Abstract
The first member of the next generation CoreValve “family” is the 23 mm CoreValve® Evolut™ (Medtronic, Minneapolis, MN, USA), which is indicated for an annulus range of 18 mm to 20 mm and extends the spectrum of patients with aortic stenosis that can be treated with the self-expanding CoreValve bioprosthesis. The Core-Valve Evolut provides several technical refinements and is designed to enable re-capturability in the future.
Here, we report on the first case in a 93-year-old female patient who was implanted at the University Hospital Bonn, Germany, on June 1st, 2012.
Introduction
More than 60,000 patients underwent TAVI procedures worldwide and the dramatic growth in TAVI will possibly continue over the next few years. The next generation of transcatheter heart valves promises to simplify TAVI and addresses TAVI-related issues such as periprosthetic aortic regurgitation and conduction disturbances.
The Medtronic CoreValve® Evolut™ (Medtronic, Minneapolis, MN, USA) provides several refinements to improve anatomical fit, annular sealing, and durability (Figure 1). In addition, the device is designed to enable re-capturability and further improvements in annular fit in the future.
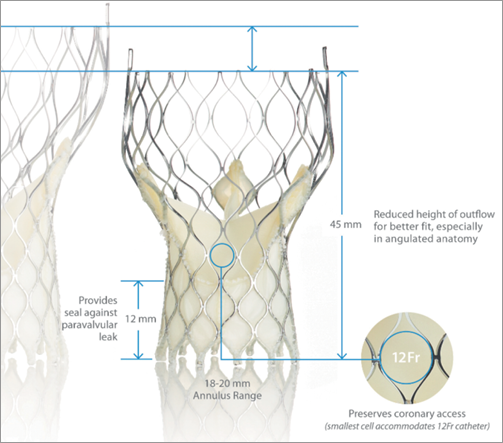
Figure 1. The new CoreValve Evolut compared to the third generation Medtronic CoreValve (Courtesy of Medtronic).
Cell design
The Evolut valve retains CoreValve’s cell design, facilitating access to the coronary arteries due to a larger cell design, while preserving conformability for fit in non-circular or calcified annuli.
Diameter
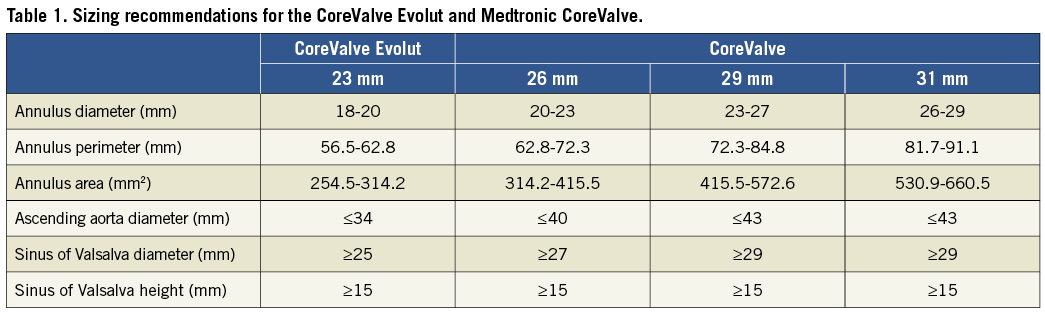
The 23 mm CoreValve Evolut valve is indicated for an annulus diameter range of 18 mm to 20 mm or a perimeter of 56.5 to 62.8 mm (Table 1). With the introduction of the full Evolut product family, annulus sizes from 18 mm to 30 mm can be treated. All valve sizes are delivered via an 18 Fr delivery catheter system.
Height
The Evolut frame is tailored to reduce overall height to 45 mm, approximately 10 mm shorter than the original CoreValve frame, while preserving the height of the pericardial skirt (12 mm) to provide a seal against paravalvular leakage. The shortened valve height is designed to optimise fit, especially in angulated anatomy.
Sealing interference and radial force
For the Evolut the sealing interference and radial force have been optimised to promote annular sealing. Sealing interference of the Evolut with the native aortic annulus is optimised within the range of the current CoreValve performance, eliminating the lowest interference range, since higher interference has been associated with reduced paravalvular leakage. In addition, the radial force for Evolut is more consistent across the full range of indicated annulus sizes.
Durability
The CoreValve Evolut retains the design characteristics that contribute to CoreValve durability, including supra-annular valve position, longer commissures, and porcine pericardial tissue. In addition, the new valve is treated with alpha-amino oleic acid (AOA). AOA binds to aldehyde groups within the pericardial tissue and, thus, inhibits calcification of the prosthetic valve leaflets. AOA has been shown to reduce both short- and long-term calcification. Positive results have been demonstrated with 20 years of clinical experience on surgical valves.
The first CoreValve Evolut was implanted in a 93-year-old female patient, who suffered from severe aortic stenosis with dyspnoea NYHA Class III, at the University of Bonn, Germany, on June 1st, 2012. This patient had a non-significant coronary 1-vessel disease with a left ventricular ejection fraction of 65%. Additional comorbidities included pulmonary hypertension (sPAP 65 mmHg) and chronic renal insufficiency resulting in a logistic EuroSCORE of 30% and a STS score of 4.9%.
Pre-interventional multislice computed tomography (MSCT) revealed an aortic annulus maximum and minimum diameter of 21.8×16.5 mm2 (mean diameter 19.2 mm) with a perimeter of 63 mm and an area of 291.2 mm2. Therefore, we decided to use the CoreValve Evolut 23 mm for transfemoral TAVI in this patient. The Evolut was delivered with the AccuTrack system via an Amplatz super stiff wire placed in the left ventricle (Figure 2). We decided to abandon pre-dilatation to reduce the risk of periprocedural complications such as annular rupture and pacemaker implantation and delivered the CoreValve Evolut uneventfully at the desired implantation depth of 4 mm (Figure 3 and Figure 4). No movement of the valve towards the ventricle or aorta was noted during delivery. With the implantation of the Evolut, a good post-procedural result with trace-to-mild paravalvular regurgitation in the final aortogram (Figure 5) was obtained with an AR Index of 32.1 and a remaining gradient of 4 mmHg (RRAorta: 140/65 mmHg; RRLV: 144/0-20 mmHg). In an orthogonal view of the prosthesis, a round-shaped inflow tract of the prosthesis with optimal frame expansion could be confirmed (Figure 6). Post-interventional echocardiography after three days confirmed a good procedural result and haemodynamic performance of the CoreValve Evolut with only trace paravalvular leakage.
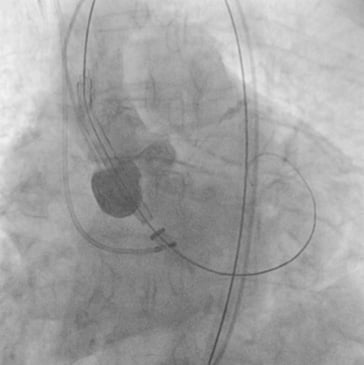
Figure 2. Aortic root angiography with the CoreValve Evolut advanced over the native aortic annulus without predilatation.
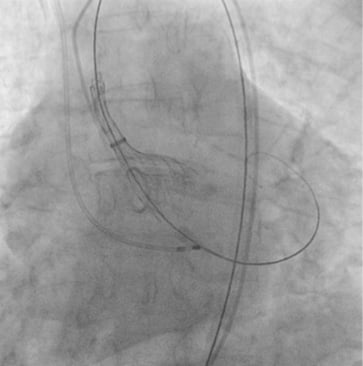
Figure 3. Deployment of the CoreValve Evolut.
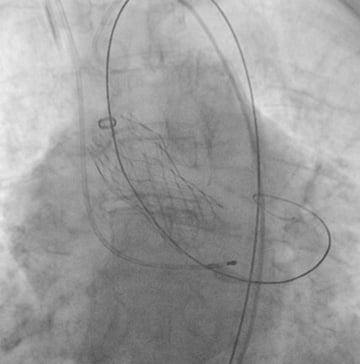
Figure 4. Final release of the CoreValve Evolut prosthesis.
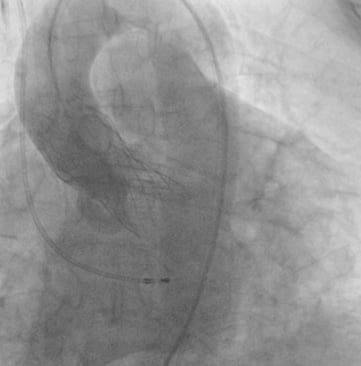
Figure 5. Aortography after full deployment of the CoreValve Evolut with trace-to-mild paravalvular regurgitation.
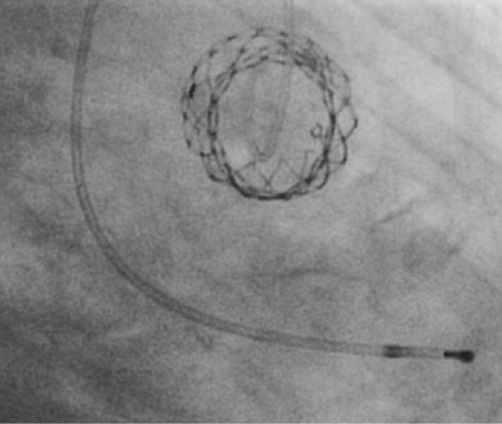
Figure 6. Orthogonal view of the CoreValve Evolut showing the round-shaped inflow tract after deployment without pre-dilatation.
To evaluate the impact of the refined valve design on TAVI-specific issues such as periprosthetic aortic regurgitation, conduction abnormalities, and even stroke –especially with respect to the small valvular anatomy– all patients who undergo TAVI with the new CoreValve Evolut 23 mm prosthesis will be prospectively included into a registry.
Conflict of interest statement
E. Grube is a proctor for CoreValve/Medtronic. The other authors have no conflicts of interest to declare.

