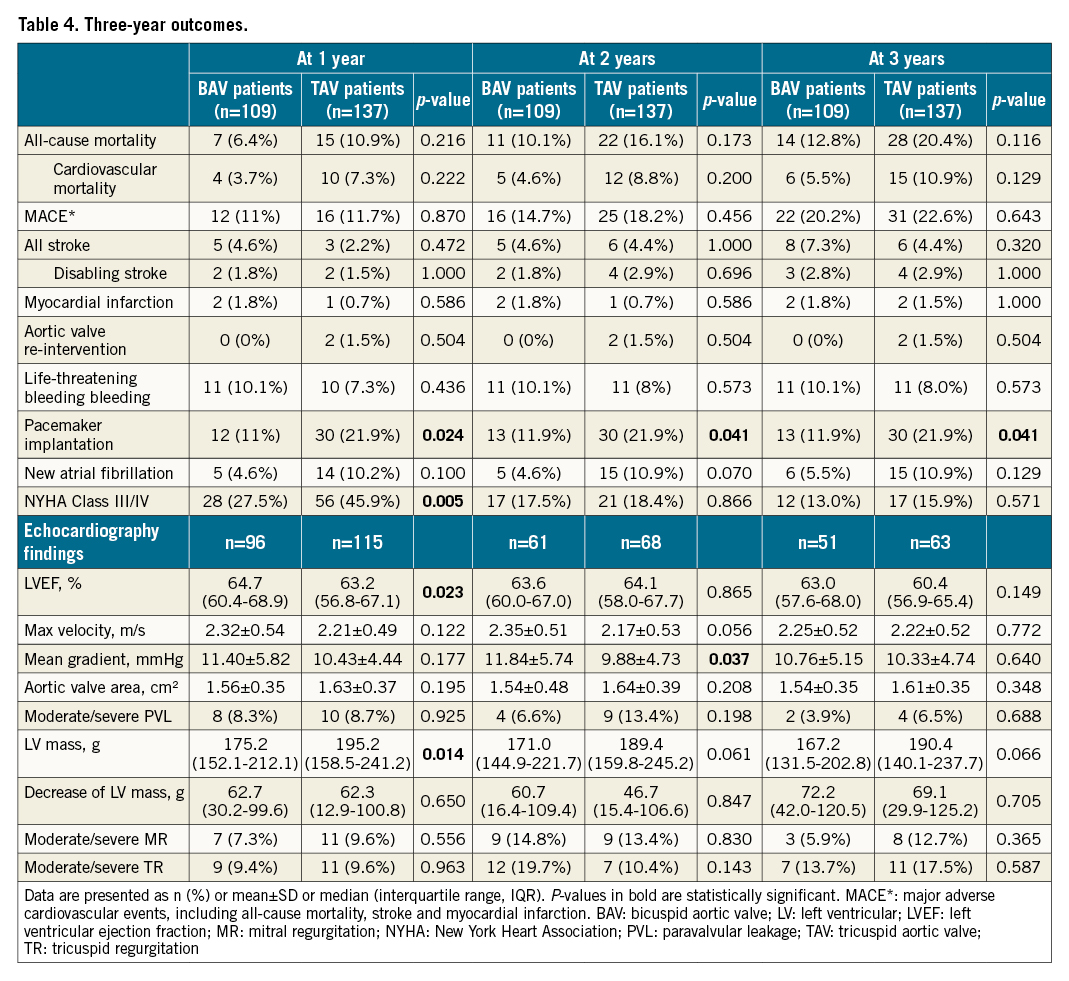
Echocardiographic findings
Both bicuspid and tricuspid AS patients had a distinct decrease in mean gradient and had an increased area of the aortic valve after the procedure, and this improvement was sustained for three years (Figure 1A, Figure 1B). The moderate or severe paravalvular leakage rate was similar after TAVI (6.5% vs 11.9%, p=0.162). There was also no difference in PVL between the two groups in the first year (8.3% vs 8.7%, p=0.925), second year (6.6% vs 13.4%, p=0.198) and third year (3.9% vs 6.5%, p=0.668) on echocardiographic examination. Although BAV patients had a lower LV mass at baseline (Figure 1C), a similar decrease of LV mass was found between BAV and TAV patients (Table 4, Figure 1D). This improvement mainly occurred during the first year after the TAVI procedure (Figure 1D, Supplementary Figure 2). The detailed annual quantification of left heart chamber size is shown in Supplementary Table 6. A comparison between baseline echocardiography and the echo data at different time points is provided in Supplementary Table 7.
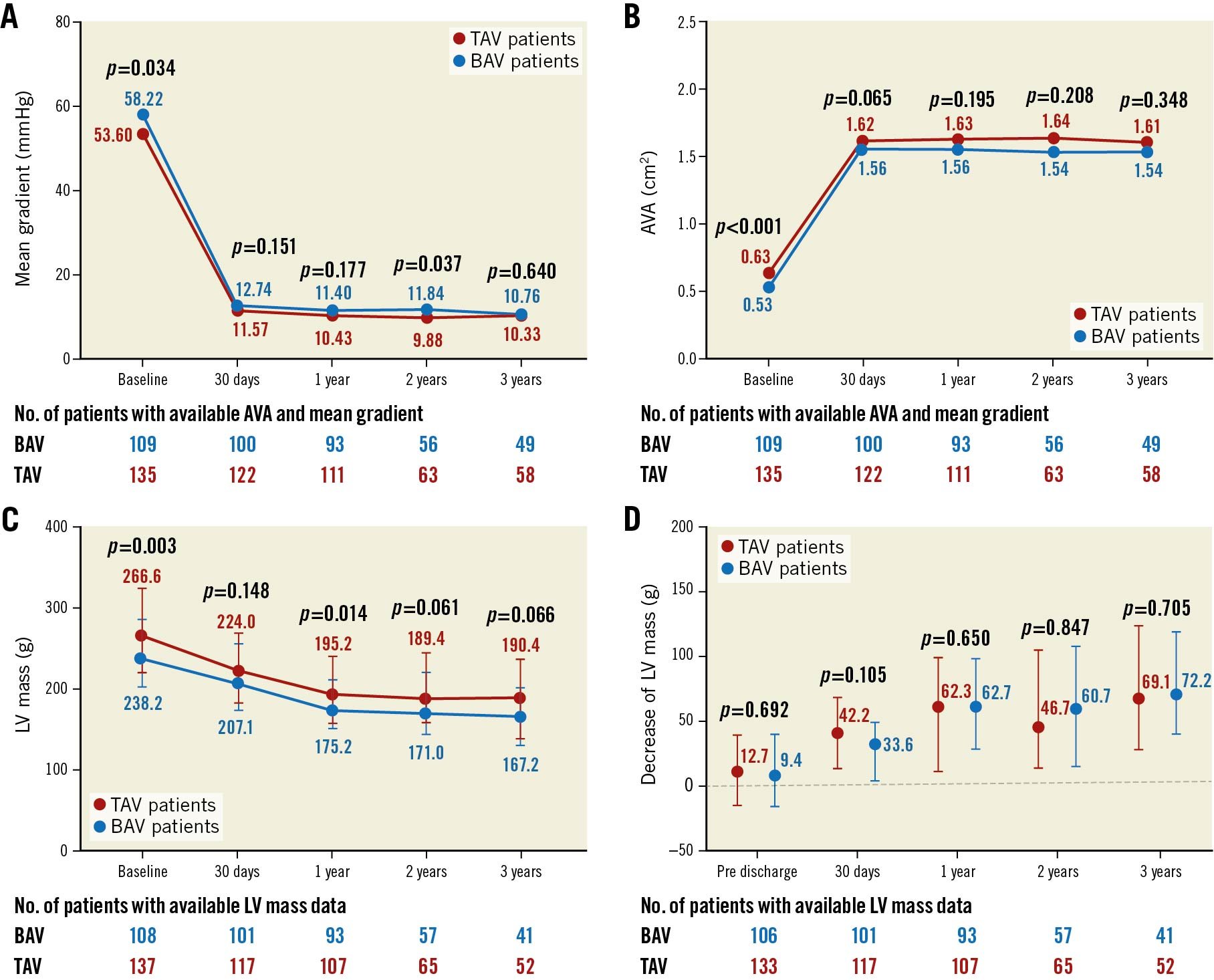
Figure 1. Echocardiographic findings up to three years. Changes in mean gradient (A), AVA (B), and LV mass (C) from baseline to 30 days, 1 year, 2 years, and 3 years in the BAV patients and TAV patients. D) Comparison of decrease in LV mass between TAV patients and BAV patients at pre-discharge, 30 days, 1 year, 2 years, and 3 years. The bars in (C) and (D) represent interquartile range. AVA: aortic valve area; BAV: bicuspid aortic valve; LV: left ventricle; TAV: tricuspid aortic valve
Univariate and multivariate analysis
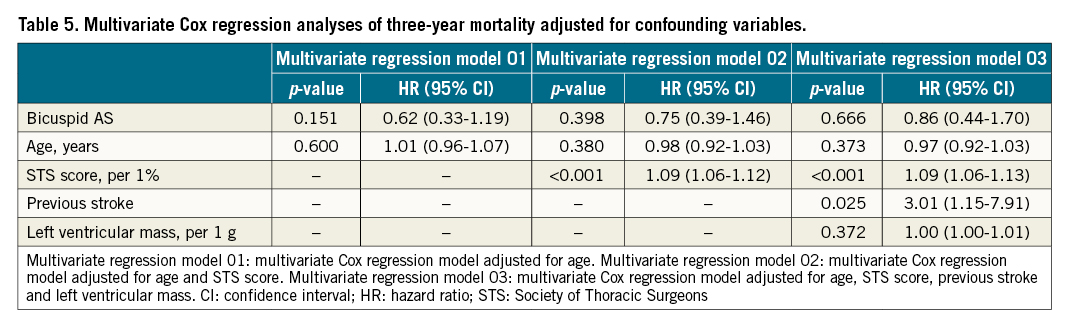
According to the univariate Cox regression analysis, bicuspid AS was not differentially associated with the all-cause mortality (hazard ratio [HR] 0.61, 95% CI: 0.32-1.16). After adjusting for potential confounding factors, including age, STS score, prior stroke, and LV mass, bicuspid AS was not associated with risk of all-cause mortality (HR 0.86, 95% CI: 0.44-1.70) (Table 5). In multivariate Cox regression analysis using a forward Likelihood Ratio method, higher STS score, history of stroke, prior pacemaker implantation, dialysis, and combination of moderate or severe mitral regurgitation were found to be the independent predictors of three-year all-cause mortality (Supplementary Table 8).
Discussion
To the best of our knowledge, this is the first study that has compared the long-term outcomes of TAVI for BAV versus TAV up to three years. The main findings of the study are: 1) except for the lower incidence of permanent pacemaker implantation that was observed in BAV patients, three-year clinical outcomes were comparable between bicuspid and tricuspid AS patients; 2) the bicuspid AS patients had a lower percentage of NYHA Class III/IV in the first year after the TAVI procedure while the percentage was similar between the BAV and TAV groups in the second and third year of follow-up; 3) BAV patients showed consistent valve haemodynamic improvement, which was similar to TAV patients; and 4) both TAV and BAV patients experienced significant LV reverse remodelling after TAVI.
Because of its anatomical characteristics, bicuspid aortic stenosis used to be considered a contraindication for TAVI. However, recent studies have suggested the satisfactory short-term efficacy and safety of TAVI in BAV patients1112. In addition, a national database analysis, which included 1,055 BAV patients who underwent TAVI and 30,840 BAV patients who received SAVR, revealed similar in-hospital outcomes between the two groups22. According to the 2020 ACC/AHA guidelines for valvular heart disease, TAVI is recommended in BAV patients as an alternative to SAVR after careful and comprehensive assessment2. Furthermore, a previous study demonstrated a high frequency (47.5%) of bicuspid AS in the Chinese population, thus suggesting many bicuspid TAVI candidates23. In our study, the proportion of BAV was 44.3%, 61.5% of whom were BAV Type 0. This revealed completely different population characteristics from those in the West1124. Therefore, the study is warranted to evaluate long-term clinical outcomes in the Chinese BAV population.
According to the K-M survival analysis, the three-year survival rates were comparable between BAV and TAV patients (p=0.126) (Central illustration). Although it showed a numerically higher three-year survival rate in the BAV population, this may be related to the younger age, lower STS score, and fewer baseline comorbidities. Consequently, three multivariate Cox analyses were performed to adjust for confounding factors, revealing that bicuspid AS was not associated with a different risk of three-year all-cause mortality (Table 5). Moreover, 5.5% of BAV patients suffered cardiovascular mortality during the three years of follow-up, which was comparable to TAV patients (p=0.129). In a subgroup analysis of patients treated with a self-expanding valve, a similar incidence of all-cause mortality, cardiovascular mortality and MACE was found in the two groups (Supplementary Table 3). Similar results could also be found in patients who underwent TAVI using balloon-expandable valves and mechanically expandable valves (Supplementary Table 4). The satisfactory clinical results provide long-term evidence for TAVI in bicuspid AS. A valuable basis was provided for large-scale randomised controlled trials to explore further the feasibility and effectiveness of TAVI for BAV. It could be assumed that, with the accumulation of evidence6925, TAVI might be gradually applied to the BAV population in the future.
Nearly 90% of the patients in our study suffered from NYHA Class III or IV symptoms at baseline. After the TAVI procedure, a significant decrease of NYHA stage could be found in both the bicuspid and tricuspid AS population. Nevertheless, the proportion of patients with NYHA Class I/II functional status was larger in BAV patients at one-year follow-up, which might be due to the worse baseline health status and more severe LV remodelling in tricuspid AS patients. The observed difference disappeared in the second and third years of follow-up, suggesting it took longer for the heart function to recover in TAV patients. Nevertheless, although TAV patients suffered more severe LV remodelling before the procedure, tricuspid and bicuspid AS patients experienced similar and notable reverse remodelling after TAVI. This recovery predominantly occurred in the first year after the procedure and remained stable at follow-up (Supplementary Figure 2). Unfortunately, as some echocardiography data were not available during follow-up, the findings need to be verified by further studies.
Aortic dissection is a devastating and potentially life-threatening complication. Notably, there was a significantly higher incidence of aortic dissection (4.6%) in BAV patients. It should be noted that the study presented here was a real-world study, which means that it includes some patients with hostile aortic anatomy for whom TAVI was performed to save their lives. According to previous studies, ascending aorta dilatation is a basic feature of bicuspid valve abnormality that can contribute to a higher risk of aortic dissection and rupture926. In addition to this, the more severe calcification and larger aortic angulation could also increase the risk of aortic dissection. Therefore, preprocedural anatomy should be carefully assessed to identify patients at high risk of these complications.
The adverse clinical effect of permanent pacemaker implantation after a TAVI procedure was highlighted in a recent pooled analysis27. In our study, most patients underwent TAVI using a first-generation self-expanding prosthesis, which was thought to have a higher risk of PPMI28. According to previous studies, bicuspid AS patients are thought to have a similar or higher risk of PPMI1129. Nevertheless, our study revealed a lower incidence of PPMI in bicuspid AS patients in three years of follow-up (11.9% vs 21.9%, p=0.041). Since bicuspid AS patients had more severe valve calcification (519.7 mm3 vs 435.2 mm3, p=0.025), which could provide radial force for anchoring, the higher prosthesis implantation depth (4.5 mm vs 6.0 mm, p<0.001) and different valve size selection strategy based on supra-annular structure might have contributed to the difference between the two groups, as described in previous studies2530. Accordingly, further exploration of different strategies for minimising pacemaker implantation in bicuspid AS patients is warranted.
In our multivariate Cox regression analysis, prior stroke and higher STS score were independent predictors of three-year all-cause mortality. As the BAV patients had a lower proportion of stroke history and lower STS scores, these two factors should be highlighted since they could contribute to the numerically higher three-year survival rate. Moreover, we found that poor health status (dialysis) and more cardiac comorbidities (with a prior pacemaker and moderate or severe mitral regurgitation) were associated with worse long-term prognosis.
Limitations
This study has some limitations that should be considered when interpreting our findings. First, the data in this study were retrospectively collected from our TAVI database. However, some patients (40.2%) were in our prospective cohort registry (the Transcatheter Aortic Valve Replacement Single Center Registry in Chinese Population [TORCH] registry, NCT02803294), which was initiated in June 2016. A prospective, multicentre, randomised controlled study for bicuspid AS patients is also ongoing (NCT04722796). Secondly, although only one patient was lost to follow-up within three years after the procedure, the non-randomised design and small sample size made it difficult to match baseline characteristics precisely. Even though similar results could be found in multivariate regression analysis adjusted for confounding factors, the finding that bicuspid and tricuspid AS patients had a similar long-term prognosis should be further verified by large-scale and longer follow-up (e.g., five- or 10-year follow-up) studies. Furthermore, echocardiographic data were not available for all patients during follow-up, which might have led to bias.
Conclusions
Except for a lower incidence of permanent pacemaker implantation, bicuspid AS patients had comparable three-year outcomes to tricuspid AS patients after the TAVI procedure. Both the BAV and TAV populations showed significant improvement in valve haemodynamics, which could be sustained for three years. Similar LV reverse remodelling was also found during follow-up. This study suggests satisfactory long-term outcomes of TAVI in BAV patients; however, these findings need to be further confirmed by large-scale and randomised studies.
Impact on daily practice
Based on the accumulated evidence, the indications for TAVI are expanding rapidly to low-risk patients. Furthermore, the NOTION 2 trial (NCT02825134) is exploring the effectiveness of TAVI in a young population. As patients with BAV are more likely to develop severe AS at a young age, it can be assumed that more BAV patients will be candidates for TAVI. However, no evidence on the long-term outcomes of TAVI exist in this population. In our study, we found that BAV patients had similar satisfactory three-year clinical outcomes, persistent valve haemodynamic improvement, and obvious cardiac reverse remodelling compared with TAV patients. The study provides evidence for clinical practice and further exploration of TAVI for bicuspid aortic stenosis.
Funding
This work was supported by the National Key R&D Program of China (2019YFA0110400 for JA. Wang, 2016YFC13010204 for JA. Wang). The National Natural Science Foundation of China (No. 81870292 for JA. Wang, No. 81570233, 81770252 for XB. Liu). The Key Social Development Program of Major Science and Technology Projects in Zhejiang Province (No. 2015C03028 for JA. Wang).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

