
Introduction
Transcatheter aortic valve implantation (TAVI) has become a well-established treatment option for patients with symptomatic severe aortic stenosis. Over the last decade, there have been many design developments aimed at improving TAVI performance, including the availability of different valve sizes. In early 2017 the new 34 mm Evolut™ R valve (Medtronic, Minneapolis, MN, USA) was introduced in Germany, extending the annulus diameter range up to 30 mm. Large annuli are more challenging for TAVI procedures, since it takes longer to achieve complete anchoring, and a later anchoring during the process of implantation increases the risk of malpositioning. Therefore, the aim of this multicentre registry was to study the early procedural results with the novel 34 mm Evolut R valve.
Methods
We performed an intention-to-treat analysis on 124 consecutive patients scheduled for TAVI with the 34 mm Evolut R valve in three experienced high-volume centres between January and September 2017. Inclusion criteria were severe symptomatic aortic stenosis, a perimeter-derived annulus size of between 26 and 30 mm, a suitable transfemoral access vessel (minimum diameter of 5.5 to 6 mm, depending on calcification patterns) and a Heart Team decision favouring transfemoral TAVI.
All patients gave informed written consent to TAVI and data collection. The study protocol was in accordance with the local ethics committees. All patients received a mandatory cardiac and vascular computed tomography scan. Statistical analysis was performed with SPSS Statistics, Version 22 (IBM Corp., Armonk, NY, USA). In each case we performed a Fisher’s exact test to test for independency between two categorical variables, using a two-sided alpha level of 0.05.
Safety-related adverse events were adjudicated according to Valve Academic Research Consortium-2 definitions.
Results
A 34 mm Evolut R implantation was attempted in 124 patients. Mean age was 81.3±5.9 years. During the study period, 52% of all TAVI patients were female; however, most patients treated with the 34 mm Evolut R were male (89.5%), due to the fact that the female patients had smaller body sizes and thus smaller annuli than the male patients. Mean perimeter-derived annulus diameter was 27.5±1.3 mm. Most patients were in the intermediate surgical risk category (STS score 5.2±3.9%; EuroSCORE II 6.4±5.9%). Supplementary Table 1 summarises all baseline characteristics. In two patients, the prosthesis could not be implanted due to severe tortuosity of the iliac artery and aorta, despite the use of an additional sheath. Only transfemoral access was used. Thirty-day all-cause mortality was 2.4%, and 30-day disabling stroke was 1.6%. While immediate angiographic paravalvular regurgitation (PVR) ≥II° was 9%, pre-discharge PVR ≥II° on echocardiography was 1.6% (Figure 1), without any statistical relationship to the size or eccentricity of the annulus.
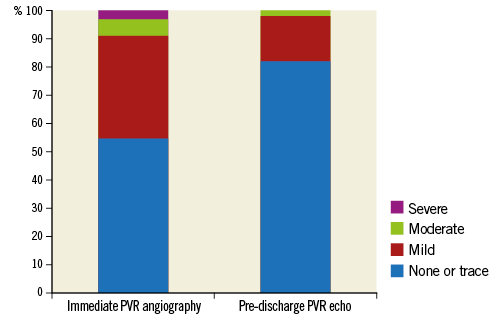
Figure 1. Immediate PVR per angiography and pre-discharge PVR per echocardiography. PVR: paravalvular regurgitation
The rate of new permanent pacemaker implantation (PPI) was 21.9%. In four patients, an additional 20 Fr sheath was used, due to tortuosity of the iliac arteries. The major vascular complications rate was 7.3%. Balloon predilatation was performed in 84.7% of cases. The resheath/recapture rate was 22.9%. An implantation depth of <4 mm was achieved in 65.6% of the patients. Implantation depth had no influence on the PVR (p=0.3) or PPI rate (p=0.6). Supplementary Table 2 summarises all procedural parameters.
Discussion
This is one of the first reports describing the early experience with the largest TAVI prosthesis presently on the market. It is well known that larger annuli pose relevant challenges for TAVI. Comparing the 34 mm Evolut R with its predecessor the 31 mm CoreValve (Medtronic), several important aspects have to be noted. The maximum diameter of a non-compressed 34 mm valve is 3 mm larger than that of the 31 mm valve, the lowest part of the valve is less conical in the 34 mm valve (thus allowing better adaptation to the left ventricular outflow tract), the overall cell and frame geometry has been altered (as seen with the other Evolut R prostheses) and, most importantly, the ability to resheath/recapture the valve has been added. Data from the predecessor 31 mm CoreValve showed partially unfavourable results: lower implantations, and higher paravalvular regurgitation and PPI rates1,2. Interestingly, in a previous publication of 54 patients receiving the 31 mm CoreValve1, most patient characteristics were similar to this registry with comparable age, gender distribution, comorbidities and risk scores. Nevertheless, this registry shows a clinically relevant reduction in pacemaker implantations and paravalvular regurgitation compared to the data on the 31 mm valve.
Most patients had an optimal implantation depth. Still, the rate of PPI was rather high compared to data of the smaller Evolut R valves3. However, compared to the predecessor 31 mm CoreValve, the numbers seem to be much lower (up to 35% in previous registries)1. A possible explanation is the more conical shape of the larger self-expanding Medtronic valves, in contrast to the more cylindrical shape of the smaller prostheses. This may cause a higher circumscribed stress to the conduction system.
Discharge PVR ≥II° on echocardiography was only 1.6%. This is most likely due to the small number of malpositionings and the strong radial force in the lowest portion of the frame. While the frame geometry would imply a higher rate of new PPI, even this number was not higher than previously reported for the smaller Evolut R valves4.
Most implantations were performed after predilatation of the valve. From a mechanical standpoint, this seems advisable, since the more conical shape of the lower portion of the frame poses the risk of pulling the valve into the left ventricle. A missing predilatation may further increase this risk if the mid portion of the frame is unable to expand fully due to strong valvular calcification. However, this is just a subjective evaluation, and only a randomised trial would be able to judge this procedural step fully.
In contrast to the smaller Evolut R valves, the 34 mm prosthesis requires a larger delivery catheter. Should an additional external sheath be required, a 20 Fr instead of an 18 Fr sheath would have to be used. However, as long as an additional sheath is not needed (in this registry just 1.4%), the vessel stress is still 2 Fr lower compared to the predecessor 31 mm CoreValve, which required an 18 Fr sheath.
Limitations
This registry is limited by its non-randomised design; however, no other valve on the market is approved for perimeter-derived diameters of up to 30 mm, which makes comparisons with other valves more difficult. Thus, this study has to function as a descriptive stand-alone registry, summarising early experiences with the 34 mm Evolut R. A comparison with studies using the 34 mm Evolut R is hardly possible, since very few data exist on this novel valve.
Conclusion
In conclusion, this registry shows comparable success and complication rates for the novel 34 mm Evolut R valve compared to the smaller Evolut R prostheses.
| Impact on daily practice This multicentre registry with the novel 34 mm Evolut R prosthesis demonstrated success and complication rates comparable to those of smaller valve sizes. Despite the challenging nature of larger annuli, ideal implantation depth and less than moderate PVR were achieved in a large proportion of these patients. |
Conflict of interest statement
R. Bekeredjian, A. Harnath, and F. Gatto report personal fees from Medtronic during the conduct of the study. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Baseline characteristics.
Supplementary Table 2. Procedural and clinical outcomes.
To read the full content of this article, please download the PDF.

