Abstract
Aims: The aim of this prospective multinational registry is to assess and identify predictors of in-hospital outcome and complications of contemporary TAVI practice.
Methods and results: The Transcatheter Valve Treatment Sentinel Pilot Registry is a prospective independent consecutive collection of individual patient data entered into a web-based case record form (CRF) or transferred from compatible national registries. A total of 4,571 patients underwent TAVI between January 2011 and May 2012 in 137 centres of 10 European countries. Average age was 81.4±7.1 years with equal representation of the two sexes. Logistic EuroSCORE (20.2±13.3), access site (femoral approach: 74.2%), type of anaesthesia and duration of hospital stay (9.3±8.1 days) showed wide variations among the participating countries. In-hospital mortality (7.4%), stroke (1.8%), myocardial infarction (0.9%), major vascular complications (3.1%) were similar in the SAPIEN XT and CoreValve (p=0.15). Mortality was lower in transfemoral (5.9%) than in transapical (12.8%) and other access routes (9.7%; p<0.01). Advanced age, high logistic EuroSCORE, pre-procedural ≥grade 2 mitral regurgitation and deployment failure predicted higher mortality at multivariate analysis.
Conclusions: Increased operator experience and the refinement of valve types and delivery catheters may explain the lower rate of mortality, stroke and vascular complications than in historical studies and registries.
Introduction
Elderly patients with severe symptomatic degenerative aortic valve stenosis are often either at high risk or not suitable candidates for surgery and have a very poor prognosis when left on medical treatment1. Europe has played a major role in the development of transcatheter valve treatment, with the first balloon valvuloplasty in 19862 and first clinical aortic valve implant in 2002 by Alain Cribier in France3. The Placement of Aortic Transcatheter Valves (PARTNER) randomised trial confirmed superiority over medical treatment or equivalency to AVR in patients deemed inoperable4 or at high surgical risk5. Large registries can be divided into industry sponsored studies limited to specific valve types6,7 and national registries reporting country specific results, often starting from the first years of TAVI implantation8-10, when operator experience and the size/design of the valves and/or delivery systems used were very different to today. Variations in national health policy and practice, device performance and definitions may account for otherwise inexplicable differences in outcome and complications, such as the rate of permanent pacemaker (PPM) implantation ranging from less than 10% in Italy11 to almost 40% in Germany4. The Sentinel Registry of Transcatheter Valve Treatment is part of the ESC EURObservational Study Programme and reports results of 4,571patients from 10 pilot countries. These are presented focusing on clinical indications, patient characteristics, procedural approach, in-hospital outcome and complications.
Methods
PARTICIPATING CENTRES AND STUDY CONDUCTION
A total of 137 centres throughout 10 European countries contributed to this registry (Online Appendix). Five countries (Czech Republic – nine centres, 141 patients, 3.08%; France – 33 centres, 2,279 patients, 49.86%; Spain – 26 centres, 689 patients, 15.07%; Switzerland – 12 centres, 129 patients, 2.82% and the United Kingdom – 25 centres, 886 patients, 19.38%) electronically transferred their entire 2011 national database after data monitoring and cleaning. Two countries (Italy, 254 patients, 5.56% and Poland, 157 patients, 3.43%) provided consecutive data from 24 active centres between January 2011 or the time of EC approval and May 2012 via a dedicated web-based CRF hosted and managed at Heart House. This generated queries to clean the database and validate entries. Consistency of variable definitions was controlled by the Executive Committee with a threshold for participation set at 90% of a core group of 112 variables. Whenever possible, and consistent with the practice of existing databases, the Valve Academic Research Consortium (VARC) definitions were applied10. Appropriate changes were performed in some of the national databases to ensure consistency. Two countries (Belgium, 20 patients, 0.44% and Israel, 4 patients, 0.09%) did not meet the 90% threshold and were asked at a later stage to participate via direct electronic submission which was limited to six centres. Germany (12 patients, 0.26%) participated only in the mitral component of the Valve Registry with the exception of two centres.
The study protocol and database was designed by the TCVT Registry Executive Committee, with members appointed to represent the various medical subspecialties involved in this multidisciplinary procedure including interventional cardiologists, cardiac surgeons, intensive cardiac care specialists, cardiac imaging specialists selected from the relevant Working Groups and Associations within the European Society of Cardiology (ESC)12. This Committee together with the 10 National Coordinators nominated by the President of each of the National Cardiological Societies, acted as the Registry Steering Committee. The EORP Oversight Committee supervised the conduct of the registry. The pilot registry had no direct or indirect sponsorship from the two companies (Edwards Lifesciences and Medtronic) manufacturing the only two commercial devices available in Europe during the study period.
ENROLMENT CRITERIA
In the participating centres all consecutive patients receiving transcatheter aortic valve implantation using approved (CE-marked) devices were prospectively entered into the registry. For the national registries, patients provided written informed consent to the TAVI procedure including consent for anonymous processing of the data. An individual specific consent approved by the hospital Ethics Committees of the participating centres was signed by patients directly entered into the TCVT database.
STUDY DEVICES
Two devices were used throughout the study period. The SAPIEN XT (Edwards Lifesciences Inc., Irvine, CA, USA) is a balloon expandable cobalt chromium open-cell stent mounting a trileaflet valve consisting of bovine pericardial tissue. The CoreValve (Medtronic Inc., Minneapolis, MN, USA) is a self-expandable nitinol stent mounting a prosthesis consisting of porcine pericardial tissue, delivered via an 18 Fr delivery catheter.
ENDPOINTS AND DEFINITIONS
The aim of this first report of the pilot TCVR is the assessment of indications, modalities of perioperative assessment and procedural technique, in-hospital results and complications. Pre-specified comparisons in the statistical plan included country of enrolment, age groups, access site and type of valve.
The definitions entered into the electronic CRF followed the VARC definitions13. The participating countries with existing national registries were encouraged to modify their database to follow these criteria. When this was not feasible endpoints were limited to unequivocal events (for instance death or stroke) without distinction of cardiovascular or non-cardiovascular causes and minor/major strokes. When various definitions were entered for an adverse event such as a haemorrhagic complication, a common unequivocal indicator present in all registries was used (for instance blood transfusions were used as a surrogate for bleeding). Individual definitions are reported in the TCVT study protocol (www.escardio.org/guidelines-surveys/eorp/surveys/Pages/tcvt.aspx).
STATISTICAL ANALYSIS
Univariate analysis was applied to both continuous and categorical variables. Continuous variables were reported as mean±SD or as median and Interquartile Range (IQR). Between-group comparisons were made using a non-parametric test (Kruskal-Wallis test). Categorical variables were reported as percentages. Between-group comparisons were made using a chi-square test or a Fisher’s exact test if any expected cell count was less than five. Multivariate analysis was used to explore relationship between mortality and baseline covariates for the TAVI population. The baseline covariates were compared between dead and alive patients at discharge (chi-squared or Kruskal-Wallis test for categorical or continuous variables respectively), and only the significant variables were included in the model. Multiple logistic regression was done using a multiple imputation procedure to overcome the limitation caused by the presence of missing data. Instead of filling in a single value for each missing value, Rubin’s (1987) multiple imputation procedure replaces each missing value with a set of plausible values that represent the uncertainty about the right value to impute. These multiplied imputed data sets are then analysed by using standard procedures for complete data and combining the results from these analyses. This procedure was performed using the programme R (http://www.R-project.org/.) and the package Hmisc (http://CRAN.R-project.org/package=Hmisc).14,15
Results
PATIENT CHARACTERISTICS
A total of 4,571 patients underwent TAVI between January 2011 and June 2012 in 137 centres of 10 European countries. Average age was 81.4±7.1 years and body/mass index was 26.6±4.9 Kg/m2. NYHA Class III or IV was present in 76.9% of patients. Patient characteristics are reported in Table 1 for the entire population (Table 1, column 1) and divided for patients with ≤80 years or >80 years (Table 1, columns 2 and 3). There is a greater incidence of diabetes, COPD, extracardiac arteriopathy (carotid, peripheral), renal dialysis, prior myocardial infarction, previous cardiac surgery or PCI and previous aortic valve replacement (valve-in-valve procedures) in the younger group.
Despite the greater prevalence of comorbidities in patients <80 years of age, they had a significantly lower mean logistic EuroSCORE (17.4±13.2 vs. 22.0±13.1; p<0.01). There were large variations in the average logistic EuroSCORE among the participating countries (Figure 1) with countries like Spain and Italy having an average of 16.2±10.0 and 17.6±13.9 and countries like the UK and the Czech Republic having an average logistic EuroSCORE of 22.6±13.3 and 23.2±15.7(p<0.01).
PREPROCEDURAL NON-INVASIVE ASSESSMENT
Transthoracic echocardiography was by far the most frequently used technique for the preprocedural assessment of aortic valve stenosis severity, presence and severity of associated regurgitation, aortic annulus size, left ventricular ejection fraction (LVEF) and concomitant mitral valve disease. Only 10.8% and 0.3% of patients are reported to have undergone multislice CT or cardiac MRI, respectively. The baseline mean aortic transvalvular gradient was 49.1±16.2 mmHg and aortic valve area 0.68±0.26 cm2 (Figure 2). Preprocedural aortic valve regurgitation was frequent with 16.5% and 1.7% reported to have grade 2 and grade 3, respectively. Mitral valve regurgitation graded ≥2 was present in 20.8% of patients. Preprocedural LVEF was preserved in the majority of patients with a mean of 54.1±13.8%, with 8.2% of patients with an ejection fraction ≤30%.
PROCEDURE
The transfemoral route was by far the preferred access site, used in 74.2% of patients. The transapical route, only applicable with the SAPIEN XT valve was used in 16.4% of patients (28.6% of all SAPIEN XT valves). Other access sites (trans-subclavian, transaxillary, direct aortic, etc.) were used in 9.4% of cases, more frequently using the CoreValve. When patients are divided according to their access route, large differences were observed in clinical characteristics, comorbidities and EuroSCORE (Table 2).
General anaesthesia (GA) was used in 62.9% of all transfemoral procedures, with wide variations among countries (Figure 1), p<0.01.
SAPIEN XT valves were implanted in 2,604 of the 4,571patients (57.3%). Valve selection was skewed towards small diameters with 23 mm SAPIEN XT and 26 mm CoreValve used in 44.2% and 40.8% of patients and intermediate diameter 26 mm SAPIEN XT and 29 mm CoreValve used in 48.7% and 53.1% of patients, respectively.
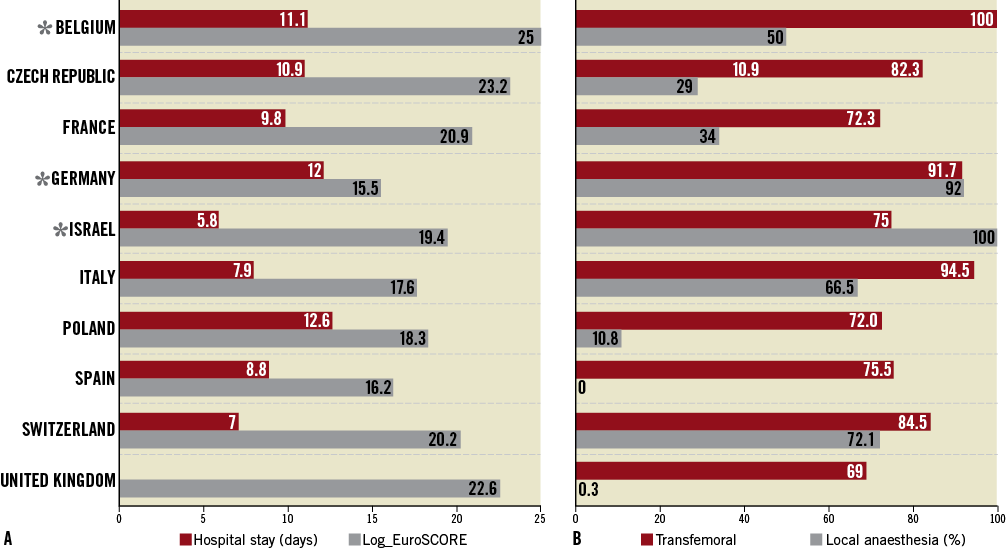
Figure 1. Variations among participating countries. A) Red columns indicate the mean in-hospital stay in days. Note the wide variation from the reported seven days in Switzerland compared to >12 days in Poland. Grey columns report the mean logistic EuroSCORE of each participating country. The Czech Republic and the UK reported the highest EuroSCORE (25) whereas Italy, Poland and Spain had <20 mean EuroSCORE. B) Red columns represent the percentage of aortic valves implanted via transfemoral access (%). All cases included from Belgium were performed via transfemoral access, compared to <70% in the United Kingdom. Grey columns show the use of local anaesthesia within different countries, ranging from less than 1% in the UK and Spain to more than half of the patients in Italy and France. Asterisks indicate the three countries (Belgium, Germany and Israel) where the number of patients and centres was insufficient to be representative of national practice in the study period.
PROCEDURAL RESULTS AND COMPLICATIONS
The valve was successfully deployed in 96.5% of patients, without significant differences based on access site (Table 3) or valve type (Table 4). A second valve was required in 2.4% of cases with surgical conversion occurring in 4.3%.
Overall in-hospital mortality was 7.4%, similar in SAPIEN XT and CoreValve, without significant differences (7.9% vs. 6.7%: Table 4, p=0.15). There were however large mortality differences according to approach (transfemoral 5.9%, transapical 12.8%, trans-subclavian and other approaches 9.7%, Table 3, p<0.01). Bleeding requiring blood transfusion(s) was the most frequent complication with an incidence greater than 20% for transapical and trans-subclavian approaches, and 15.0% for transfemoral (p<0.01). The need for implantation of a PPM was significantly greater for the CoreValve than for the SAPIEN XT valve (23.4% vs. 6.0%, p<0.01). There was a low and similar incidence of in-hospital major vascular complications (3.1%), stroke (1.8%) and myocardial infarction (0.9%).
ECHOCARDIOGRAPHIC CHANGES POST-PROCEDURE
The aortic valve area increased from 0.68±0.26 cm2 preprocedure to 1.81±0.55 cm2 postprocedure, with a concomitant reduction in mean gradient (Figure 2). Aortic valve regurgitation in the pre-discharge control echocardiogram (available in 2,522 patients) showed a reduction in the incidence of grade 2 regurgitation to 7.7% and of grade 3 regurgitation to 1.3% following TAVI. Post-procedural LVEF remained stable at 55.2±12.6%.
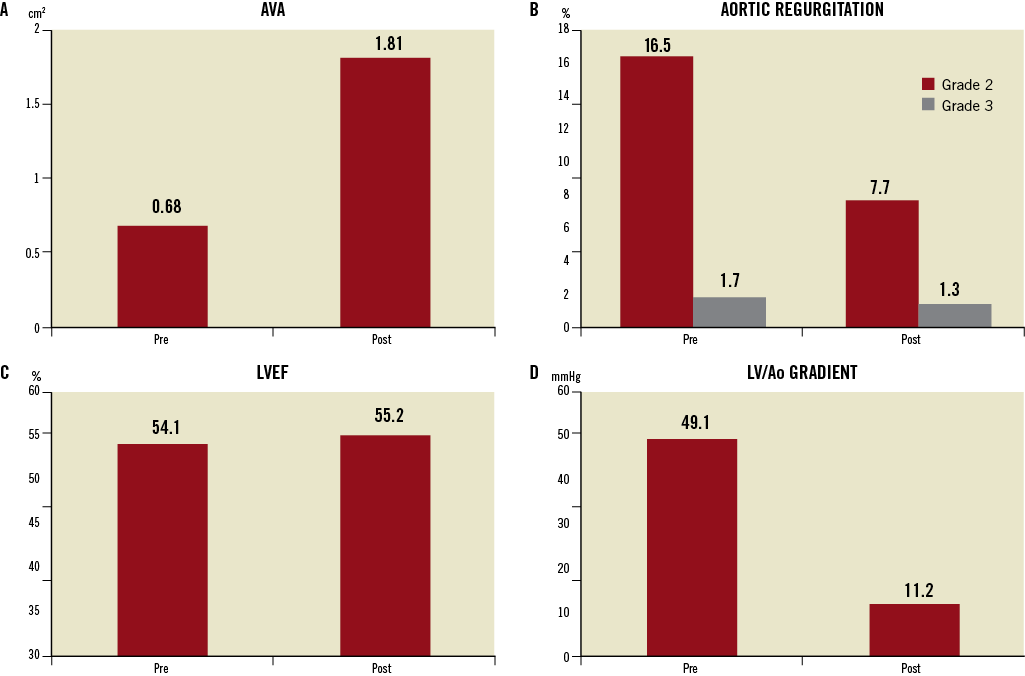
Figure 2. Haemodynamic changes after transcatheter aortic valve implantation. A) Mean aortic valve area (AVA) before and after the procedure. B) Presence of grade 2 and 3 aortic valve regurgitation (%) before and after the procedure. C) Left ventricular ejection fraction (LVEF) before and after the procedure. D) Left ventricular (LV)/Aortic (Ao) transvalvular mean gradient measured before and after the procedure, with an expected large decrease after valve implantation.
MULTIVARIATE PREDICTORS OF MORTALITY
Advanced age, high logistic EuroSCORE, pre-procedural ≥grade 2 mitral regurgitation and failure to deploy the valve were associated with a significant increase in mortality (Table 5).
IN-HOSPITAL STAY
Duration of hospital stay for the TAVI procedure was 9.3±8.1 days. Great variability was shown in the practice in different countries (Figure 1) with a marked prolongation of hospital stay in patients receiving GA (10.2±8.7days vs. 7.9±6.1days, p<0.01), and/or treated using a transapical or other surgical approaches (43.8% and 39.5% stayed in hospital >10 days vs. 22.0% of patients treated transfemorally, p<0.01).
Discussion
The general purpose of the Sentinel Registries of the ESC EuroObservational Research Study is to independently monitor the application of new technologies in Europe, detect regional differences in indication and technique and assess adherence to guidelines. This registry, also in its pilot phase, appears to have reached its goal as it reports results from the largest TAVI database presented so far and has the unique characteristic of being limited to procedures performed between January 2011 and June 2012 using contemporary valves and/or delivery systems.
The first reassuring message provided by this registry concerns indications. The fear that TAVI was going to be indiscriminately applied to younger and low-risk patients in the absence of supportive studies is dispelled by this large registry of contemporary practice, showing an average age approaching 82 years and a EuroSCORE >20. As indicated by the recently presented German Registry (GARY), including both TAVI and surgical AVR procedures, the average age of TAVI patients is much older than surgical AVR cohorts, indicating that TAVI in Europe remains principally reserved for very old patients who are known to have not only higher mortality, but also more prolonged hospitalisation and slower recovery after conventional AVR. Data from a previous ESC registry on valve treatment16 showed that these patients were often left to medical therapy, which carries very poor prognosis in symptomatic severe aortic valve stenosis. The routine application of this revolutionary technique is likely to prolong survival and improve the quality of life in these patients17. In patients younger than 80 years the presence of comorbidities is more frequent than in older patients. This indicates that a stringent selection process has been followed, restricting TAVI in younger patients to those with significant comorbid conditions that confer a markedly increased surgical risk. Very few patients younger than 70 years undergo TAVI in Europe, and only for “special” indications such as valve-in-valve or for prohibitive comorbidities.
The transfemoral approach clearly represents the preferred route in Europe, but the threshold to move towards other routes and the type of alternative varies, with the transapical approach utilised in more than 25% of patients in Spain or Poland, and seldom applied in Italy or the Czech Republic. In some countries (UK, Italy), patients with peripheral vascular disease, and a risk profile similar to the traditional candidates for a transapical approach, are considered for a variety of “surgical” alternatives, with the direct exposure of the aorta18, subclavian19 and axillary artery20. These techniques appear more frequently applied with the CoreValve, not suitable for a transapical approach, but they are also becoming more commonly applied with the SAPIEN XT valve.
Transthoracic echocardiography (TTE) appears the prevalent modality to confirm the severity of stenosis, assess the diameter of the valve annulus, confirm resolution of the obstruction and determine the presence and severity of post-procedural paravalvular regurgitation21. The anteroposterior annulus diameter measured with TTE was still the main guide for valve sizing. One hypothesis is that the use of this technique may be responsible for a systematic underestimation of valve size. Reliance on TTE may therefore aggravate the main problem of transcatheter valves which is paravalvular AR22,23. MSCT was used in only 13.1% of patients and currently evidence suggests that perimetric measurements may be more accurate for valve selection. The somewhat surprisingly high proportion of smaller valve sizes seen in this registry might support this concept. In spite of the possibly suboptimal valve-to-annulus diameter ratio, this registry showed an incidence of ≥2 aortic regurgitation somewhat lower than in PARTNER or in other registries, with significantly lower regurgitation using the SAPIEN XT valve.
Direct comparison of mortality in this large contemporary registry with previous trials and registries is limited by the incompleteness of 30-day data, forcing us to limit analysis to in-hospital mortality. Overall, the in-hospital mortality of 7.3% still qualifies TAVI as a high-risk procedure. Transfemoral procedures had a much lower mortality at 3.9%, which was significantly different in the univariate analysis to the transapical cohort. This may be related to the presence of confounders such as comorbidities contraindicating femoral access and it is important to note that this difference ceased to be significant at multivariate analysis. When the other surgical vascular access approaches are considered, there was a trend, despite the similar patient characteristics, for mortality to be lower than with the transapical approach, with an incidence in between transfemoral and transapical. Only procedural failure has a solid 7.3 odds ratio for in-hospital mortality. EuroSCORE and age were also significantly associated with mortality, but the overall predictive value of those two variables was too low to be of practical help to estimate individual survival. Preprocedural mitral regurgitation ≥2 was the fourth parameter shown at multivariate analysis to correlate with in-hospital survival.
In patients with an average age greater than 81 years, life expectancy is already low and the relative importance of quality of life over survival increases. The percentage of stroke is less than 2% in both valve groups, a reassuring finding when compared with the much higher figures in the PARTNER Trial24 and in line with other published European experiences22. Major vascular complications were also less frequent than previously reported, probably reflecting improved operator skills five years after the TAVI programme had been started in most centres, as well as smaller catheter size. It should be noted that it is very likely that the incidence of self-reported and unadjudicated complications in registries is likely to be lower than in controlled clinical trials. The most striking difference reported in the various European national registries concern the need for PPM implantation. The average implantation rate observed is 13.2% but this complication appears very device specific with the CoreValve requiring a pacemaker in 23.4% of patients and the SAPIEN XT showing a much lower incidence of 6.0%, not far from percentages reported after surgical AVR. Higher CoreValve implantation enabled by increased operator experience and the new AccuTrak delivery system has been claimed to reduce the need for permanent pacemaker implantation and aortic regurgitation, more frequent with suboptimal low (ventricular) implants. This claim may be correct compared with the historical CoreValve series, but such improvement is still not sufficient to align the need for pacemaker implantation and the severity of aortic regurgitation with the results achieved with the SAPIEN XT valve.
Mortality and other major adverse events including vascular complications and bleeding were similar for the two valve types.
Hospital stay approaches 10 days on average and it is difficult to explain differences. Planning of outpatient preliminary investigations and availability of rehabilitation centres with early transfer in the post-operative period may represent confounding organisational variables. General anaesthesia is still favoured by most operators and is the standard in many centres and countries. It certainly offers the operator and the patient a more relaxed experience during the procedure, with the benefit of continuous transoesophageal echocardiographic guidance, but there is a price to pay in terms of duration of procedure and hospital admission.
Based on our results, the recommendation of using aspirin and clopidogrel post TAVI is adopted in only 30% of patients. This lack of adherence probably reflects the paucity of data upon which this recommendation is based.
This registry has strengths and weaknesses. The electronic transfer of large national databases has obvious advantages in terms of data quality since it ensures consecutive enrolment in all centres with country-based data monitoring and quality control. Still, the use of different definitions or the fact some parameters were not collected forced us to look for a minimum common denominator, which necessarily requires exclusion of some of the variables or acquisition limited to some of the countries. We candidly report the available data for each variable and adopted a statistical method allowing corrections for missing data in multivariate analysis. The goal of this pilot phase was also to raise awareness of these discrepancies and promote the adoption of uniform definitions across Europe. This registry has accelerated the process of creation of national databases in countries such as Spain, Switzerland and the Czech Republic, with this last country adopting almost entirely the web based database of this study stimulating others to follow (Poland, Italy). The absence of a centralised analysis process and independent adjudication is of greater concern for some more subtle adverse events such as post-procedural aortic regurgitation and stroke. Investigators naturally tend to minimise adverse events and magnify success, which may explain the better outcome observed compared with the PARTNER Trial and previous registries. Still it is highly unlikely that investigators willingly report unchecked or false data in a registry reviewed by national authorities, and it is more likely that only clearly evident complications, possibly the most clinically relevant, are picked up and reported. Finally, this initial report of the pilot phase is limited to in-hospital data, making difficult the comparison with studies focused on 30-day events, a time-point recommended as a primary endpoint in the VARC definitions. The poor completeness observed at 30 days is not only a consequence of the fact that centres were allowed enrolment till May 2012, but also reflects possible difficulties maintaining high rates of follow-up in a voluntary initiative. Other registries and studies have succeeded in exploring predictors of late survival. Even if this registry will be continued as a permanent initiative, its main strength is likely to remain the unbiased independent comparison of indications, technique and complications in different European countries, with a database potentially far larger than any other registry, a goal already achieved in this pilot phase.
Conclusions
This truly contemporary registry, using the most widely used valve types and delivery catheters, suggests that TAVI in Europe is still reserved to very old patients or patients with severe comorbidities and high surgical risk. The technique is in evolution with transfemoral procedures more often done only under local anaesthesia, and novel approaches (trans-subclavian and direct aortic access) challenging the traditional transapical route for patients who are not suitable for a transfemoral approach. Mortality and other adverse events such as stroke, vascular complications and severe aortic insufficiency appear slightly lower than in previous studies and registries, especially for transfemoral procedures.
Acknowledgements
Data collection and statistical analysis coordinated and conducted at the European Heart House by Gerard Gracia, Cecile Laroche and Malika Manini and by the EORP Oversight Committee (Angeles Alonso, Luigi Paolo Badano, Jean Fajadet, Roberto Ferrari, Michel Komajda, Piotr Ponikowski, Luigi Tavazzi, Panos Vardas, David Wood).
From the EURObservational Research Programme of the European Society of Cardiology, Sophia Antipolis, France.
Funding
European Society of Cardiology.
Conflict of interest statement
C. Di Mario has been sponsored by Edwards Lifesciences and Medtronic to participate in courses in Nyon and Tolochenaz as part of the mandatory certification process for the implantation of both the SAPIEN and the CoreValve. He also receives speakers’ fees, grants for clinical trials and sponsorship for the organisation of congresses and courses from Medtronic UK and Medtronic Vascular Europe. E. Eltchaninoff receives Proctor fees from Edwards Lifesciences. N. Moat receives lecture fees from Medtronic. G.P. Ussia received Proctorship fees from both Medtronic and Edwards Lifesciences. P. Wenaweser receives lecture, Proctor and Consultant fees from Medtronic and Edwards Lifesciences. G. Nickenig receives lecture fees from Medtronic. B. Prendergast received speakers’ fees from Edwards Lifesciences. S. Windecker received lecture, Proctor and Consultant fees from Medtronic and Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Online data supplement
Appendix. Sentinel Registry of Transcatheter Valve Treatment: participating centres
TCVT Executive Committee: Carlo Di Mario, UK (Chairman), European Association of Percutaneous Cardiovascular Interventions (EAPCI); Aortic Valve Registry: Neil Moat, UK, Bernard Iung, France, ESC WG on Valve Disease; Gerhard Schuler, Germany, EAPCI; Pepe Zamorano, Spain, European Association of Echocardiography (EAE). Mitral Valve Registry: Ottavio Alfieri, Italy, ESC WG of Cardiovascular Surgery; Olaf Franzen, Denmark, EAPCI; Susanna Price, UK, ESC WG Acute Cardiac Care, L. Tavazzi (Oversight Committee), A. Maggioni (Board consultant for the EURObservational Research Programme)
National Coordinators:
BELGIUM E. Schroeder, M. Claeys; CZECH REPUBLIC P. Kala; FRANCE H. Eltchaninoff; GERMANY G. Nickenig; ISRAEL H. Danenberg; ITALY F. Romeo, GP. Ussia; POLAND M. Zembala; SPAIN J. Goicolea; SWITZERLAND R. Corti, P. Wenaweser; UNITED KINGDOM B. Prendergast.
EURObservational Research Programme team:
M. Manini (Head of Dept); G. Gracia, V. Missiamenou, M. Konte (data monitors); C. Laroche (statistician), C. Taylor (IT specialist), E. Fiorucci, E. Folkesson, M. Glemot, PA. McNeill (assistants)
FUNDING
The following companies are supporting the EURObservational Research programme: GOLD: Abott Vascular, Bayer Pharma, Bristol Myers Squibb (BMS), Pfizer, Boehringer Ingelheim, Daiichi Sankyo Europe, Menarini international Operations, Novartis Pharma, Sanofi-Aventis, Servier International. SILVER: Amgen. BRONZE: Boston Scientific International, Merck & Co. (MSD). The contribution of NICOR to the collection and transfer of the 2011 UK TAVI Registry is gratefully acknowledged.
Participating Centres with local PIs:
BELGIUM Aalst: M. Vanderheyden, Edegem: M. Claeys, Liège: V. Legrand, J. Beckers, J. Boland, Q. Desiron, D. Henroteaux, S. Pourbaix, O. Vanderperren, Yvoir: M. Buche, P. Chenu, A. Guédès, E. Schroeder.
CZECH REPUBLIC Brno: P. Kala, P. Nemec, M. Tretina, Ceske Budejovice: L. Pesl, Hradec Kralove: J. Stasek, J. Bis, Usti n. Labem: P. Cervinka, Olomouc: R. Stipal, Trinec: M. Branny, Praha: V. Kocka, P. Widimsky, M. Zelizko, M. Mates, J. Horak.
FRANCE Angers: J. Debrux, S. Delepine, A. Furber, F. Pinaud, Besançon: S. Chocron, N. Meneveau, F. Schiele, Bois-Bernard: O. Fabre, A. Gommeaux, P. Hochart, M. Pecheux, Brest: E. Bezon, J. Boschat, JN. Choplain, M. Gilard, Bron: R. Dauphin, G. Durand De Gevigney, G. Finet, O. Jegaden, JF. Obadia, G. Rioufol, Caen: G. Grollier, T. Lognone, M. Massetti, R. Sabatier, Caluire-et-Cuire: JP. Claudel, F. Gabrielle, F. Pelissier, G. Tremeau, Clermont-Ferrand: K. Azarnouch, N. Durel, A. Inorta, JR. Lusson, G. Souteyrand, Créteil: JL. Dubois Rande, M. Kirsch, E. Teiger, Grenoble: B. Bertrand, O. Chavanon, P. Porcu, G. Vanzetto, Le Chesnay: O. Bical, P. Deleuze, X. Favereau, A. Jegou, Le Plessis Robinson: A. Azmoun, C. Caussin, S. Ghostine, R. Nottin, Lille: F. Juthier, T. Modine, A. Sudre, E. Van Belle, Marseille: JL. Bonnet, F. Collart, T. Cuisset, D. Grisoli, F. Collet, A. Vaillant, J. Vicat, O. Wittenberg, R. Houel, P. Joly, R. Rosario, Massy: B. Chevalier, A. Farge, P. Garot, T. Hovasse, T. Lefevre, MC. Morice, M. Romano, Monaco: F. Bourlon, Montpellier/Nimes/Perpignan: B. Albat, M. Krastevich, F. Leclercq, C. Piot, L. Schmutz, Neuilly-sur-Seine: JC. Dib, B. Gerardin, T. Waldmann, Paris: D. Blanchard, JN. Fabiani, A. Lafont, R. Zegdi, F. Beygui, J. Collet, P. Leprince, A. Pavie, D. Himbert, P. Nataf, Pessac: E. Choukroun, P. Dos Santos, JP. Guibaud, L. Leroux, Rennes: M. Bedossa, D. Boulmier, H. Le Breton, JP. Verhoye, Rouen: JP. Bessou, A. Cribier, H. Eltchaninoff, C. Tron, Saint-Denis: N. Bonnet, F. Digne, P. Guyon, P. Mesnidrey, Saint-Herblain: D. Crochet, R. Gaudin, JC. Roussel, A. Tirouvanziam, Strabourg: M. Kindo, JP. Mazzucotelli, P. Ohlmann, Toulouse: D. Carrie, G. Fournial, B. Marcheix, D. Tchetche, Tours: O. Bar, C. Barbey, D. Blanchard, S. Chassaing, D. Chatel, O. Le Page, A. Tauran, Vandoeuvre les Nancy: M. Angioi, JP. Carteaux, F. Moulin, JP. Villemot, Villeurbanne: D. Champagnac, V. Doisy, Y. Lienhart, R. Roriz.
GERMANY Bad Krozingen: M. Bockstatt, HJ. Buettner, T. Comberg, R. Hoewisch-Pengel, E. Maurer, B. Steiger, Bonn: K. Brunsmann, C. Hammerstingl, P. Lang, M. Lennarz, G. Nickenig, R. Schueler, JM. Sinning, C. Zimmermann, Frankfurt: M. Bürgstein, S. De Bruijn, R. Eckhardt, S. Esfajani, M. Kroth, H. Sievert, M. Sirotina, N. Staiger, R. Talea, Hamburg: S. Baldus, K. Heitmann, B. Kolodzick, Lubeck: P. Radke, H. Schunkert, Münster: H. Baumgartner, A. Hellige, G. Kaleschke, G. Kerckhoff, München: M. Block, D. Brill, A. Erbsloeh, M. Fueller, Neuss: H. Degen, M. Haude, A. Kezic, L. Schürmann, Wuppertal: G. Haltern, M. Seyfarth, J. Szymanski, K. Tiroch.
ISRAEL Jerusalem: H. Danenberg, S. Loncar, G. Perlman.
ITALY Ospedale Ferrarotto Catania, C. Tamburino; Villa Maria Cecilia, Cotignola-Ravenna, A. Cremonesi; C. Grattoni, Lecce Hospital, Lecce, A. Liso; F. Castriota, Ospedale Arnas Civico, Palermo, A. Stabile; Ospedale Cisanello, Azienda Ospedaliera Universitaria, Pisa, A. Petronio; Policlinico Tor Vergata, Roma, F. Romeo; Istituto Humanitas, Milano, P. Presbitero; Policlinico Umberto I, Roma, G. Sardella.
POLAND Bialystok: S. Dobrzycki, M. Frank, T. Hirnle, P. Kralisz, A. Lukaszewicz, B. Sobkowicz, Bydgoszcz: L. Anisimowicz, J. Kubica, G. Lau, W. Ogorzeja, W. Pawliszak, M. Radomski, A. Sukiennik, I. Swiatkiewicz, M. Woznicki, Gdansk: D. Ciecwierz, D. Jagielak, R. Lango, G. Laskawski, J. Rogowski, R. Targonski, A. Wojtowicz, Katowice: M. Lelek, A. Ochala, R. Parma, G. Smolka, P. Weglarz, W. Wojakowski, Krakow: D. Dudek, B. Kapelak, P. Kleczynski, J. Konstanty-Kalandyk, J. Sadowski, R. Sobczynski, D. Sorysz, J. Trebacz, K. Zmudka, Poznan: M. Grygier, M. Jemielity, M. Lesiak, Warszawa: EK. Biernacka, Z. Chmielak, M. Dabrowski, M. Demkow, Z. Juraszynski, K. Kusmierski, J. Pregowski, W. Ruzyllo, A. Witkowski, Warszawa: R. Chichon, Z. Huczek, J. Kochman, M. Marchel, M. Michalak, G. Opolski, P. Scislo, R. Wilimski, K. Byczkowska, RJ. Gil, T. Kulawik, A. Pawlak, K. Suwalski, Wroclaw: W. Banasiak, P. Kubler, K. Reczuch, Zabrze: P. Chodor, Z. Kalarus, M. Krason, A. Lekston, T. Podolecki, R. Przybylski, W. Streb, P. Trzeciak, K. Wilczek, M. Zembala, J. Zembala-John.
SPAIN La Coruña: JJ. Cuenca Castillo, J. Salgado Fernandez, N. Vazquez Gonzalez, Alicante: P. Llamas, V. Mainar, Asturias: R. Del Valle, C. Moris De La Tassa, Badajoz: F. Gonzalez De Diego, J. Gonzalez Rodriguez, JR. Lopez Minguez, A. Merchan, Barcelona: A. Cequier Fillat, R. Romaguera, M. Castella, J. Mulet, M. Sabate Tenas, M. Jimenez, JM. Padro, A. Serra, F. Mauri, C. Oliete, X. Ruyra, B. Garcia, A. Igual, V. Serra Garcia, Bilbao: J. Alcibar, JI. Aramendi, JM. Aguirre Salcedo, L. Andraka, AA. Morist, J. Zuazo Meabe, Granada: E. Molina Lerma, Guipuzcoa: I. Gallo, J. Goiti, M. Larman, A. Saenz, Huelva: J. Diaz Fernandez, A. Gomez Menchero, Leon: M. Alvarez García, M. Castano, F. Fernandez Vazquez, J. Gualis Cardona, Madrid: F. Navarro Del Amo, A. Albarran Gonzalez-Trevilla, JM. Cortina, J. Tascon, J. Cobiella, E. Garcia, R. Hernandez Antolin, E. Rodriguez Hernandez, L. Martinez Elbal, F. Fernandez-Aviles Diaz, A. Gonzalez Pinto, J. Rodriguez Roda, JL. Zunzunegui, O. Al-Razzo, JM. Mesa, R. Moreno, P. Salinas, J. Goicolea Ruigomez, JF. Oteo Dominguez, J. Ugarte, A. Epeldegui, I. Garcia Andrade, J. Pey Yllera, Malaga: OG. Albarova, JH. Alonso Briales, JM. Hernandez Garcia, AJ. Munoz Garcia, C. Urbano Carrillo, Murcia: R. Arcas, E. Pinar, M. Valdes, Salamanca: I. Cruz, J. Martin Moreiras, Santander: JM. Revuelta, J. Zueco, Santiago de Compostela: AL. Fernandez Gonzalez, D. Lopez, R. Trillo Nouche, Sevilla: JM. Barquero Aroca, RR. Salmeron, JM. Borrego, JM. Cubero Gomez, A. Sanchez, Toledo: A. Canas, J. Moreu Burgos, Valencia: O. Albarova, A. Berenguer, S. Canovas Lopez, Valencia: J. Anastasio Romero, Vigo: A. Iniguez, V. Jimenez, J. Montoto Lope, G. Pradas.
SWITZERLAND Bern: P. Wenaweser, C. Huber; Basel: R. Jeger, F. Rütter; Geneve: M. Roffi, S. Noble; Lugano: G. Pedrazzini, F. Siclari; Lausanne: D. Locca, E. Ferrari Triemlispital, Zurich: David Tueller, Michele Genoni; Zurich: L. Altwegg, J. Grünenfelder.
UNITED KINGDOM Belfast: G. Manoharan, R. Jeugnathan; Birmingham: S. Doshi, P. Ludman, J. Mascaro; Brighton: D. Hildick-Smith, U. Trevedi; Bristol: A. Baumbach, M. Turner; Blackpool: DH. Roberts, A. Tang; Cambridge: C Densem, S. Tsui; Cardiff: Derriford; Leeds: D. Blackman, M. McLenegham, P. Kaul; Leicester: J. Kovac, T. Spyt; Liverpool: R. Stables, M. Kuduvalli; London: C. Knight; C. Di Mario, N. Moat, S. Price, T. Snow; M. Dalby, M. Amrani; I. Mallik, G. Michail, A. Chuckwemeka; P. McCarthy, O. Wendler; S. Brecker, M. Jahangiri; S. Redwood, M. Thomas, C. Young; M. Mullen, J. Yap; Manchester: F. Ordoubadi; Middlesborough: M. de Belder, A. Goodwin; Morriston: A. Chase, A. Youhana; Newcastle; Nottingham: R. Henderson, I. Mitchell; Oxford: B. Prendergast, R. Sayeed; Plymouth: I. Cox, C. Lloyd; Southampton: A. Calver, N. Curzen, G. Tsang; Stoke-on-Trent: M. Gunning, A. Levive; Wolverhampton: S. Khogali, B. Moninder.

