Abstract
Aims: Transcatheter aortic valve implantation (TAVI) is performed through a transarterial approach with encouraging results in «one-type valve» registries. We report 30-day data from a mixed population of patients treated with either Medtronic CoreValve® (MCV) or Edwards SAPIEN™ (ES) valves.
Methods and results: Forty-five patients had TAVI via the transarterial approach (21 MCV and 24 ES). Mean age was 81.8±4.2 years, Logistic EuroSCORE was 25.2±8.4%. Procedural success rate was 97.8%. In-hospital death rate was 4.4%. Vascular complication rate was 8.9%. Of MCV patients, 28.6% had a permanent pacemaker vs. 4.2% of ES patients; p=0.02. No additional deaths were observed between discharge and 30 days. NYHA functional class was improved at 30-days: 2.07±0.4 vs. 3.09±0.05, p<0.0001. Mean transvalvular gradient was lower: 9.5±3.28 mmHg vs. 41.9±14 mmHg, p<0.0001. Overall 30-day MACE rate was 8.9%, similar between MCV and ES patients.
Conclusion: A routine policy of TAVI using both MCV and ES valves is feasible without any worsening of procedural success rates and 30-day outcomes. A wider population of high risk patients with aortic stenosis can be offered a transarterial treatment. This could be the next standard of care for teams performing TAVI.
Abbreviations
AS: aortic stenosis
CFA: common femoral artery
MCV: Medtronic CoreValve®
EIA: external iliac artery
EOA: effective orifice area
ES: Edwards SAPIEN™
LVEF: left ventricular ejection fraction
MACE: major adverse cardiovascular events
PM: pacemaker
STS: Society of Thoracic Surgery
TAVI: transcatheter aortic valve implantation
TEE: transoesophageal echocardiography
TTE: transthoracic echocardiography
Background
Transcatheter aortic valve implantation (TAVI) is a recently developed technique for patients with symptomatic AS, deemed inoperable or at too high risk for open chest surgery1. Alain Cribier performed the first-in-man implantation of a percutaneous aortic valve prosthesis in a 57 year-old man in cardiogenic shock in 20022. Since this initial experience, after important improvements in technique and devices, more than 5,000 TAVI procedures have been performed worldwide3. Two prostheses received CE mark in 2007: Medtronic CoreValve® (MCV) and Edwards SAPIEN™ (ES) valves. Both valves can be implanted through a transarterial approach (MCV and ES), a transapical approach (ES) or subclavian access (MCV). For transarterial implantation, ES requires a surgical cut-down of the skin and exposure of the common femoral artery (CFA) or external iliac artery (EIA) before insertion of a 22 Fr or 24 Fr sheath4, or at least a surgical repair of the entry artery at the end of the procedure. MCV is delivered in a fully percutaneous transarterial procedure, without any surgical cut-down before insertion of 18 Fr sheath5. In the target high risk and aged population, TAVI can be performed through a transarterial approach with good procedural success rates and acceptable short term outcomes. Procedural success rates up to 92% have been reported, with a 30-day mortality range between 9.3 and 15%6,7. Considering the large diameter of introducer sheaths, vascular complications significantly contribute to 30-day mortality6,8. Full surgical management of entry artery and use of a pre-closure device have been suggested to reduce the vascular complication rate. However, there are no published data comparing both strategies. Due to the relatively long learning curve for TAVI, most teams select one manufacturer to develop a sufficient skill set with one type of device. Therefore, most of the available data are related to patients treated with either ES or MCV. We present the 30-day major adverse cardiovascular events (MACE) and vascular complications of 45 patients from a mixed cohort treated with both ES and MCV valves, with respectively surgical arterial management or full percutaneous approach.
Method
Between July 11, 2007 and June 30, 2009, 65 selected patients were treated with TAVI at two French centres in Toulouse: Clinique Pasteur and Hôpital Rangueil. Forty and 25 patients were respectively implanted in both hospitals. Clinique Pasteur started to implant MCV prosthesis in July 2007 then, after 15 patients, added the ES valve to its standard practice in December 2008 while continuing to implant MCV. Hôpital Rangueil started its implantation in July 2008 with ES prosthesis and referred patients for MCV, if unsuitable for ES due to large aortic annulus or small CFA. Both centres, working as one multidisciplinary team, decided to pool their data in a unique registry due to the similarity of their cardiological and surgical standards of care. A common database was constructed and prospectively updated. From this initial cohort, the results of 45 patients treated through transarterial access are presented. All events were locally adjudicated during regular multidisciplinary meetings. The results concerning 19 patients treated through transapical and one patient treated through subclavian artery are not discussed here.
Patient selection
Consecutive patients referred to either hospital for discussion of TAVI were screened for feasibility of the procedure. The screening process included transthoracic (TTE) and/or transoesophageal echocardiography (TEE), coronary angiography, aortic and iliofemoral angiography or CT scan, pulmonary functional tests, blood tests assessment and carotid artery Doppler. A CFA diameter of at least 6 mm for MCV and 8-9 mm for ES was required. Patients with smaller arteries were considered for a transapical or subclavian approach using respectively ES and MCV. The final decision on TAVI as well as the access site was taken at a multidisciplinary meeting including cardiologists (interventional and echocardiographer), anaesthesiologists and cardiovascular surgeons (Figure 1). Eligible patients were those with symptomatic AS and a EuroSCORE>20% or STS score>10%. Those with lower risk scores had comorbidities contraindicating open heart surgery, e.g., porcelain aorta, history of chest radiation, severe lung disease or renal impairment. Patients were finally selected according to the feasibility of TAVI procedure with an acceptable risk of MACE. This selection was mandatory due to a lack of evidence for treating other patients and the lack of reimbursement for MCV and ES valves in France, resulting in a limited number of available devices.
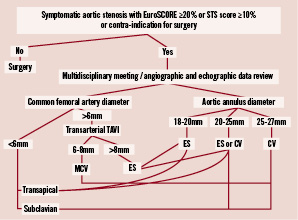
Figure 1. This is the flowchart – used during the most recent period of time – for transcatheter aortic valve decisions, choice of the bioprosthesis and access site selection. TAVI: transcatheter aortic valve implantation; MCV: Medtronic CoreValve®; ES: Edwards SAPIEN™.
Echocardiographic measurements
A TTE was performed during the screening process. Standard measurements were done with special care for the aortic annulus diameter. A diameter ranging between 18 and 27 mm was required to be eligible for TAVI. In case of an annulus deemed to be too small or too large, a TEE was performed for a precise measurement.
Procedure
All implantations were performed under general anaesthesia and TEE guidance in a conventional catheterisation laboratory. A “heart team” composed of a cardiovascular surgeon, one or two interventional cardiologists, an echocardiographer and an anaesthesiologist collaborated to treat each patient.
MCV is a self-expanding nitinol stent containing a trileaflet porcine pericardium valve. It is designed for aortic annulus ranging from 20 to 27 mm. MCV prosthesis is available in two sizes, 26 and 29 mm, both inserted in an 18 Fr sheath. The common femoral artery is punctured using the Seldinger technique under fluoroscopy guidance. A Prostar XL™ 10 Fr preclosure device (Abbott Vascular, Redwood City, CA, USA) is inserted and stitches prepared for the end of the procedure. The Prostar XL™ sheath is then replaced by an 18 Fr sheath. After MCV delivery, the 18 Fr sheath is removed and the common femoral artery sutured with pre-placed strings. This ensures arterial haemostasis.
ES is a tubular slotted balloon expandable stainless steel stent containing a bovine pericardium trileaflet valve. It is designed for aortic annulus ranging from 18 to 25 mm. ES prosthesis is available in two sizes, 23 and 26 mm, respectively inserted in a 22 Fr or 24 Fr sheath. A surgical exposure of CFA or EIA is performed before insertion of the dedicated sheath. After valve delivery the artery is surgically repaired. The choice of the access artery is left to the surgeon’s discretion.
Definitions
Procedural success is defined as bioprosthesis delivery in native aortic annulus without death or any immediate valve-related complication or aortic regurgitation more than grade 2/4.
MACE is a combination of cardiovascular death, myocardial infarction, stroke and vascular complication.
Deaths are classified as cardiac or non-cardiac, and device-related or procedure-related.
Myocardial infarction is a CK-MB rise of at least three fold the upper limit of normal or Q-wave appearance on 12 leads surface ECG.
Stroke is a permanent neurologic deficit with contributive CT scan or MRI imaging abnormalities.
Vascular complications comprise flow-limiting dissection, need for surgical arterial repair either after suture with a closure device or after first surgical suture of the artery, uncontrolled vascular bleeding, arterio-venous fistula and false aneurysm.
Bleeding controlled with blood transfusion only was not included in vascular complications because the need for transfusion was left to the physician’s decision. This was unpredictable and variable from one physician to another.
Renal insufficiency is a baseline creatinine clearance inferior to 50 ml/min as calculated with the Cockcroft-Gault formula. Acute renal impairment is a creatinine rise superior to 25% or 36 µmol/l compared to baseline values.
Aortic regurgitation is classified according to TTE or TEE, in four grades of increasing severity. It can be central or paravalvular.
Iliac or common femoral artery calcification grade is determined by CT scan or angiography: grade 0: no calcification; grade 1: mild calcification; grade 2: moderate calcification; grade 3: severe calcification.
Statistical analysis
ANOVA t-test and chi-square test are used for statistical analyses. Continuous variables are expressed as mean + standard deviation and categorical variables as percentages. A p value < 0.05 is considered statistically significant.
Results
Study population
Patient’s baseline characteristics are listed in Table 1 and 2.
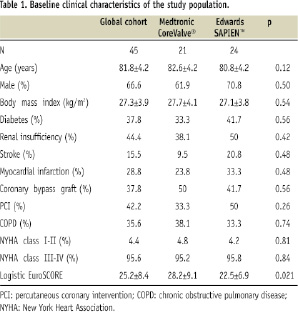
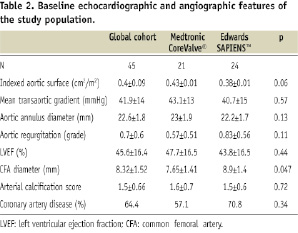
Medtronic CoreValve® (CV) and Edwards SAPIEN™ (ES) valves were used respectively in 46.7% (21/45) and 53.3% (24/45) of patients. The average EuroSCORE was 25.2±8.4% and higher in the MCV group: 28.2±9.1% vs. 22.5±6.9%, p=0.021. One explanation for a higher EuroSCORE in the MCV group is the fact that the initial patients of this group were also the first of our experience. At that time, we used to treat only very high risk patients. Indeed, when comparing 17 patients treated in the early period (from July 2007 to December 2008) to 28 patients treated more recently (January 2009 to June 2009), the EuroSCORE was significantly higher in the first group: 29.1±9.6 % vs. 22.8±6.8%; p=0.015. Except for the EuroSCORE, the only statistically significant difference between MCV and ES groups was the average diameter of CFA. It was lower in MCV group: 7.6±1.9 vs. 8.9±1.9 mm, p:0.047. This difference is explained by the smaller CFA diameter required for the MCV sheath which tended to direct patients with small CFA or EIA to transarterial MCV before proposing a transapical or subclavian approach. Eight patients had an aortic annulus between 24 and 27 in combination with a common femoral artery diameter between 6 and 8 mm. These patients could only be treated with MCV.
Procedure
Successful valve delivery was achieved in 97.8% of procedures (44/45 patients). One valve (CV) could not be delivered (2.22%; 1/45 patients) due to an apical perforation with the stiff 0.035» exchange guidewire resulting in a cardiac tamponade. This complication followed a complex manipulation to recapture a first valve positioned too high in the aortic root. A pericardial drainage was successfully carried out, but the patient subsequently died 24 hours later.
Global MACE
In-hospital global MACE rate was 20% (9/45) including two cardiac deaths, three myocardial infarctions and four vascular complications (Table 3). Myocardial infarctions were only CK MB and troponin rise without any ECG changes. No stroke was documented. There was no statistical difference between MCV and ES groups: 28.6% (6/21) vs. 12.5% (3/24) respectively, p=0.18.
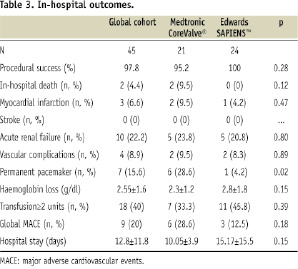
In-hospital mortality
There were two in-hospital deaths. The first is described above, due to a cardiac tamponade. The second death occurred at day five after a successful MCV delivery. It was due to a cardiogenic shock of undetermined origin, with acute left ventricle failure and refractory complete heart block. A right ventricular pacing lead, inserted through right femoral vein, had been taken out 24 hours before, but the patient was still under electrocardiographic monitoring before this event. No autopsy was carried out.
Vascular complications
Vascular complications were observed in four patients (8.9%). There was no statistically significant difference in vascular complication rates between MCV and ES groups: 9.5% (2/21) vs. 8.3% (2/24) respectively, p=0.89. Arterial injury was seen in two patients after completion of MCV implantation with CFA suture using a PROSTAR XL™ requiring a subsequent surgical arterial repair. These cases were due to a failure of the PROSTAR XL™ in patients with an arterial calcification score grade 2. One ES patient had an iliofemoral bypass for extensive injury of CFA and EIA after surgical insertion of a 24 Fr sheath prior to delivery of a 26 mm ES valve. The CFA diameter measured by CT scan was 8 mm with a calcification score grade 3. Another patient underwent a subsequent repair of CFA after completion of a transfemoral ES procedure and initially successful surgical repair. This redo vascular surgery was necessary because of retroperitoneal bleeding. All four patients with vascular complications had a good secondary outcome and could be discharged from hospital.
Blood transfusion
Even if the need for a post-procedure surgical arterial repair was not frequent, a high transfusion rate was seen in the global cohort. Of the patients, 40% (18/45) required at least two units of red cells. The decision to do a transfusion was left to the physician’s assessment according to the level of blood loss and the haemodynamic status of the patient. Different indications for transfusion were observed: five patients with a known history of coronary disease and potentially unstable coronary lesions had a systematic transfusion when haemoglobin level was under 10 g/dl, nine patients had blood transfusion consecutive to an important periprocedural blood loss without any need for a surgical management of the access site and four patients had blood transfusion consecutive to a surgical repair of the entry artery for uncontrolled bleeding. There was no difference between MCV and ES patients in terms of transfusion rate: 33.3% (7/21) vs. 45.8% (11/24) respectively, p=0.39. There was no difference between patients treated with PROSTAR XL™, those treated through surgical exposure of CFA or EIA: 33.3% (7/21), 53.8% (7/13) and 36.4% (4/11) respectively, p=0.47.
Other complications
A permanent pacemaker (PM) was implanted in seven patients (15.6%). There was significantly more PM implantation after MCV than ES valves: 28.6% (6/21) vs. 4.2% (1/24) respectively, p=0.02.
The rate of of myocardial infarction was 4.45% (2/45) without any statistical difference between both groups of prostheses. Both cases were asymptomatic elevation of CK-MB.
The overall rate of acute renal failure was 22.2% (10/45) without any statistical difference between both groups. No patient required dialysis.
Hospital stay
The average duration of hospitalisation for transarterial TAVI was 12.8 days without any statistical difference between groups of prostheses: 10.05±3.9 days vs. 15.17±15.5 days respectively, p=0.15. This duration seemed to be significantly higher when the EIA was surgically exposed as compared to a percutaneous pre-closure of the CFA: 13.54±5.6 days vs. 10.05±0.5 days respectively, p=0.049.
Thirty-day outcome
The 30-day clinical follow-up was available in all patients (Table 4).
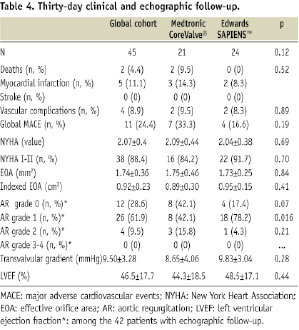
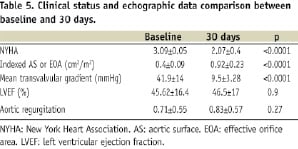
Overall 30-day MACE rate was 24.4% (p=0.19 between groups). No additional deaths were seen between hospital discharge and one month. Thirty-day survival rate was 97.5%. There was no difference between patients treated with MCV and ES (90.5% vs. 100%, p=0.12). One additional case of acute coronary syndrome with significant CK MB and troponin rise was observed in each group. The first case occurred in an 86-year old female, three weeks after a successful Medtronic CoreValve® implantation and was due to distal right coronary stenosis successfully treated with angioplasty plus stent. The second case occurred in an 84-year old male, two weeks after a successful Edwards SAPIEN™ implantation, due to a diffuse disease of the left circumflex artery treated medically without any further angioplasty. No stroke or post-discharge vascular complications were observed. A large majority of patients were in NYHA class 1 or 2 (88.4%), with a significant improvement compared to baseline: 3.09±0.05 vs. 2.07±0.4, p<0.0001 (Table 5).
Thirty-day echographic data
Echographic data were available at 30-day in 97.7% (42/43) of patients discharged from the hospitals (table 5). The average aortic surface was 1.74±0.36 cm2, which is consistent with reports from previous registries10. The average indexed effective orifice area was 0.92±0.23 cm2 and was significantly improved as compared to the baseline indexed aortic surface (0.40±0.09 cm2, p<0.0001). The mean transvalvular gradient was also dramatically improved after TAVI: 41.9±14 vs. 9.5±3.3, p<0.0001. Grade 1 paravalvular leaks were significantly more frequent after Edwards SAPIEN™ (78.2% vs. 42.1%, p=0.016), without any difference concerning grade 2 leaks. No patient had a central or paravalvular leaks greater than grade 2, without any difference between patients treated with MCV or ES valves. There was no major change in LVEF values as compared to the baseline: 45.6±16 vs. 46.5±17%, p=0.9).
Discussion
Transcatheter aortic valve implantation is clearly becoming a valid option for inoperable or high risk patients with severe aortic stenosis. We report in-hospital outcomes and 30-day MACE in a cohort of patients treated with both the Medtronic CoreValve® and Edwards SAPIEN™ TAVI valves. The main interest of our report is to be one of the first to provide data concerning a mixed population treated with two different prostheses and two different access site techniques9. We observed that it is feasible and safe to integrate both TAVI valves into a routine practice. Indeed, we didn’t notice major differences in terms of procedural success and in-hospital MACE when compared to registries considering one type of valve. Published data concerning transarterial and transapical TAVI indicate a slightly higher mortality rate with the transapical approach, while data concerning the subclavian approach with Medtronic CoreValve® are preliminary7,10,11. So in a majority of centres, most of the patients are treated through the transfemoral approach with either type of valve. The possibility of treating patients with both Medtronic CoreValve® and Edwards SAPIEN™ bioprostheses allowed us to perform 69.2% (45/65) of our TAVI procedures through the transarterial approach. Nineteen patients had transapical TAVI and one patient had a subclavian procedure. Using both valves can be a key to treat patients with various aortic root anatomies and different common femoral artery diameters. Procedural success rate was very high (97.8%) and encouraging, considering the aged and high-risk population treated. Thirty-day mortality and MACE rate was also relatively low. The decision to combine both MCV and ES valves didn’t impair the outcome of our study population. One of the reasons is the careful selection of patients. It is noteworthy that the two in-hospital deaths observed in our registry, both occurred in patients from the initial cohort. This was part of the classical learning curve for TAVI procedures. Consequently, we had to select «ideal» candidates, even if remaining at high risk for surgery. This highlights the need for a multidisciplinary «heart team» approach, including different specialists, to select and treat these fragile patients12.
Vascular complications are not rare in the transarterial approach, and can concur significantly to global MACE and 30-day mortality6,8. In our study population, access site complications were equally frequent whatever the technique: full percutaneous approach with pre-closure or surgical management and repair of the artery. Sizes of arterial sheaths remain a major limitation of transarterial TAVI, even with 18 Fr. The frequency of blood transfusions was also equivalent between both groups and higher than previously reported. This blood loss is due to the fact that TAVI procedures remain quite lengthy, with non-negligible per-procedural bleeding. There were few postoperative uncontrolled bleeding, but there may be a role of gastrointestinal bleeding as intestinal angiodysplasia is not rare in patients with aortic stenosis13. Vascular closure devices have been proposed to reduce the vascular complication rate. Another interest of closure devices is that it allows early mobilisation, therefore lowering hospital stay and cost. There was a trend toward shorter hospital stays after fully percutaneous procedures. The success rate of percutaneous arterial closure was only 90% in our cohort because some of the arteries were probably too calcified. In our local experience, even if designed for a sheath of up to 10 Fr, pre-closure devices can be used for 24 Fr procedures, e.g., percutaneous repair of abdominal aortic aneurysms14. Translating the pre-closure concept to Edwards SAPIEN™ 22 Fr or 24 Fr procedures has been recently proposed, but there is no reported data clearly demonstrating the safety of this approach within this set-up of patients. Thus, the main challenges for manufacturers remain to improve the TAVI valves’ profile, to lower sheath sizes and make arterial pre-closure more feasible15.
Concordant with previous data, we observed a low 30-day mortality rate with significant NYHA functional class improvement after TAVI. Although predicted mortality is certainly overestimated by the logistic EuroSCORE, 30-day mortality seen was dramatically low when compared to the values predicted by those risk scores16. There was no difference in 30-day echographic findings between patients treated with Medtronic CoreValve® and Edwards SAPIEN™, except for the rate of grade 1 paravalvular leaks, which were more frequent after Edwards SAPIEN™. Paravalvular leaks were frequent (71.4%) in our study population, but no patient had regurgitation more than grade 2/4 on 30-day echocardiography. The clinical impact of aortic regurgitation grade 1 or 2/4 in this population has to be investigated and should be assessed in registries with longer follow-up. A determination of the cover index (100 [prosthesis diameter-TEE annulus diameter]/prosthesis diameter) has recently been proposed to anticipate the risk of significant paravalvular leaks; this kind of tool can help to adequately match the size of the prosthesis to the native aortic annulus diameter17. A definitive pacemaker was necessary for an important number of patients treated with Medtronic CoreValve®. This is consistent with previous reports18. There is a clear need for accurate tools to detect risk factors for this event, and improvement in device characteristics to lower the frequency of heart block. This event remains rare with the Edwards SAPIEN™19.
In conclusion, a routine policy of TAVI using both Medtronic CoreValve® and Edwards SAPIEN™ valves is feasible without impairment in procedural success rates and 30-day outcomes compared to previous data from registries using one type of valve. Vascular complications are not rare, and seem to happen as frequently whatever the technique to access the common femoral artery. Despite a probably longer learning curve, a wider population of high-risk or inoperable patients with aortic stenosis can be offered a transarterial treatment. This policy could be the next standard of care for teams performing TAVI.
Our study has several limitations making it mostly observational. The size of the study population is quite small. Even if it was a mixed population, we didn’t perform any randomisation for valve type or access site techniques. Moreover, the strategy of using both devices was not tested in the whole cohort, since the initial patients were all treated with the Medtronic CoreValve® prosthesis. A larger population and longer follow-up will be necessary to confirm our preliminary findings.

