Abstract
Background: Prospective data about transcatheter aortic valve implantation (TAVI) in bicuspid aortic valve (BAV) patients are limited.
Aims: We aimed to evaluate the clinical impact of the Evolut PRO and R (34 mm) self-expanding prostheses in BAV patients and explore the impact of different computed tomography (CT) sizing algorithms in a prospective registry.
Methods: A total of 149 bicuspid patients were treated in 14 countries. The primary endpoint was the intended valve performance at 30 days. Secondary endpoints were 30-day and 1-year mortality, severe patient-prosthesis mismatch (PPM) and the ellipticity index at 30 days. All study endpoints were adjudicated according to Valve Academic Research Consortium 3 criteria.
Results: The mean Society of Thoracic Surgeons score was 2.6% (1.7-4.2). Type I L-R BAV was observed in 72.5% of the patients. Evolut valve sizes 29 and 34 mm were utilised in 49.0% and 36.9% of the cases, respectively. The 30-day cardiac death rate was 2.6%; the 1-year cardiac death rate was 11.0%. Valve performance at 30 days was observed in 142/149 (95.3%) patients. The mean aortic valve area post-TAVI was 2.1 (1.8-2.6) cm2, and the mean aortic gradient was 7.2 (5.4-9.5) mmHg. No patient had more than moderate aortic regurgitation at 30 days. PPM was observed in 13/143 (9.1%) surviving patients and was severe in 2 patients (1.6%). Valve function was maintained at 1 year. The mean ellipticity index remained 1.3 (interquartile range 1.2-1.4). Overall, 30-day and 1-year clinical and echocardiography outcomes were similar between the two sizing strategies.
Conclusions: BIVOLUTX demonstrated a favourable bioprosthetic valve performance and good clinical outcomes after TAVI with the Evolut platform in patients with bicuspid aortic stenosis. No impact from the sizing methodology could be identified.
Introduction
Transcatheter aortic valve implantation (TAVI) is an established alternative to surgery for elderly patients with symptomatic aortic valve stenosis (AS) across the surgical risk spectrum1. Bicuspid aortic valve (BAV) is the most frequent congenital valvular disease affecting up to 2% of the general population2. Among patients undergoing surgical aortic valve replacement (SAVR) for AS in Western countries, BAV was found in 0.5% of cases3. The scientific background for TAVI in the context of BAV stenosis has, for a long time, been restricted to observational data with relatively small datasets. Studies evaluating first-generation transcatheter heart valves (THV) reported high rates of more than mild paravalvular leak (PVL), pacemaker implantation and aortic dissection and underscored the importance of multislice computed tomography (MSCT) planning in improving sizing and clinical outcomes456. TAVI results appeared comparable in selected BAV and regular tricuspid AS patients treated with newer-generation THV78. A recent, prospective study evaluating a self-expanding THV underscored the safety and efficacy of TAVI in low-risk patients with BAV9. However, selection bias and scarce prospective data are intrinsic limitations and preclude overall generalisability of the studies on TAVI in BAV. Bicuspid phenotypes with heavily calcified leaflets and a calcified raphe seem to be associated with impaired procedural success and worse clinical outcome. More research is needed to understand the role of MSCT in the identification and risk stratification of patients with BAV who may be suitable for TAVI.
The aim of the Bicuspid Aortic Stenosis With Evolut Platform International Experience (BIVOLUTX) registry was to evaluate the valve performance and clinical outcomes of TAVI with the self-expanding, supra-annular Evolut PRO prosthesis (Medtronic) in BAV patients and explore the impact of different computed tomography (CT) sizing algorithms510. ClinicalTrials.gov: NCT03495050.
Methods
BIVOLUTX was an investigator-initiated, international, multicentre, prospective registry including 150 consecutive patients with BAV who were scheduled for TAVI with the Evolut PRO (23, 26, 29 mm) and Evolut R (34 mm) THVs (Medtronic), in 14 countries across Europe and Canada. BAV anatomy was classified according to the Sievers classification1112. The indication for TAVI was based on local Heart Team consensus. Each patient provided written informed consent for the TAVI procedure, anonymous data collection and analysis. All data were collected in an electronic clinical report form (eCRF). The anatomical eligibility for TAVI with the Evolut platform was left to the operators’ discretion. From June 2018 to January 2020, a total of 3,777 patients underwent TAVI. Among them, 206 had BAV, and 152 were deemed eligible for inclusion in the BIVOLUTX trial. Two patients were excluded because of bailout TAVI with a balloon-expandable valve, and one patient withdrew consent. Finally, 149 were treated with the Evolut PRO and R 34 mm platforms and included in the registry (Supplementary Figure 1).
Inclusion criteria
Patients were included in the registry if they had a symptomatic BAV AS indicated for transfemoral TAVI, were over 18 years old, had anatomical suitability for the Evolut platform and had a life expectancy >1 year.
Study endpoints
The primary endpoint was valve performance, defined as a mean aortic valve gradient <20 mmHg or peak velocity <3 m/s and no moderate or severe prosthetic valve regurgitation at 30 days. An independent echocardiography core laboratory (Mayo Clinic) evaluated all echocardiograms.
Secondary endpoints were 30-day and 1-year all-cause and cardiovascular mortality, severe patient-prosthesis mismatch (PPM), defined as an aortic valve area (AVA) <0.6 cm2/m2, and the ellipticity index at 30 days. All study endpoints were adjudicated by an independent clinical event committee according to the updated Valve Academic Research Consortium 3 (VARC-3) criteria13.
Investigational transcatheter heart valve
Evolut PRO is the next iteration of the Medtronic CoreValve Evolut R platform. Briefly, it is a nitinol THV with a trileaflet porcine pericardium valve sutured in a supra-annular position. Evolut PRO has an external porcine pericardial wrap at the inflow level, designed to mitigate paravalvular regurgitation. The device is fully repositionable and retrievable before final detachment of the paddles and is available in three sizes (23, 26, 29 mm), covering aortic annuli from 18 to 26 mm. Evolut PRO and Evolut R XL were CE (European conformity)-marked in January and July 2017, respectively.
Sizing based on multislice computed tomography (MSCT)
BAV type was characterised according to the Sievers classification. The presence of a raphe was captured, including raphe length. The total amount of calcium in the aortic root was quantified and expressed as calcium volume. The perimeter and perimeter-derived diameter of the aortic annulus were the dimensions considered for THV size selection. The intercommissural distance (ICD) was measured at 4 mm above the aortic annulus, in an effort of standardisation and according to the Bicuspid Aortic Valve Anatomy and Relationship With Devices (BAVARD) algorithm (Figure 1)14. All analyses were performed using a dedicated software package (3Mensio; Pie Medical Imaging).
The ellipticity index was calculated as the ratio of the maximum and minimum annulus diameters (Dmax/Dmin). The more circular the aortic annulus is, the closer to 1 the ellipticity index is.
We prospectively recorded, for each patient, if sizing was based on the aortic annulus dimension alone, the ICD at 4 mm or an integration of both dimensions (combined sizing).
All MSCTs were locally analysed by the physicians for procedural planning and sent to a dedicated MSCT core laboratory (Medtronic) for additional uniform study-specific analyses.
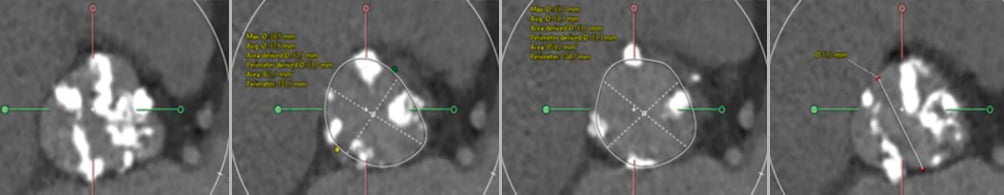
Figure 1. MSCT sizing. Various measurements at the level of the aortic root. From left to right: sinus of Valsalva, aortic annulus, LVOT and intercommissural distance (at 4 mm above the annulus). LVOT: left ventricular outflow tract; MSCT: multislice computed tomography
Statistical analysis
All patient data were anonymised and collected in a central eCRF. Statistical analyses were performed at the Erasmus University Medical Center, Rotterdam, the Netherlands. For continuous variables, normality of the distribution was assessed by means of a Kolmogorov-Smirnov test. Normally distributed continuous variables were expressed as mean±standard deviation; non-normally distributed continuous variables were expressed as median and interquartile range (IQR, 25th-75th percentile). Categorical variables were expressed as numbers and percentages. Baseline, procedural and follow-up data of the two sizing-strategy groups were compared using the independent samples t-test for normally distributed continuous variables, the Mann-Whitney U test for non-normally distributed continuous variables, and the Chi-square test or Fisher’s exact test for categorical variables, as appropriate. We indicated the number of available measurements in the tables, and, in case of missing data, the nature of the missing data was further specified in the footnotes. All analyses were conducted using SPSS software, version 20.0 (IBM).
Results
Baseline clinical characteristics of the study population
Between June 2018 and January 2020, a total of 3,777 patients were treated with TAVI in 14 countries across Europe and Canada, and 206 (5.45%) had a BAV. Among these BAV patients, 149 (72.3%) were treated with the Evolut PRO and R 34 platforms and included in BIVOLUTX. Baseline characteristics of the study population are presented in Table 1. The patients were predominantly male (63.1%), with a mean age of 78.4±7.5 years. The mean Society of Thoracic Surgeons (STS) score was 2.6% (1.7-4.2) and the mean European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 2.8% (1.7-4.2). The mean aortic valve area (AVA) was 0.7 (0.6-0.9) cm2, with a mean peak velocity of 4.2 (3.9-4.5) m/s, and the mean aortic valve gradient was 45.5 (38.3-52.8) mmHg.
Table 1. Baseline characteristics.
| Baseline data | n=149 | Annular sizing n=77 | Combined sizing n=72 | p-value | |
|---|---|---|---|---|---|
| Age at procedure, years | 78.4±7.5 | 76.2±8.0 | 80.7±6.1 | 0.03 | |
| Male gender | 94 (63.1) | 47 (61.0) | 47 (65.3) | 0.6 | |
| BMI, kg/m2 | 25.9±5.6 | 26.1±5.1 | 25.6±6 | 0.4 | |
| BSA, m2 | 1.8±0.2 | 1.8±0.2 | 1.8±0.2 | 0.9 | |
| Ischaemic heart disease | 47 (31.5) | 24 (31.2) | 23 (31.9) | 0.6 | |
| PAD | 14 (9.4) | 6 (7.8) | 8 (11.1) | 0.6 | |
| Previous valve surgery | 1 (0.7) | 1 (1.3) | 0 (0) | - | |
| Other valve disease | 19 (12.8) | 14 (18.2) | 5 (6.9) | 0.1 | |
| Previous valvuloplasty | 2 (1.3) | 1 (1.3) | 1 (1.4) | - | |
| Ascending aorta, mm | 37.0±5.5 | 39.0±5.9 | 35.6±4.5 | 0.1 | |
| Porcelain aorta | 4 (2.7) | 3 (3.9) | 1 (1.4) | 0.4 | |
| Frailty | 62 (41.6) | 38 (49.4) | 24 (33.3) | 0.1 | |
| GFR <60 ml/min | 63 (42.3) | 30 (38.9) | 33 (45.8) | 0.4 | |
| COPD | 21 (14.1) | 12 (15.6) | 9 (12.5) | 0.6 | |
| NYHA Class | NYHA I | 7 (4.7) | 3 (3.9) | 4 (5.6) | 0.6 |
| NYHA II | 78 (52.3) | 40 (51.9) | 38 (52.8) | ||
| NYHA III | 59 (39.6) | 30 (38.9) | 29 (40.3) | ||
| NYHA IV | 5 (3.4) | 4 (5.2) | 1 (1.4) | ||
| STS score | 2.6 [1.7-4.2] | 2.2 [1.6-3.3] | 3.4 [2.2-5.2] | 0.001 | |
| EuroSCORE II | 2.8 [1.7-4.2] | 2.1 [1.4-3.9] | 3.2 [2.4-5.2] | 0.003 | |
| Syncope | 6 (4.0) | 1 (1.3) | 5 (6.9) | 0.1 | |
| Angina | 19 (12.8) | 16 (20.8) | 3 (4.2) | 0.003 | |
| ECG | |||||
| PM/ICD | 12 (8.1) | 6 (7.8) | 6 (8.3) | 1 | |
| Conduction disturbance/arrhythmias | Sinus rhythm | 127 (85.2) | 65 (84.4) | 62 (86.1) | 0.6 |
| Atrial fibrillation | 18 (12.1) | 9 (11.7) | 9 (12.5) | ||
| Other | 4 (2.7) | 3 (3.9) | 1 (1.4) | ||
| Left bundle branch block | 19 (12.8) | 6 (7.8) | 13 (18.1) | 0.1 | |
| Right bundle branch block | 10 (6.7) | 7 (9.1) | 3 (4.2) | 0.3 | |
| First-degree AV block | 16 (10.7) | 7 (9.1) | 9 (12.5) | 0.6 | |
| Coronary angiography | Coronary artery disease* | 61 (40.9) | 32 (41.6) | 29 (40.3) | 0.6 |
| Echocardiography data | |||||
| Septum, mm | 12.0 [10.9-14.0] | 12.0 [10.0-13.8] | 12.0 [11.0-14.0] | 0.1 | |
| LVEDD, mm | 51.0±9.0 | 52.3±8.9 | 50.4±9.1 | 0.5 | |
| Left ventricular EF, % | 60 [45-65] | 60 [45-65] | 60 [50-65] | 1 | |
| Low-flow, low-gradient | 35 (23.8) | 15 (19.5) | 20 (28.6) | 0.2 | |
| Aortic valve area, cm2 | 0.7 [0.6-0.9] | 0.8 [0.6-0.9] | 0.7 [0.6-0.9] | 0.2 | |
| Indexed aortic valve area, cm2 | 0.4 [0.3-0.5] | 0.4 [0.3-0.5] | 0.4 [0.3-0.5] | 0.4 | |
| Peak aortic velocity, m/s | 4.2 [3.9-4.5] | 4.2 [4.0-4.5] | 4.2 [4.0-4.5] | 0.9 | |
| Mean aortic gradient, mmHg | 45.5 [38.3-52.8] | 45.9 [39.0-52.9] | 45 [37.4-53.0] | 0.8 | |
| Aortic regurgitation* | 100 (67.1) | 54 (70.1) | 46 (63.9) | 0.5 | |
| - Trivial | 26 (17.6) | 16 (29.6) | 10 (22.2) | 0.2 | |
| - Mild | 56 (37.8) | 31 (57.4) | 26 (56.5) | ||
| - Mild to moderate | 13 (8.8) | 5 (9.3) | 8 (17.8) | ||
| - Moderate | 3 (2.0) | 1 (1.9) | 2 (4.4) | ||
| - Moderate to severe | 1 (0.7) | 1 (1.9) | 0 (0) | ||
| - Severe | 0 (0) | 0 (0) | 0 (0) | ||
| Pulmonary hypertension (>60 mmHg) | 5 (3.4) | 0 (0) | 5 (6.9) | 0.04 | |
| Data are presented as mean±SD, n (%) or median [IQR]. *One missing patient. AV: atrioventricular; BMI: body mass index; BSA: body surface area; COPD: chronic obstructive pulmonary disease; ECG: electrocardiogram; EF: ejection fraction; EuroSCORE: European System for Cardiac Operative Risk Stratification; GFR: glomerular filtration range; ICD: implantable cardiac defibrillator; IQR: interquartile range; LVEDD: left ventricular end-diastolic diameter; NOAC: non-oral anticoagulant; NYHA: New York Heart Association; PAD: peripheral arterial disease; PM: pacemaker; SD: standard deviation; STS: Society of Thoracic Surgeons | |||||
Baseline MSCT and sizing strategies
Preprocedural MSCT details are synthesised in Table 2 and Figure 1. Overall, the sizing strategy followed two different methodologies, as per operators’ discretion. For 77 patients (51.7%), the sizing was according to the annulus size; for 72 patients (48.3%), THV size was determined by the combination of annular and supra-annular dimensions. The most frequent BAV phenotype was Type 1 L-R (72.5%), while Type 0 and Type 2 phenotypes were encountered in 10.1% and 3.3% of the patients, respectively (Figure 2). A calcified raphe was identified in 52 patients (34.9%). The mean perimeter-derived diameter (PDd) was 24.6±2.0 mm. The annular baseline ellipticity index was 1.3±0.1. The mean ICD 4 mm above the aortic annulus was 27.4±1.7 mm.
Table 2. MSCT baseline characteristics.
| Baseline MSCT analysis | n=149 | |
|---|---|---|
| Sizing strategy | Based on annulus | 77 (51.7) |
| Combination of annulus and ICD | 72 (48.3) | |
| BAV anatomy | - Type 0 | 15 (10.1) |
| - Type 1 L-R | 108 (72.5) | |
| - Type 1 R-N | 20 (13.4) | |
| - Type 1 L-N | 1 (0.7) | |
| - Type 2 | 5 (3.4) | |
| Calcified raphe | 52 (34.9) | |
| Perimeter-derived annulus diameter, mm | 24.6±2.0 | |
| Area-derived annulus diameter, mm | 25.0±2.3 | |
| Ellipticity index at the aortic annulus | 1.3±0.1 | |
| ICD 4 mm above annulus, mm | 27.4±1.7 | |
| LVOT 4 mm below annulus, mm | 25.8±3.0 | |
| Calcium score 650 HU, mm3 | 1,423.8±628.8 | |
| Sinus of Valsalva diameter, mm | 33.5±2.2 | |
| Right coronary artery height, mm | 17.5±3.5 | |
| Left coronary artery height, mm | 11.5±2.1 | |
| Sinotubular junction diameter, mm | 31.5±3.5 | |
| Ascending aorta, mm | 35.8±3.7 | |
| Data are presented as n (%) or mean±SD. BAV: bicuspid aortic valve; HU: Hounsfield units; ICD: intercommissural distance; LVOT: left ventricular outflow tract; MSCT: multislice computed tomography; SD: standard deviation | ||
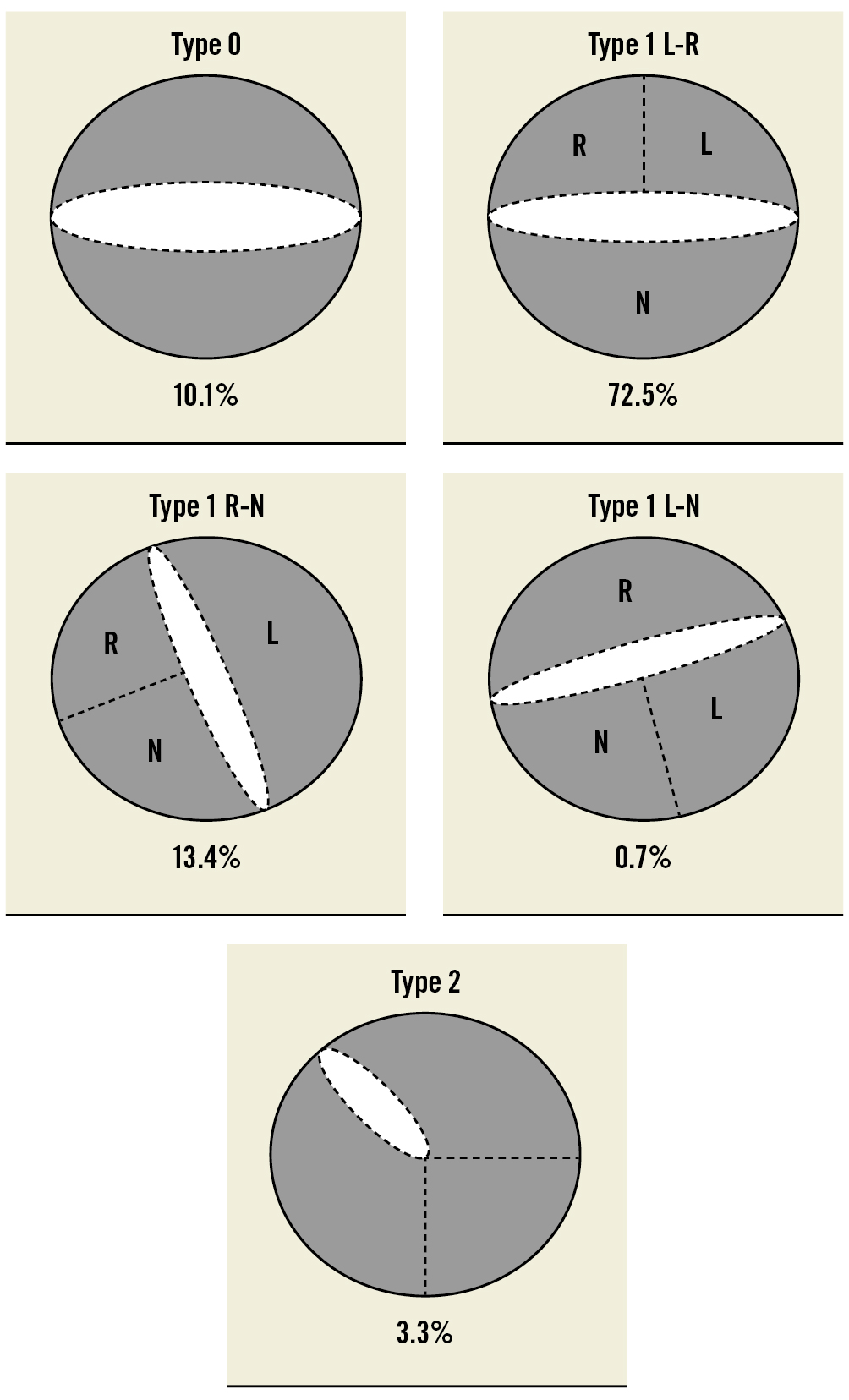
Figure 2. Distribution of BAV phenotypes. BAV: bicuspid aortic valve
Procedural details
Procedural details are reported in Supplementary Table 1. The predominant Evolut valve sizes utilised were 29 and 34 mm (49.0% and 36.9%, respectively). Balloon predilatation was applied in 130 cases (87.2%). THV repositioning for implant depth optimisation was required in 45 patients (30.2%). Three patients (2.0%) received more than one THV due to initial malpositioning. Post-dilatation was performed in 83 patients (55.7%). One patient died during the procedure because of a wire-induced left ventricle perforation. Overall procedural details were similar for patients with annular-based sizing and combined sizing strategies.
Thirty-day and one-year clinical outcomes
Thirty-day and 1-year clinical outcomes are summarised in Supplementary Table 2 and Table 3.
The 30-day cardiac and non-cardiac death rates were 2.6% and 1.3%, respectively. Seven patients (4.6%) experienced a stroke, categorised as disabling in 5. Six patients (4.0%) experienced a major vascular complication. New pacemaker implantation was necessary for 29 patients (19.5%). Device success was achieved in 136/149 (91.3%) cases. The reasons for not achieving device success were TAVI-in-TAVI (3 patients, 2.0%), cardiac tamponade (4 patients, 2.7%) necessitating conversion to open surgery in two cases, procedural mortality (1 patient, 0.7%), ventricular septal perforation (2 patients, 1.3%) and mitral valve apparatus damage (3 patients, 2.0%). Most patients were in NYHA Functional Class I/II at 30 days (145/97, 3%).
At 1 year, 13 patients were lost to follow-up, and 15 patients (11.0%) had died. Deaths were cardiovascular in 5 patients (3.3%). Stroke occurred in 9 patients (7.1%), disabling in 6. A new pacemaker implantation was needed for 33 patients (25.6%), mostly due to high-degree AV block post-procedure. Two patients underwent an uneventful percutaneous coronary revascularisation. Three patients (2.4%) underwent a redo-TAVI procedure, and two patients (1.4%) required correction of a symptomatic ventricular septal defect, one patient had a percutaneous closure of a septal defect. One patient had a pericardiocentesis for cardiac tamponade post-pacemaker implantation. Two patients (1.6%) had endocarditis and one patient (0.8%) had subclinical valve thrombosis.
Table 3. One-year clinical and echocardiographic follow-up.
| Clinical data | n=136 | Annular sizing n=70 |
ICD/combined sizing n=66 |
p-value |
|---|---|---|---|---|
| Mortality | 15 (11.0) | 9 (12.9) | 6 (9.1) | |
| - Cardiovascular mortality | 5 (3.3) | 3 (3.8) | 2 (2.7) | - |
| - Non-cardiovascular mortality | 7 (4.6) | 4 (5.1) | 3 (4.1) | - |
| - Unknown | 3 (2.0) | 2 (2.5) | 1 (1.3) | |
| n=126 | n=66 | n=60 | ||
| Myocardial infarction | 1 (0.8) | 1 (1.5) | 0 (0) | - |
| n=129 | n=67 | n=62 | ||
| Neurological events | - | |||
| - TIA | 1 (0.8) | 1 (1.5) | 0 | - |
| - Non-disabling stroke | 2 (1.6) | 1 (1.5) | 1 (1.6) | - |
| - Disabling stroke | 6 (4.7) | 4 (6.0) | 2 (3.2) | - |
| n=124 | n=65 | n=59 | ||
| Acute kidney injury | 3 (2.4) | 3 (4.6) | 0 | - |
| n=130 | n=68 | n=62 | ||
| Bleeding | - | |||
| - Major bleeding | 10 (7.7) | 5 (7.4) | 5 (8.1) | - |
| - Minor bleeding | 7 (5.4) | 2 (2.9) | 5 (8.1) | - |
| - Life threatening bleeding | 5 (3.8) | 4 (5.9) 1 (1.6) | - | |
| n=125 | n=65 | n=60 | ||
| Vascular complications | 13 (10.4) | 7 (10.8) | 6 (10.0) | - |
| - Major vascular complications | 6 (4.8) | 3 (4.4) | 3 (5.0) | |
| - Minor vascular complications | 7 (5.6) | 4 (5.8) | 3 (5.0) | - |
| - Percutaneous closure device failure | 0 (0) | 0 (0) | 0 (0) | |
| n=129 | n=68 | n=61 | ||
| Conduction disturbances and arrhythmias | 57 (44.2) | 34 (50) | 23 (37.7) | |
| n=129 | n=67 | n=62 | ||
| Pacemaker implantation | 33 (25.6) | 15 (22.4) | 18 (29) | - |
| High-degree AV block | (13.1) | 9 (15.0) | 8 (12.9) | |
| Atrial fibrillation | 0 (0) | 0 (0) | 0 (0) | |
| Bradycardia | 4 (3.1) | 3 (5.0) | 1 (1.6) | |
| LBBB or RBBB + first-degree AV block, n (%) | 9 (6.9) | 3 (5.0) | 6 (9.6) | |
| Other, n (%) | 2 (1.5) | 0 (0) | 2 (3.2) | |
| n=125 | n=65 | n=60 | ||
| Coronary obstruction | 1 (0.8) | 0 (0) | 1 (1.7) | - |
| n=124 | n=65 | n=59 | ||
| Endocarditis | 2 (1.6) | 1 (1.5) | 1 (1.7) | |
| n=126 | n=65 | n=61 | ||
| Valve thrombosis | 1 (0.8) | 0 (0) | 1 (1.6) | |
| n=129 | n=66 | n=63 | ||
| Rehospitalisation | 36 (27.9) | 19 (28.8) | 17 (27.0) | |
| n=125 | n=65 | n=60 | ||
| TAVI-in-TAVI (all periprocedural) | 3 (2.4) | 3 (4.6) | 0 (0) | - |
| n=127 | n=66 | n=61 | ||
| Cardiac tamponade | 4 (3.1) | 3 (4.5) | 1 (1.6) | |
| n=126 | n=66 | n=60 | ||
| Conversion to open surgery | 2 (2.6) | 2 (3) | 0 (0) | - |
| n=126 | n=65 | n=61 | ||
| New onset or worsening of heart failure | 5 (4) | 4 (6.2) | 1 (1.6) | |
| n=125 | n=65 | n=60 | ||
| New cardiac intervention | 3 (2.4) | 2 (3.1) | 1 (1.7) | |
| Echocardiography data | n=149 | Annular sizing n=77 | ICD/combined sizing n=72 | p-value |
| n=101 | n=51 | n=50 | ||
| Septum, mm | 11 [10.0-13.0] | 11 [10.0-12.9] | 12 [9.8-14.0] | 0.2 |
| n=104 | n=53 | n=51 | ||
| LVEDD, mm | 49.1±8.4 | 48.3±9.1 | 51.0±7.5 | 0.4 |
| n=16 | n=58 | n=58 | ||
| LVEF, % | 61 [55-65] | 60 [50-65] | 62 [57-65] | 0.5 |
| n=99 | n=50 | n=49 | ||
| AVA, cm2 | 2.1 [1.8-2.5] | 2.0 [1.8-2.5] | 2.2 [1.8-2.5] | 0.5 |
| n=97 | n=50 | n=47 | ||
| AVA index | 1.1 [0.9-1.4] | 1.1 [0.9-1.4] | 1.2 [1.0-1.4] | 0.3 |
| n=109 | n=52 | n=57 | ||
| Peak aortic velocity, m/s | 2.0 [1.7-2.2] | 2.0 [1.7-2.3] | 1.9 [1.6-2.2] | 0.3 |
| n=118 | n=55 | n=58 | ||
| Mean aortic gradient, mmHg | 8.1 [6.2-11.1] | 8.7 [6.4-11.3] | 8.0 [5.3-10.9] | 0.3 |
| n=115 | n=58 | n=57 | ||
| Prosthetic valve regurgitation | 62 (53.9) | 31 (53.4) | 31 (54.4) | 0.5 |
| - Trivial | 26/62 (22.6) | 17/31 (54.8) | 9/31 (29.0) | 0.5 |
| - Mild | 29/62 (25.2) | 10/31 (32.3) | 19/31 (61.3) | |
| - Mild to moderate | 3/62 (2.6) | 3/31 (9.7) | 0 (0) | |
| - Moderate | 3/62 (2.6) | 1/31 (3.2) | 2/31 (6.5) | |
| - Moderate to severe | 1/62 (1.6) | 0 (0) | 1/31 (3.2) | |
| - Severe | 0 (0) | 0 (0) | 0 (0) | |
| n=78 | n=45 | n=33 | ||
| Patient-prosthesis mismatch | 9 (11.5) | 6 (13.3) | 3 (9.1) | 0.4 |
| - PPM BMI ≤30 | ||||
| Moderate 0.85-0.65 | 6 (7.6) | 5 (11.1) | 1 (3.0) | |
| Severe <0.65 | 3 (3.8) | 1 (2.2) | 2 (6.0) | |
| - PPM BMI >30 | 0 (0) | 0 (0) | 0 (0) | |
| n=96 | n=52 | n=44 | ||
| Doppler velocity index | 0.5 [0.4-0.6] | 0.5 [0.4-0.6] | 0.5 [0.4-0.6] | 1 |
| Data are presented as n (%), mean±SD, or median [IQR]. AV: atrioventricular; AVA: aortic valve area; BMI: body mass index; EOA: effective orifice area; ICD: intercomissural distance; IQR: interquartile range; LBBB: left bundle branch block; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; PM: pacemaker; PPM: patient-prosthesis mismatch; SD: standard deviation; RBBB: right bundle branch block; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack | ||||
Transcatheter valve performance
At 30 days, the primary endpoint of valve performance was observed in 142/149 (95.3%) patients: 74/77 (96.1%) vs 68/72 (94.4%); p=0.9, in the annular and combined sizing groups, respectively. The mean AVA post-TAVI was 2.1 (1.8-2.6) cm2, the mean indexed AVA index was 1.2 (1.0-1.5) cm2, and the mean aortic gradient was 7.2 (5.4-9.5) mmHg. Aortic regurgitation, predominantly paravalvular, was trivial for 8 patients (7.5%), mild in 86 patients (81.1%), mild to moderate in 9 patients (8.5%) and moderate in 3 patients (2.8%). Patient-prosthesis mismatch (PPM) was observed in 13/143 (9.0%) surviving patients and was severe in 2 patients (1.3%). Valve function was maintained at 1 year as illustrated by a mean AVA of 2.1 (1.8-2.5) cm2, an indexed AVA of 1.1 (0.9-1.4), and a mean aortic gradient of 8.1 (6.2-11.1) mmHg (Supplementary Figure 2). One patient (1.6%) had moderate to severe regurgitation. Three patients (4.8%) had severe PPM.
Post-TAVI MSCT was obtained at 30 days for 101/149 patients. The mean ellipticity index remained 1.3 (IQR 1.2-1.4).
Overall, 30-day and 1-year clinical and echocardiography outcomes were similar between the sizing strategies (Central illustration).
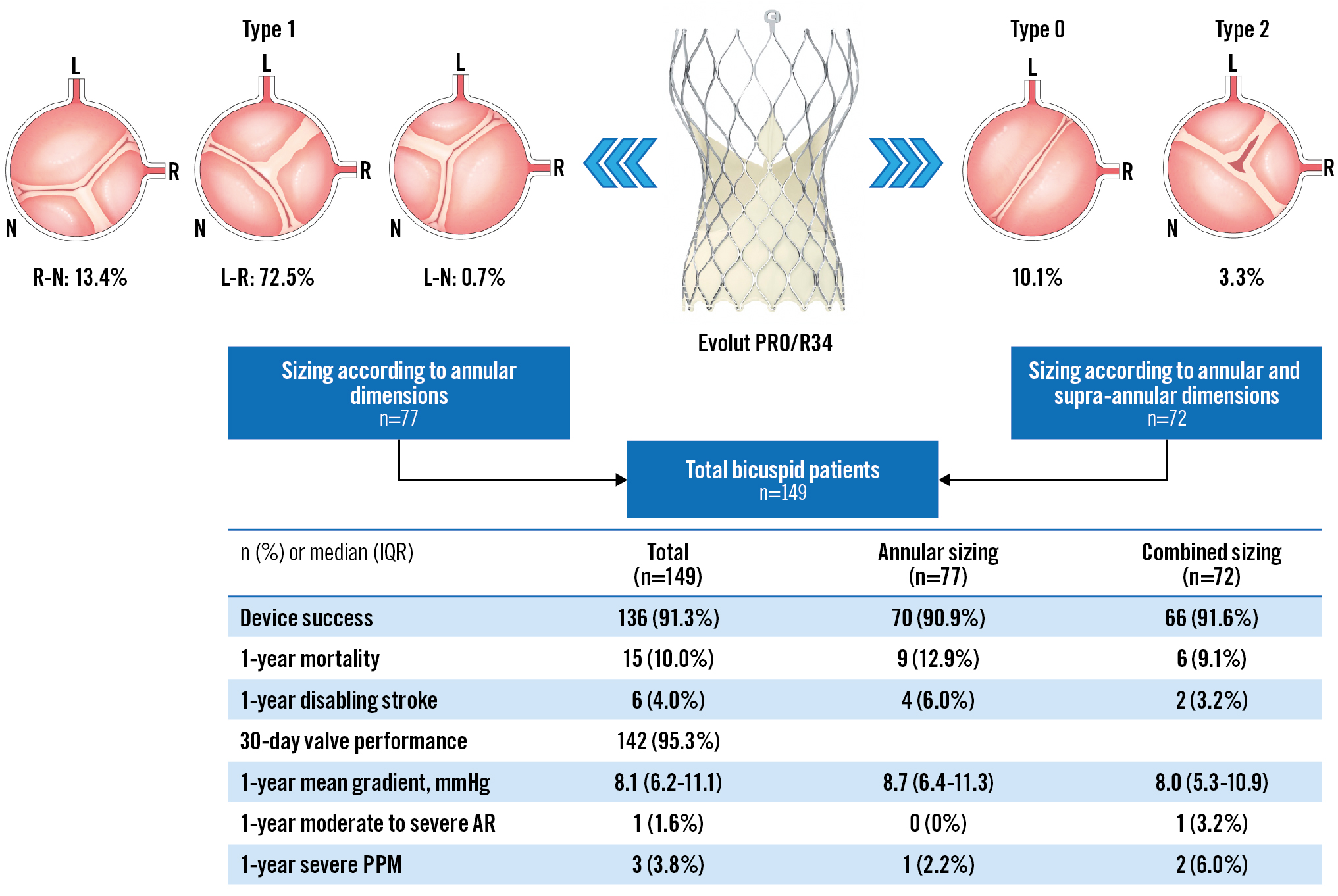
Central illustration. Clinical outcomes after TAVI with Evolut PRO/R34 in bicuspid patients. AR: aortic regurgitation; IQR: interquartile range; PPM: patient-prosthesis mismatch; TAVI: transcatheter aortic valve implantation
Discussion
BIVOLUTX evaluated TAVI with the self-expanding supra-annular Evolut PRO and Evolut R34 in patients with symptomatic severe bicuspid aortic valve stenosis. The main findings of our registry may be summarised as follows: 1) TAVI with this self-expanding platform for BAV resulted in a device success rate of more than 90%. 2) Optimal haemodynamic valve performance was confirmed by a mean transvalvular gradient <10 mmHg and a peak velocity <2 m/s at 30-day and 1-year follow-up. 3) There were no differences in clinical outcome or device success rate between the sizing strategies at the annular level or with the combination of annular and intercommissural dimensions. 4) Neurological events and conduction disorders requiring new pacemakers were, however, quite significant.
The prospective, single-arm Medtronic Transcatheter Aortic Valve Replacement (TAVR) Low Risk Bicuspid Study enrolled 150 patients, with a mean age of 70 years and an STS Predicted Risk of Mortality (PROM) of 1.4, who underwent TAVI with the Evolut platform for severe BAV AS. All patients were evaluated by a screening committee to ensure patient eligibility for the study and anatomical suitability15. In BIVOLUTX, there was no screening committee, patients were on average 8 years older and had higher operative risk, which may have resulted in a better representation of current clinical practice. Similar haemodynamics (effective orifice area [EOA], mean gradient and regurgitation) were observed in both registries, strengthening the suitability of the Evolut platform for TAVI in various BAV anatomies. Several Evolut PRO features may help explain this excellent valve performance. THV repositioning to optimise the final implant depth was carried out in one-third of the procedures; the supra-annular position of the leaflets potentially helped to achieve large EOAs and low transprosthetic gradients; the sealing wrap mitigated perivalvular regurgitation. The overall circularity of the device may be favoured by the combination of systematic predilatation and frequent post-dilatation1617.
Overall, the outcomes after TAVI in BAV patients may be affected by anatomical characteristics, such as the calcium burden and the presence of a calcified raphe, which can preclude optimal device expansion and impact short- and long-term outcomes. Recently, Yoon et al analysed the calcium burden and distribution in 1,034 Type 0 and Type 1 BAV patients who underwent TAVI with commercially available TAVI platforms. The presence of a calcified raphe and excessive leaflet calcification were associated with increased rates of aortic root injury, moderate or severe paravalvular regurgitation, stroke, and all-cause mortality at 30 days. The mean calcium volume was an independent predictor of 2-year mortality7. Thus, valve morphology and calcification need to be thoroughly analysed before performing TAVI in BAV patients. Calcium burden and distribution are thought to be a risk factor for stroke7. We reported a 4.6% stroke rate in BIVOLUTX, which is almost equal to the 4% stroke rate in the Medtronic TAVR Low Risk Bicuspid Study, although higher than the rates reported in other recent studies18. Reassuringly, the Transcatheter Valve Therapy (TVT) Registry, which compared mortality and stroke at 30 days in 3,243 bicuspid and 34,417 tricuspid patients undergoing TAVI with a third-generation balloon-expandable THV, reported no significant differences between the two groups of patients19. The stroke rate in the BIVOLUTX population could be related not only to the high calcium amount but also to the high percentage of pre- and post-dilation required in more than 50% of patients.
Indeed, the risk and impact of disabling stroke in younger BAV patients may be an argument for the use of cerebral embolic protection devices20.
Implantation of a new pacemaker was required in 19% of patients at 30 days and 25% at 1 year, which is more frequent than what is usually reported for tricuspid AS patients; but was similar to the 15% pacemaker rate at 30 days in the Medtronic TAVR Low Risk Bicuspid Study. The systematic use of an adapted cusp overlap technique may be a way to further decrease the need for a permanent pacemaker post-TAVI in BAV patients21. This technique, not systematically utilised at the time of enrolment in our registry, promotes high implants with minimal interaction of the inflow portion of the stent frame with the conduction system. A dedicated study focusing on bicuspid aortic valves using the cusp overlap technique is required.
To date, BAV anatomy is not yet included as a variable for the decision-making between TAVI and SAVR for AS, and the existing data are mostly derived from retrospective studies comparing surgery and percutaneous treatment, since there are no randomised trials comparing SAVR and TAVI for BAV. Thus, the decision to perform TAVI in BAV is currently based on expert opinion and may require experienced TAVI operators2223.
The most appropriate MSCT sizing algorithm in the context of TAVI for BAV remains a matter of debate. As proposed by the BAVARD registry, some operators select the valve size based solely on the aortic annulus dimension, others combine annular and supra-annular dimensions. In our registry, similar procedural and clinical outcomes were observed when comparing annular-based sizing and combined sizing.
Another topic which remains controversial is the type of device to utilise in BAV patients. A study, retrospectively comparing self-expanding versus balloon-expandable THVs (SAPIEN 3 [Edwards Lifesciences] and Evolut R/PRO platforms) in BAV, identified a higher rate of moderate to severe paravalvular regurgitation with self-expanding THVs and a higher rate of annular rupture with balloon-expandable THVs at 1-year follow-up24. Our data confirm improved procedural and clinical outcomes with contemporary techniques using the Evolut PRO and R 34 platforms in BAV.
Limitations
Our study has several limitations. The decision to proceed with TAVI using the Evolut platform was per the local Heart Team’s discretion and, therefore, introduces selection bias. BAV phenotypes were predominantly Sievers Type 0 or Type 1 (including a raphe between the left and right cusps), which are more common in the Western world. Our findings should not be extrapolated to other BAV phenotypes and/or other TAVI platforms. The sample size did not allow any comparison between bicuspid phenotypes. MSCT on depth sizing and outcomes analyses will be the focus of a dedicated manuscript.
Conclusions
BIVOLUTX demonstrated a favourable bioprosthetic valve performance and good clinical outcomes after TAVI with the Evolut platform in patients with bicuspid aortic stenosis. Further efforts should focus on reducing conduction disorders and neurological events.
Impact on daily practice
Selected patients with severe bicuspid aortic stenosis can be effectively treated with TAVI using the self-expanding Evolut platform.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Funding
Medtronic provided a research grant to Clinique Pasteur to conduct the trial. The clinical research organisation was Axiodis, France.
Conflict of interest statement
N. Van Mieghem has received research grant support from Abbott Vascular, Biotronik, Boston Scientific, Medtronic, Edwards Lifesciences, Daiichi Sankyo, AstraZeneca, Teleflex, and PulseCath BV; and advisory fees from Anteris, JenaValve, Abiomed, Abbott Vascular, Biotronik, Boston Scientific, Medtronic, Amgen, Daiichi Sankyo, AstraZeneca, Teleflex, PulseCath BV, and Siemens. D. Mylotte is a consultant for Medtronic, Boston Scientfic, and MicroPort. N. Piazza is a consultant to Medtronic, Peijia, and PiCardia. L. Leroux is a proctor for Medtronic and Abbott and a consultant for Edwards Lifesciences. O. De Backer has received institutional research grants and consulting fees from Abbott, Boston Scientific, and Medtronic. N. Dumonteil has received consultancy and proctoring fees from Abbott Vascular, Boston Scientific, Edwards Lifesciences, and Medtronic. D. Tchétché is a consultant for Abbott Vascular, Edwards Lifesciences, Medtronic, Boston Scientific, T-Heart, and Caranx Medical. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

