Abstract
Aims: To evaluate short-term clinical outcomes following transcatheter aortic valve implantation (TAVI) using CE-mark approved devices in Switzerland.
Methods and results: The Swiss TAVI registry is a national, prospective, multicentre, monitored cohort study evaluating clinical outcomes in consecutive patients undergoing TAVI at cardiovascular centres in Switzerland. From February 2011 to March 2013, a total of 697 patients underwent TAVI for native aortic valve stenosis (98.1%), degenerative aortic bioprosthesis (1.6%) or severe aortic regurgitation (0.3%). Patients were elderly (82.4±6 years), 52% were females, and the majority highly symptomatic (73.1% NYHA III/IV). Patients with severe aortic stenosis (mean gradient 44.8±17 mmHg, aortic valve area 0.7±0.3 cm2) were either deemed inoperable or at high risk for conventional surgery (STS 8.2%±7). The transfemoral access was the most frequently used (79.1%), followed by transapical (18.1%), direct aortic (1.7%) and subclavian access (1.1%). At 30 days, rates of all-cause mortality, cerebrovascular events and myocardial infarction were 4.8%, 3.3% and 0.4%, respectively. The most frequently observed adverse events were access-related complications (11.8%), permanent pacemaker implantation (20.5%) and bleeding complications (16.6%). The Swiss TAVI registry is registered at ClinicalTrials.gov (NCT01368250).
Conclusions: The Swiss TAVI registry is a national cohort study evaluating consecutive TAVI procedures in Switzerland. This first outcome report provides favourable short-term clinical outcomes in unselected TAVI patients.
Introduction
Aortic valve stenosis is the most clinically relevant valvular heart disease in the elderly patient population and is associated with worse clinical outcomes once symptoms occur1. Surgical aortic valve replacement (SAVR) was for decades the standard treatment for patients with symptomatic, severe aortic stenosis, and resulted in effective alleviation of symptoms, improvement of health-related quality of life and overall prognosis2. Following the introduction of transcatheter aortic valve implantation (TAVI) in 2002 as a less invasive treatment for symptomatic severe aortic stenosis3, TAVI has evolved into a reliable therapeutic alternative with comparable results to SAVR in well-defined patient subgroups4-6. Currently, the indication for TAVI is limited to carefully selected patients deemed inoperable or at excessive risk for SAVR2. A successful TAVI procedure is preceded by a complex selection process of patients, requiring detailed imaging information of the aortic valve anatomy and the peripheral vasculature, and also a meticulous clinical assessment by an interdisciplinary Heart Team7,8.
In the era of the Heart Team, the Swiss Working Group of Interventional Cardiology in collaboration with the Swiss Society of Cardiac Surgery started a nationwide, prospective cohort study in 2011, with the intention of assessing the safety and efficacy of unselected and consecutive TAVI procedures in Switzerland. As the first TAVI in Switzerland was performed in August 2007, this registry mainly focuses on contemporary results of experienced cardiovascular centres in consecutive patients treated with TAVI in Switzerland.
Methods
The Swiss TAVI registry is a national, prospective cohort study aiming for consecutive patient enrolment, data monitoring and endpoint adjudication by a dedicated clinical events committee according to the recommendations of the Valve Academic Research Consortium (VARC)9,10. The Swiss TAVI registry was designed to provide short-term clinical outcomes and long-term clinical data of TAVI patients treated with CE-approved devices.
The aim of the present report was to describe the clinical and procedural characteristics of patients treated with TAVI in Switzerland using different CE-approved devices and various access routes as well as to stratify short-term outcomes according to device type and access route. The study protocol was approved by the local cantonal ethics committee at each participating centre and all patients provided written informed consent. The Swiss TAVI registry is performed under the lead of the Swiss Cardiovascular Centre Bern at Bern University Hospital in cooperation with the Clinical Trials Unit Bern responsible for data management and independent statistical analysis.
Patient population
A total of 697 patients were enrolled into the Swiss TAVI registry between February 2011 and March 2013, and eight centres participated during this first period of inclusion. Consecutive patient enrolment was mandatory. All participating centres are listed in the Online Appendix.
Patients were eligible for inclusion in case of symptomatic, severe aortic stenosis, degenerated aortic bioprosthesis or severe aortic regurgitation treated with CE-approved TAVI devices. Patient screening and selection was recommended to be performed within a multidisciplinary Heart Team using detailed clinical and anatomical imaging information. The exclusion criterion for participation in the cohort study was the absence of cardiac surgery on-site.
Procedure and devices
Transcatheter aortic valve procedures were performed using CE-approved devices only. During the inclusion period, the following CE-approved devices were available in Switzerland and used for patient treatment: Medtronic CoreValve® (Medtronic Inc., Minneapolis, MN, USA), Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA), Symetis Acurate TA™ (Symetis, Lausanne, Switzerland), JenaValve (JenaValve Technology GmbH, Munich, Germany) and the Portico™ THV (St. Jude Medical, Minneapolis, MN, USA). There was no pre-specified recommendation regarding access route selection, and the decision as to general anaesthesia or conscious sedation was left to the discretion of the Heart Team and according to local expertise. In addition, there was no specific recommendation for the type and duration of antiplatelet or antithrombotic medication, which was left to the discretion of the operator and according to local expertise.
Definitions and endpoints
The primary study endpoint with respect to procedural safety was all-cause mortality at 30 days of follow-up. Secondary outcome measures included cardiovascular mortality, cerebrovascular events, myocardial infarction, bleeding complications, vascular or access-related complications and acute kidney injury. Serious adverse events were site reported and checked for plausibility. All events were adjudicated by a clinical events committee, consisting of interventional cardiologists and cardiac surgeons according to the standardised endpoint definitions proposed by the Valve Academic Research Consortium (VARC)9.
Data collection and quality control
Data were collected using standardised case-report forms available on a web-based database (www.swisstaviregistry.ch). The database was maintained by the Clinical Trials Unit at the University of Bern. Apart from baseline, procedural and in-hospital characteristics, the Swiss TAVI registry prospectively collects follow-up data at 30 days, 12 months and also after three and five years following the procedure. Follow-up was performed individually by each centre on the basis of phone calls or clinical visits. Central monitoring by an independent monitor and statistician was performed to verify completeness and accuracy of data entry at each site. Monitoring included all patients; however, no on-site monitoring or patient data validation was performed.
Statistical analysis
Continuous data are reported as mean ± standard deviation (SD), and categorical variables are reported as number of patients (% of patients) where appropriate. In-hospital events are reported as counts of first occurrence per (sub-) type of event (% of all patients). Thirty-day event rates are reported using time-to-first-event data, graphically presented using Kaplan-Meier curves, with incidence rates calculated from life tables. Event rates at 30 days were compared for patients treated with transvascular vs. surgical access, and also within transfemoral patients comparing the two main devices Medtronic CoreValve and Edwards SAPIEN XT bioprosthesis, using Cox’s regressions. Reported are crude hazard ratios (HR; with 95% confidence intervals) with p-value from Wald chi-square test, or continuity corrected risk ratios (RR; 95% CI) with p-value from Fisher’s exact test in case of zero events. Reported are adjusted HR (95% CI), where groups are compared including adjustment for age, gender, previous cardiac surgery, peripheral vascular disease, and coronary artery disease. Two-sided p-values <0.05 were considered statistically significant. All analyses were performed with Stata version 12 (StataCorp, College Station, TX, USA).
Results
PATIENT POPULATION
A total of 697 patients underwent TAVI between February 2011 and March 2013 for native aortic valve stenosis (98.1%), degenerative aortic bioprosthesis (1.6%) and native aortic regurgitation (0.3%) and were entered in the Swiss TAVI registry. Elective treatment was performed in 94.9% of cases, while 5.1% of patients underwent urgent or emergent intervention due to haemodynamic instability. A multidisciplinary decision for TAVI was reached in 97.0% of procedures. Mean age was 82.4±6.2 years and 51.5% were female. Patients were highly symptomatic, with 73.1% presenting in NYHA functional Class III and IV. The mean aortic valve area was 0.74±0.3 cm2 and the transvalvular mean gradient was 44.8±17.4 mmHg. Patients were considered to be at high surgical risk or inoperable with an estimated risk of mortality at 30 days of 20.2±12.7% according to the logistic EuroSCORE and 8.2±7.1% according to the STS score. Detailed information on baseline clinical characteristics are summarised in Table 1.
PROCEDURAL CHARACTERISTICS AND RESULTS
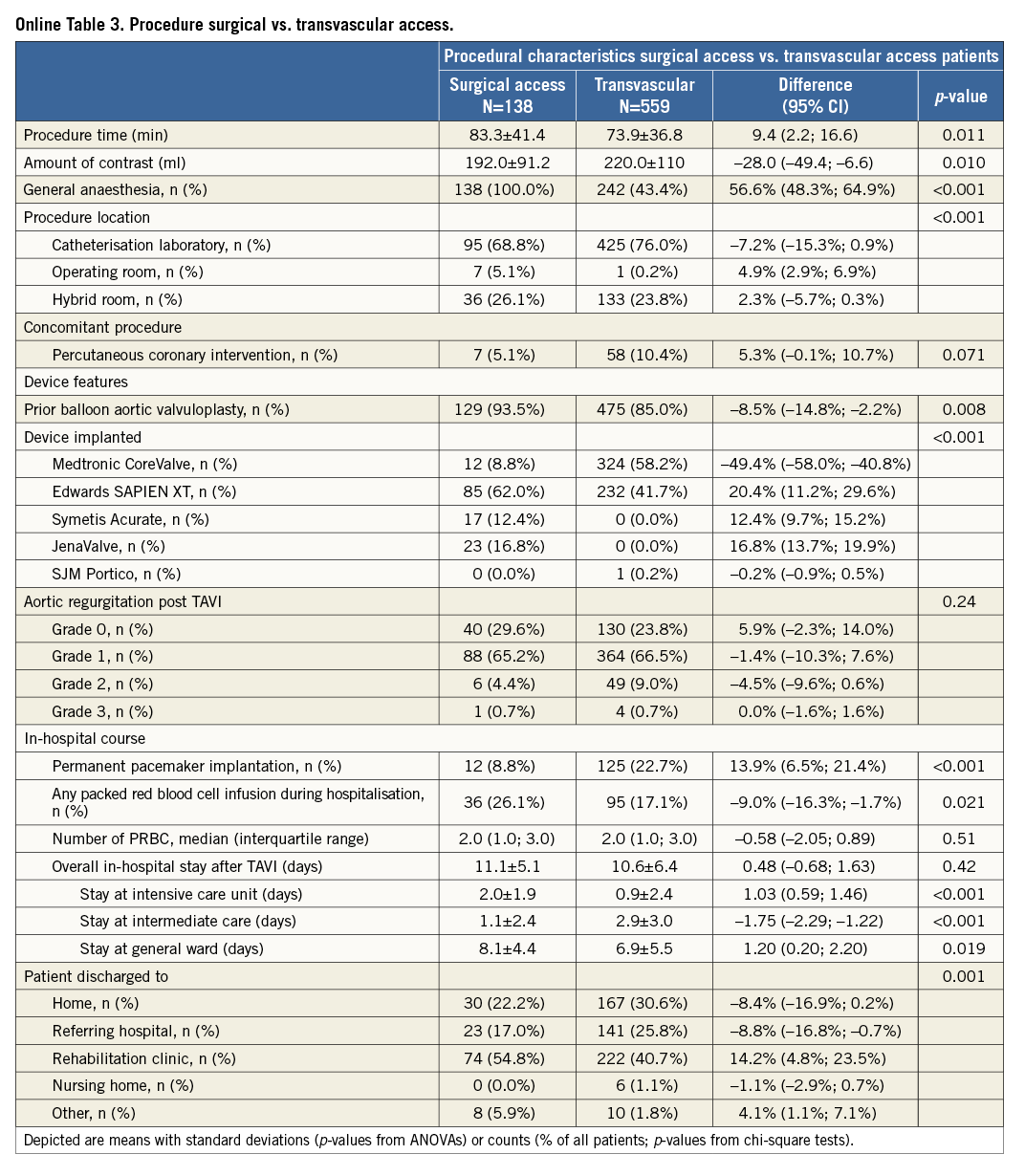
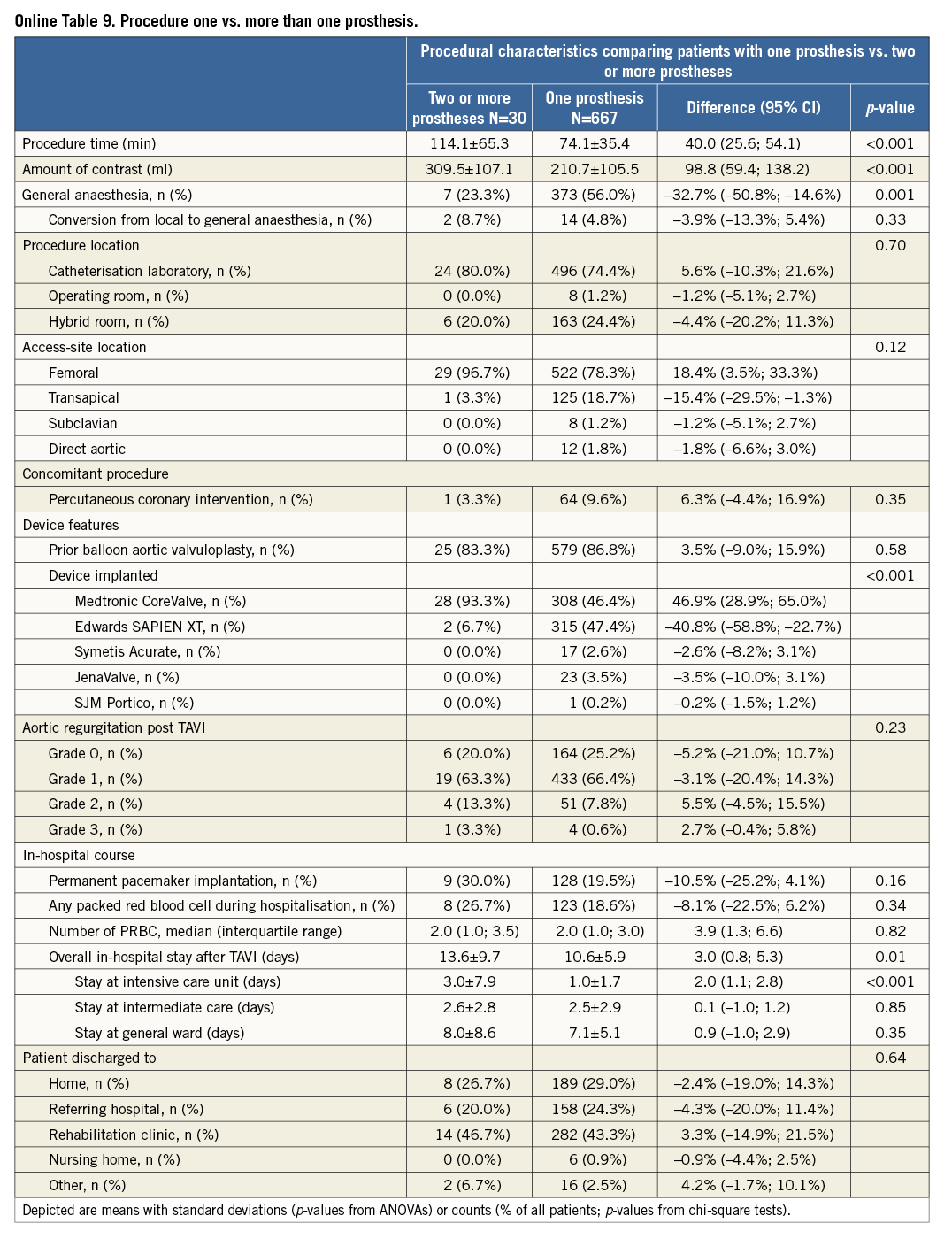
Procedural characteristics and results are presented in Table 2. Most TAVI procedures were performed in the catheterisation laboratory (74.6%), the hybrid room (24.2%), and the operating room (1.1%) using either general anaesthesia (54.6%) or conscious sedation (45.4%). Transfemoral implantation was performed in 79.1%, transapical in 18.1%, the direct aortic access was used in 1.7% and the subclavian approach in 1.1%, respectively. The majority of patients received balloon aortic valvuloplasty prior to valve insertion (86.7%).The Medtronic CoreValve was implanted in 48.4%, the Edwards SAPIEN XT in 45.7%, JenaValve in 3.3%, the Symetis Acurate TA in 2.4%, and the Portico THV prosthesis in 0.1% of patients. Most patients received only one prosthesis (95.5%); however, more than one prosthesis was required in 4.5% of patients during the intervention. A total of three patients (0.4%) did not receive any TAVI prosthesis: one patient was treated with balloon aortic valvuloplasty only, one patient was converted to surgical aortic valve replacement due to heavy calcification of the native aortic valve, and one patient died following balloon aortic valvuloplasty and prior to any valve insertion. Conversion to surgery occurred in 0.9% of patients.
Information on the in-hospital course after TAVI is provided in Table 3. Cumulative mean duration of hospital length of stay was 10.7±6.1 days, comprising 1.1±2.4 days in the intensive care unit, 2.5±2.9 days in the intermediate or coronary care unit and on average 7.1±5.3 days in the general ward. The majority of patients were discharged to a rehabilitation clinic (43.5%), while one third of patients (28.9%) were discharged home and another quarter of patients were sent back to the referring hospital (24.1%).
CLINICAL OUTCOMES
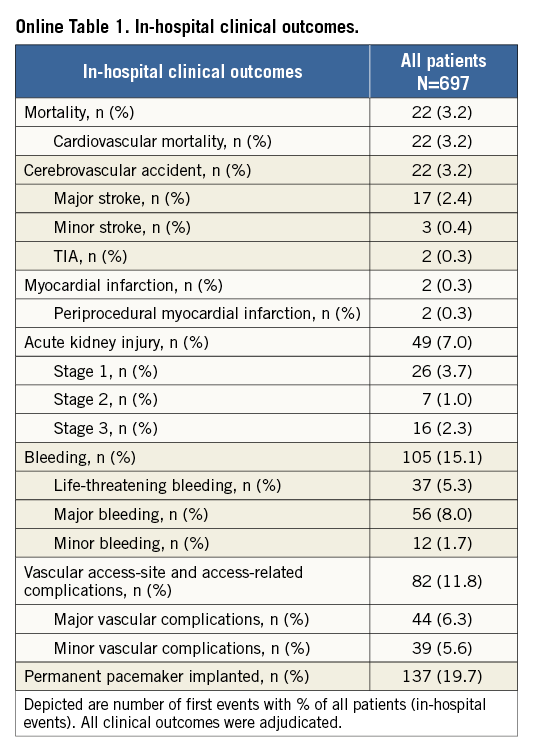
Short-term clinical outcomes within the first 30 days after TAVI are presented in Table 4 (In-hospital clinical outcomes are provided in Online Table 1). Cumulative all-cause mortality was 4.8%. All deaths were due to cardiovascular causes. The overall rate of cerebrovascular accidents after 30 days was 3.3% with the majority being major strokes (2.5%). The myocardial infarction rate was less than 1%. TAVI was effective in symptomatic alleviation (Figure 1) showing a reduction of dyspnoea from 73.1% of patients in NYHA Class III/IV at baseline to 11.0% at 30-day follow-up. Two main subanalyses were performed:
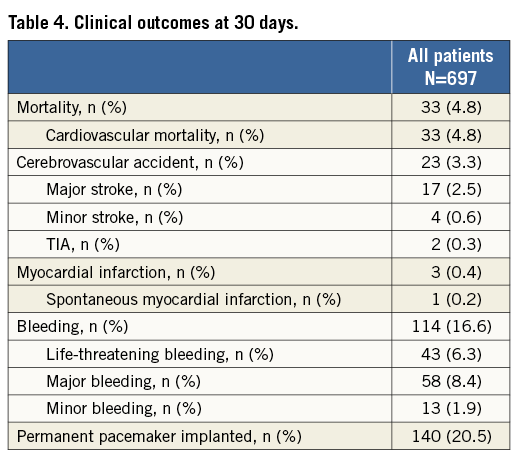
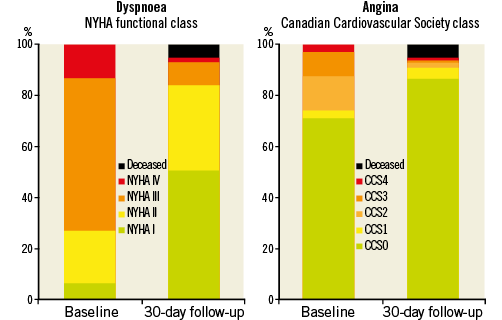
Figure 1. Symptom status at baseline evaluation and at 30-day follow-up after TAVI.
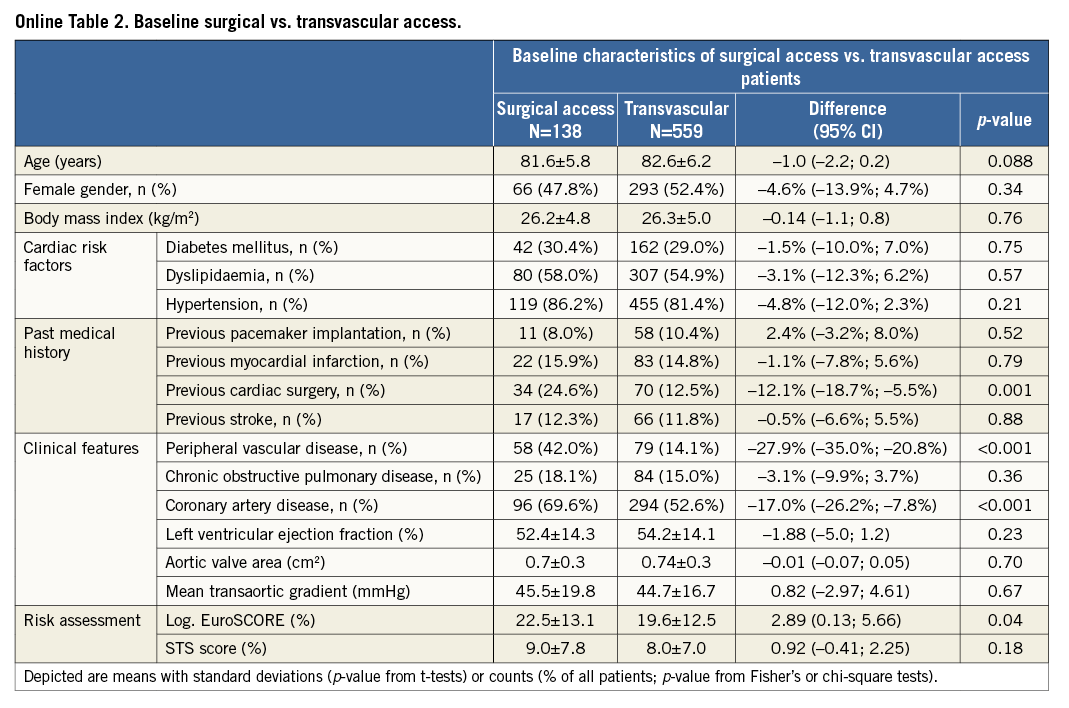
Transvascular (transfemoral and transsubclavian) versus surgical access (transapical and transaortic): baseline and procedural characteristics according to access route are provided in the Online Table 2 and Online Table 3. Clinical outcomes at 30 days of follow-up are summarised in Table 5. There were no differences with regard to cerebrovascular accidents, myocardial infarction, acute kidney injury and life-threatening bleeding complications between surgical access and transvascular patients; however, vascular access-site and access-related complications (0.7% vs. 14.7%; HRadjusted 0.05, 95% CI: 0.01-0.35) as well as major bleeding complications (3.7% vs. 9.6%; HRadjusted 0.33, 95% CI: 0.13-0.84) were found to be less frequent among surgical access patients. The increased rate of permanent pacemaker implantation between surgical access and transvascular treated patients (10.5% vs. 22.9%; HRadjusted 0.41, 95% CI: 0.23-0.72) is mainly explained by differences in device type and design, as permanent pacemaker implantation was found to be less frequent with the Edwards SAPIEN XT when compared with the Medtronic CoreValve. Furthermore, patients with surgical access were at higher risk of death after 30 days compared to patients treated by the transvascular route (Figure 2; 9.5% vs. 3.6%; HRadjusted 2.39, 95% CI: 1.13-5.04).
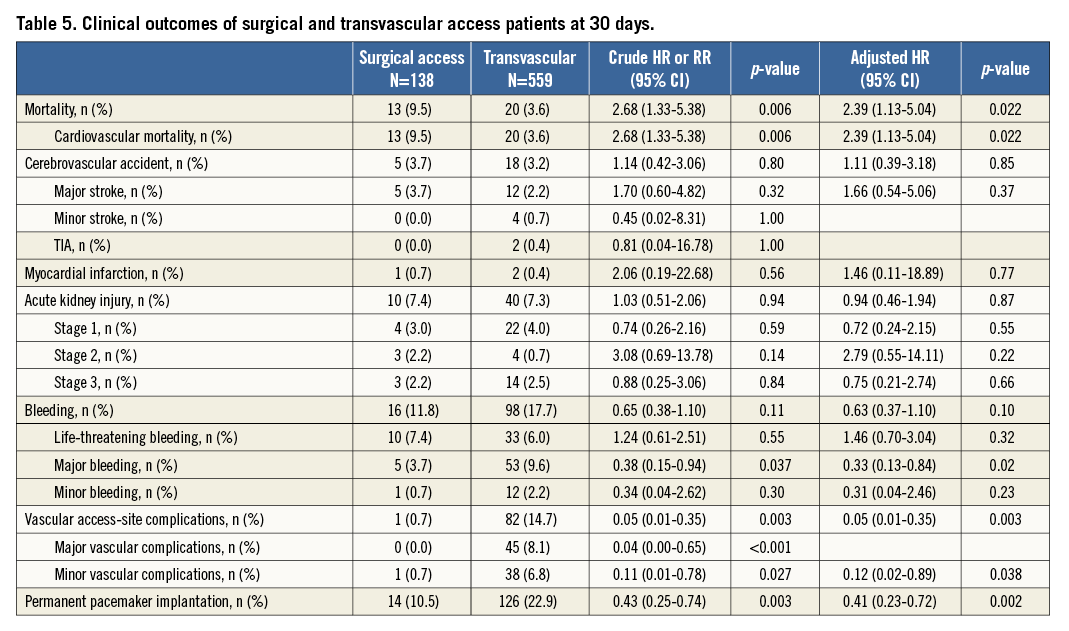
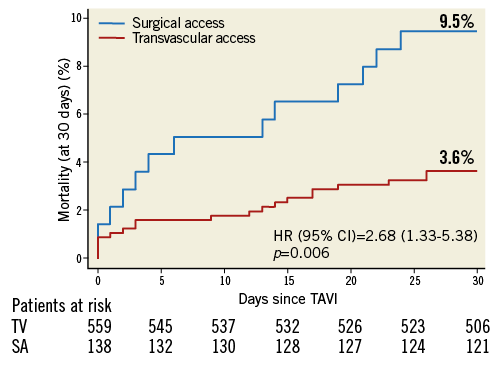
Figure 2. Cumulative incidence of all-cause mortality among patients undergoing TAVI according to type of access at 30-day follow-up. Of note, surgical access interventions include transapical and direct aortic access patients, and transvascular interventions include transfemoral and subclavian access patients. SA: surgical access
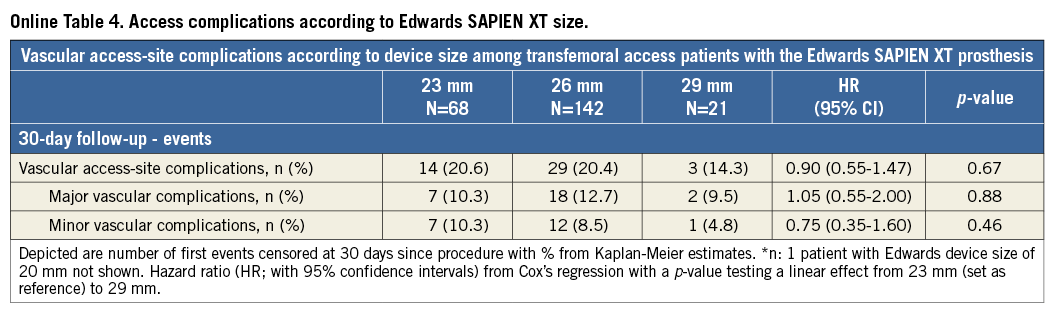
Comparison of the two most frequently used devices using the transfemoral access only: clinical outcomes at 30 days according to device type are summarised in Table 6. No differences were observed with regard to major cardiac and cerebrovascular events (Figure 3). However, there was an increased risk of vascular access-site complications when using the Edwards SAPIEN XT prosthesis (19.8% vs. 11.1%; HR 1.80, 95% CI: 1.16-2.76), which was mainly due to major vascular complications (11.7% vs. 5.6%; HR 2.12, 95% CI: 1.17-3.84). In this patient population, there was no relationship between device size and vascular access-site complications among patients receiving the Edwards SAPIEN XT prosthesis (Online Table 4). In contrast, permanent pacemaker implantation was more frequently required after the implantation of the Medtronic CoreValve prosthesis (11.4% vs. 31.3%; HR 0.33, 95% CI: 0.21-0.51).
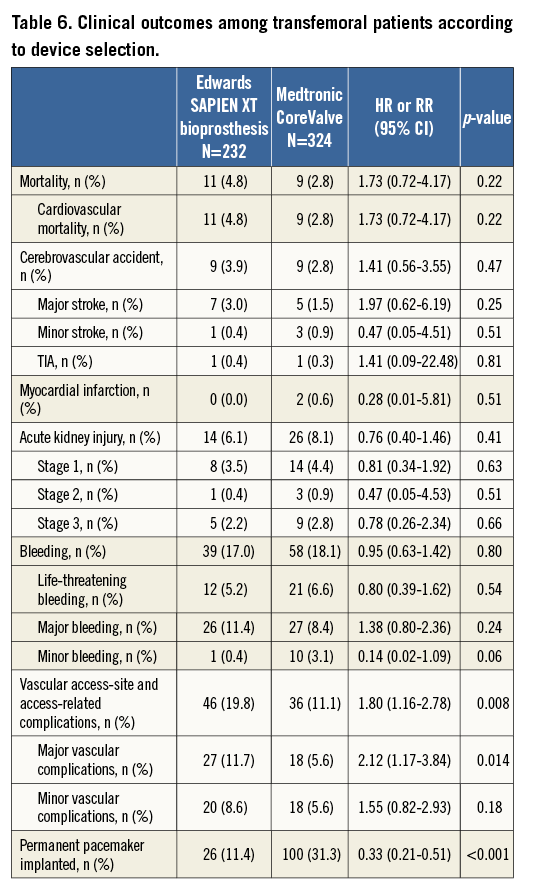
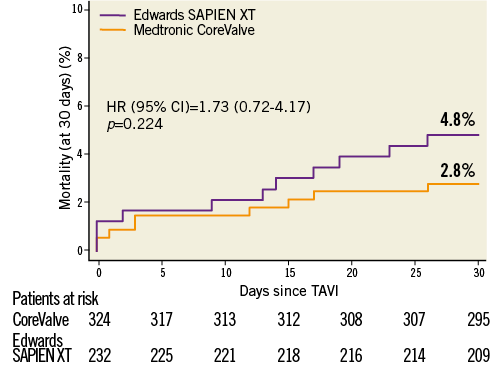
Figure 3. Cumulative incidence of all-cause mortality among patients undergoing transfemoral TAVI according to type of device implanted. Medtronic CoreValve and Edwards SAPIEN XT prosthesis implantations represent 94.1% of TAVI implantations in Switzerland.
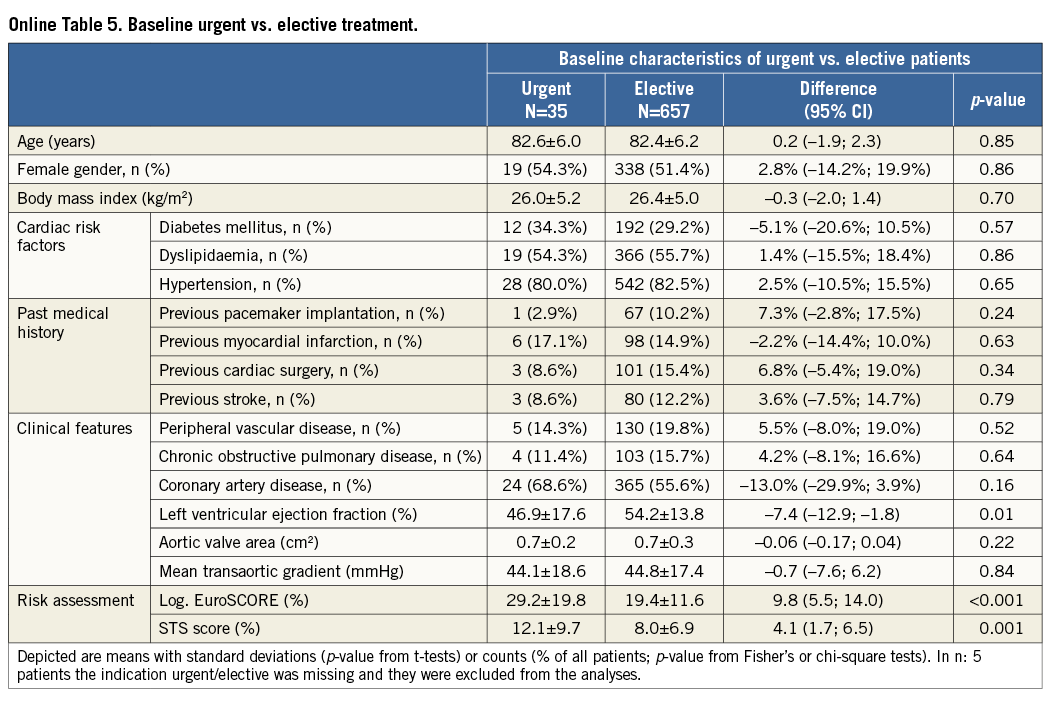
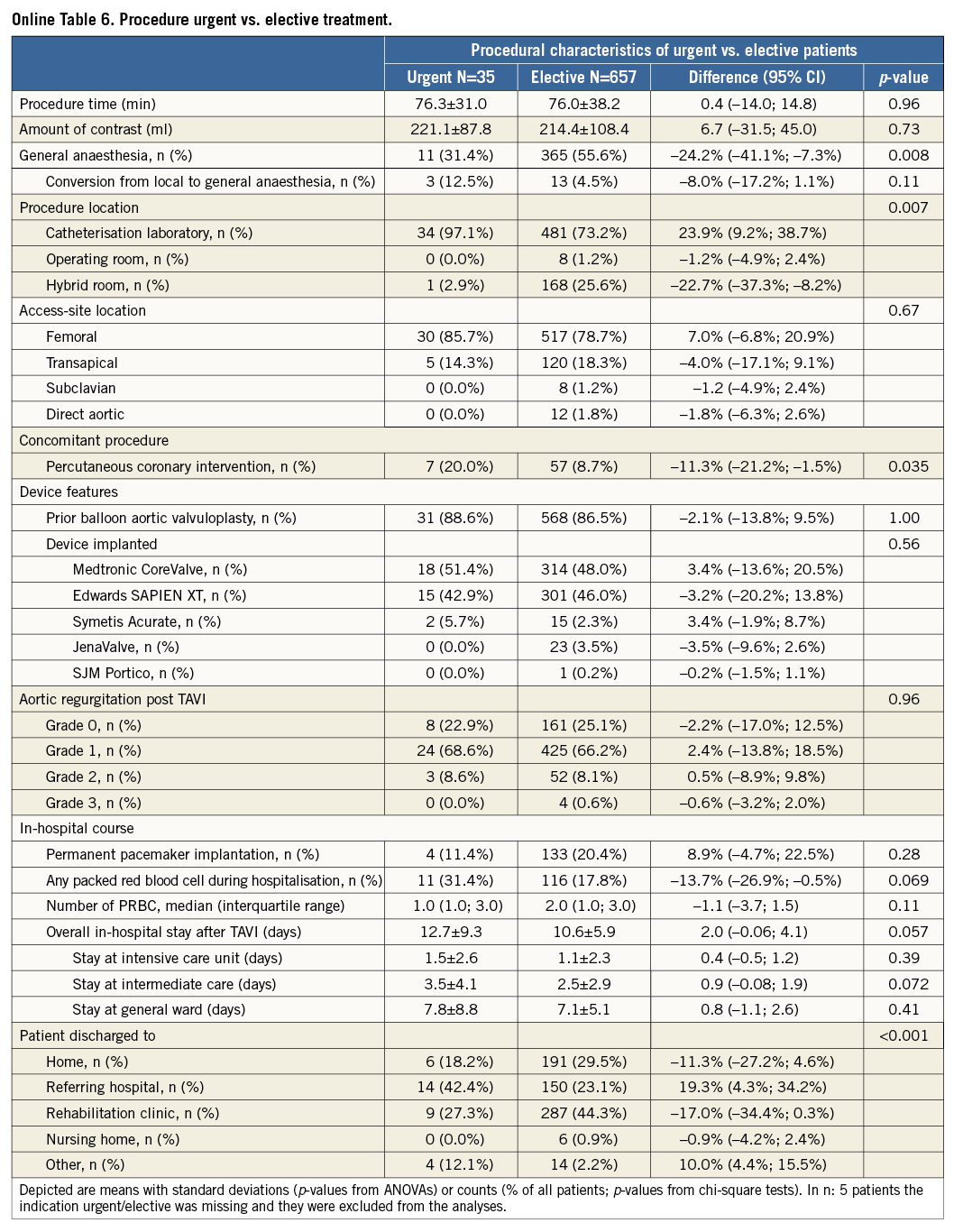
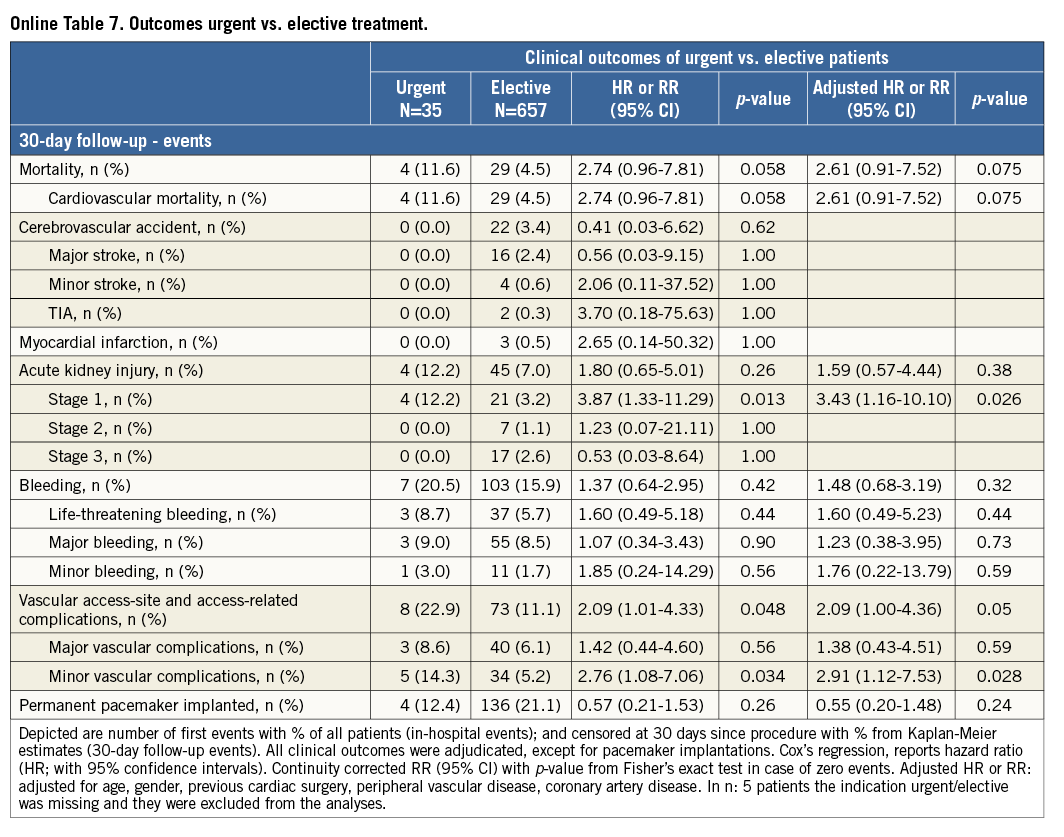
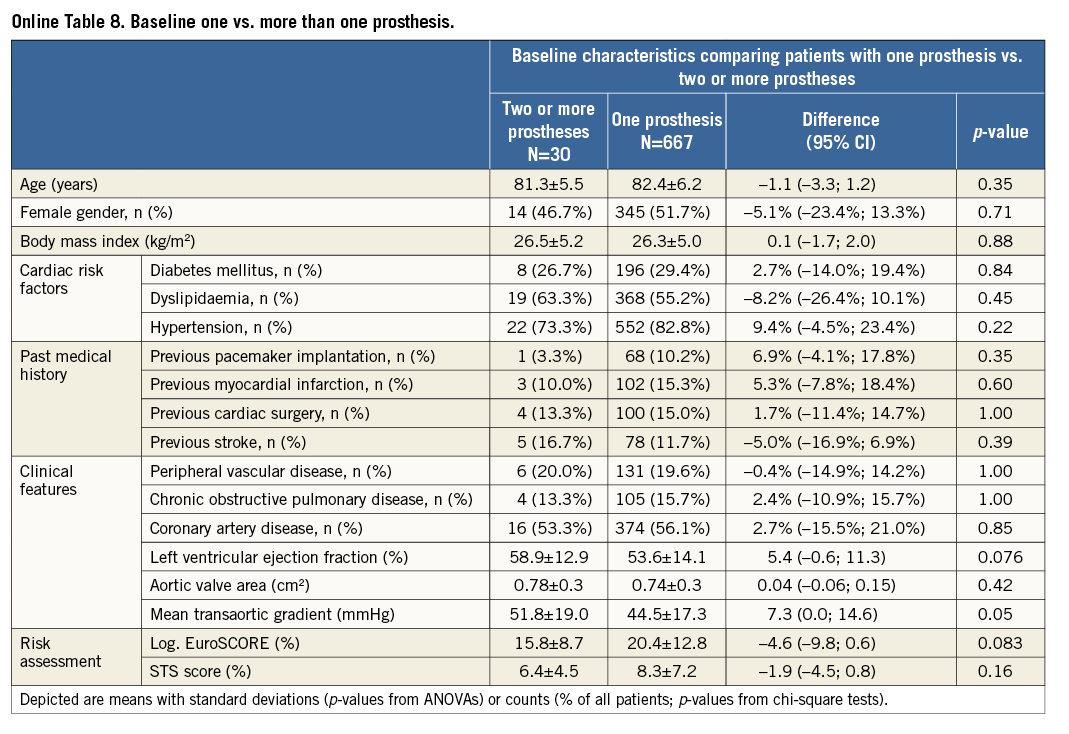
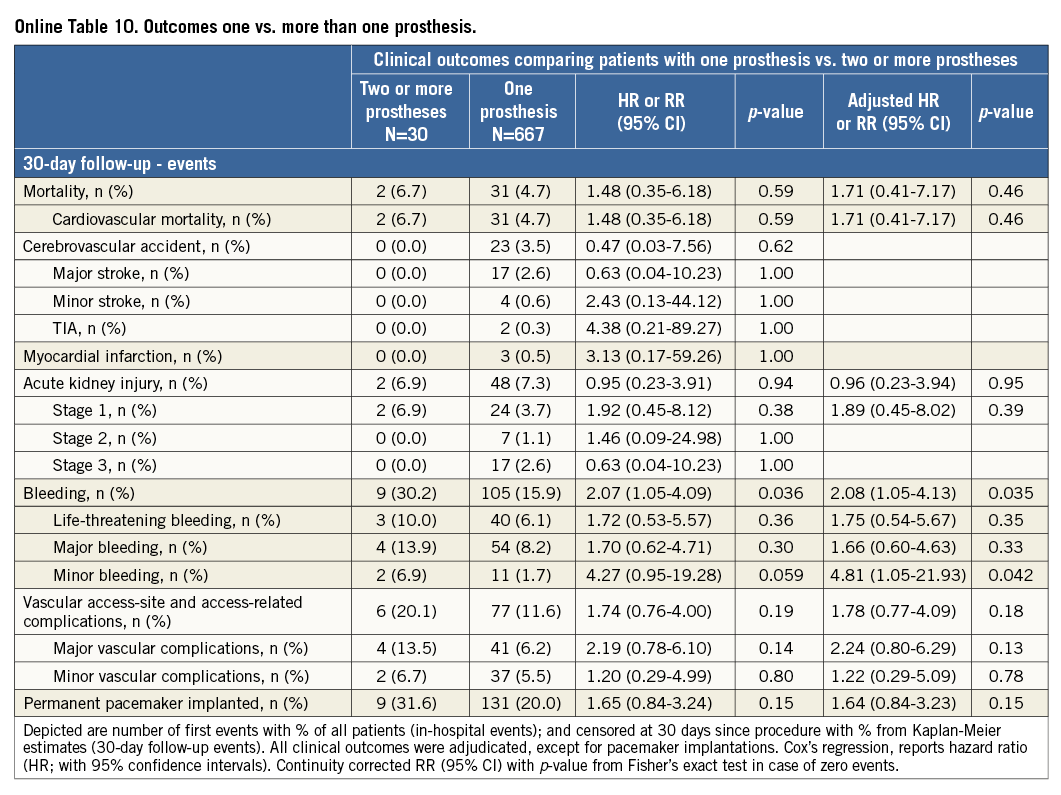
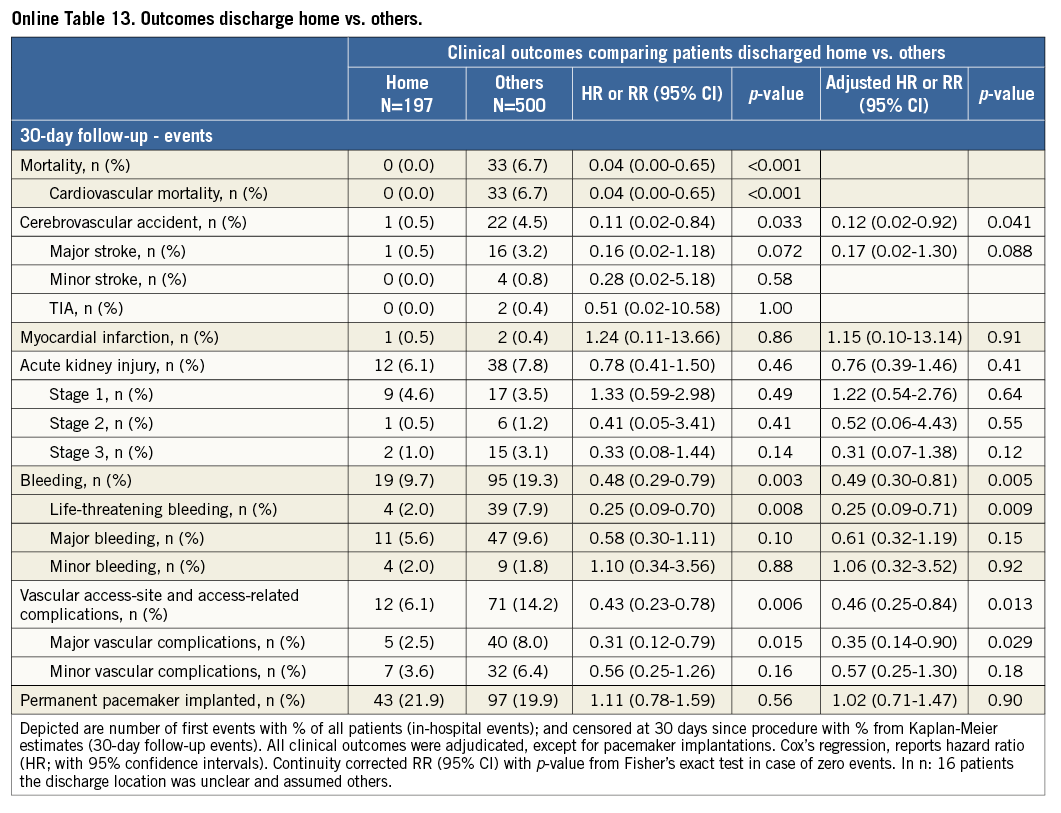
Additional subgroup analyses revealed a trend of a higher overall mortality among patients undergoing urgent TAVI in comparison with elective procedures (Online Table 5-Online Table 7). Moreover, patients receiving more than one prosthesis had a comparable outcome apart from a higher incidence of bleeding (Online Table 8- Online Table 10). Finally, patients discharged directly home (28% of the overall patient population) had very low event rates up to 30 days (mortality: 0%, myocardial infarction: 0.5%, cerebrovascular events: 0.5%) (Online Table 11-Online Table 13).
Discussion
This first report of the Swiss TAVI investigators provides detailed information on in-hospital and 30-day clinical outcomes. The main findings are:
– Carefully selected patients undergoing TAVI with different devices and access routes showed favourable short-term clinical outcomes with lower mortality rates than predicted with the logistic EuroSCORE and the STS risk score.
– Comparison of different access routes suggests a higher risk of all-cause mortality via the transapical or transaortic route even in an adjusted analysis.
– A comparative analysis between the most frequently used devices (Medtronic CoreValve and the Edwards SAPIEN XT) demonstrates no significant difference with respect to mortality, major cardiac or cerebrovascular events. However, there was a threefold increased risk of permanent pacemaker implantation for the Medtronic CoreValve and an almost twofold increased risk of vascular access-site and access-related complications among patients receiving the Edwards SAPIEN XT bioprosthesis.
In this first analysis of the Swiss TAVI registry, the overall 30-day mortality rate remains below the estimated risk using the two most frequently used risk scores for the evaluation of TAVI patients. With an estimated risk of mortality of 8.2% according to the STS score and 20.2% according to the logistic EuroSCORE, the risk of TAVI patients in Switzerland is comparable to other European or national registries11-14, and confirms the overestimation of periprocedural risk for patient selection in contemporary clinical practice. While the STS score is currently used as the standard risk assessment tool in TAVI trials, the discrepancy between the estimated risk and the observed rate of mortality after a TAVI procedure is still remarkable and highlights the urgent need for a dedicated TAVI risk score for appropriate patient selection.
All deaths within the first 30 days after TAVI were adjudicated to be due to cardiovascular causes, and the rate of 4.8% compares favourably to the large-scale CoreValve ADVANCE study (4.6%, 30-day all-cause mortality)14 and the SOURCE registry (8.5%, 30-day all-cause mortality)13, which are considered high-quality databases of contemporary TAVI experience with second-generation devices. While no significant difference was observed for all-cause mortality between the Edwards SAPIEN XT and the Medtronic CoreValve in the transfemoral cohort, there was a significantly higher risk for mortality among patients undergoing TAVI via a surgical access route. This observation was independent of baseline confounders and is consistent with previous reports from the SOURCE registry (transfemoral vs. transapical 4.3% vs. 9.9%)13, the FRANCE 2 registry (transfemoral vs. transapical 8.5% vs. 13.9%, p<0.001)11 and the UK TAVI registry (transfemoral vs. other routes 5.5% vs. 10.7%, p=0.006)12. The reason for this difference is unclear, but is most probably related to the higher risk of patients in the surgical access group. Notably, the majority of TAVI centres in Switzerland follow a “transfemoral first” strategy and the transapical access remains second choice only for patients not eligible to be treated via a transfemoral access. Knowing this, a substantial selection bias to the disadvantage of transapical patients might be mirrored in the results of this report.
The incidence of cerebrovascular accidents in the periprocedural phase after TAVI was comparable to rates reported in other registries. In Germany, periprocedural stroke after TAVI is reported with an incidence rate of 1.7 to 2.3%15, whereas the stroke rates amounted to 3.4% in France11, 4.1% in the UK (in-hospital)12, and 5% in Belgium16. In contrast to a recent meta-analysis with more than 10,000 TAVI patients, indicating a decrease in cerebrovascular accidents among patients undergoing transapical TAVI (2.7% vs. 4.2%)17, the periprocedural stroke rate was similar among surgical access and transvascular patients in our patient population. A 30-day rate of 3.3% of cerebrovascular accidents still appears relatively high and requires further action to minimise this risk. Technological improvement, more standardised procedural techniques and cerebral protection during the procedure may help in reducing these disabling adverse events. However, recent studies evaluating cerebral protection devices have so far failed to demonstrate a beneficial effect18. Last but not least, alternative antiplatelet and anticoagulation regimens (e.g., bivalirudin) might also be of help in this regard.
In this patient population, the Medtronic CoreValve was associated with a higher risk for permanent pacemaker implantation compared with the Edwards SAPIEN XT prosthesis, corroborating previous reports. Conversely, more vascular access-site complications were observed with the use of the Edwards SAPIEN XT as compared with the Medtronic CoreValve without differences in terms of bleeding. The latter observation is difficult to explain but is possibly related to the larger sheath size (outer diameter) when using the Edwards SAPIEN XT. Other confounding factors like vessel quality, tortuosity and calcification might also have contributed to this difference. Most importantly, the difference in permanent pacemaker implantation or vascular access-site complications did not translate into a higher risk of mortality.
Limitations
Several limitations need to be acknowledged before interpreting the results of this study. First, as the Swiss TAVI registry was designed to generate data on contemporary clinical practice and outcomes with newer-generation devices in Switzerland, there is no information on the learning curve or the evolution of TAVI devices, procedures or outcomes over time. Second, clinical practice and expertise might be different in the participating centres. Finally, serious adverse event reporting within Swiss TAVI is left to the discretion of each centre and, although the monitoring process includes a systematic plausibility and inconsistency check, we are not able to exclude a certain underreporting of events and bias.
Conclusion
The Swiss TAVI registry is a prospective, national cohort study assessing clinical outcomes of consecutive patients undergoing TAVI in Switzerland. This first report based on adjudicated events shows favourable short-term clinical outcomes in unselected TAVI patients.
| Impact on daily practice The Swiss TAVI registry provides favourable clinical outcomes and reflects contemporary clinical practice with CE-approved TAVI devices in patients carefully selected by the Heart Team. The transfemoral route was the preferred access for the majority of patients and was associated with low rates of mortality and cerebrovascular events. However, further improvement of the technology is required to reduce the rate of vascular access-site and bleeding complications. |
Funding
The Swiss TAVI registry is supported by a study grant from the Swiss Heart Foundation and the Swiss Working Group of Interventional Cardiology and Acute Coronary Syndromes, and is sponsored by unrestricted funds from Medtronic, Edwards Lifesciences, Symetis, JenaValve and St. Jude Medical. The study sponsors had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest statement
P. Wenaweser has received honoraria and lecture fees from Medtronic and Edwards Lifesciences and has received an unrestricted grant from Medtronic to the institution (University of Bern). F. Nietlispach serves as consultant to Edwards Lifesciences and St. Jude Medical. R. Jeger has received reimbursement for travel expenses from Medtronic, St. Jude Medical and Edwards Lifesciences. E. Ferrari is a proctor for Edwards Lifesciences. S. Toggweiler has received speaker’s honoraria from Medtronic. T. Carrel has received fees as referee from Medtronic and Edwards Lifesciences. S. Noble serves as consultant for Medtronic. P. Jüni is an unpaid steering committee or statistical executive committee member of trials funded by Abbott Vascular, Biosensors, Medtronic and Johnson & Johnson. CTU Bern, which is part of the University of Bern, has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in design, conduct or analysis of clinical studies funded by Abbott Vascular, Ablynx, Amgen, AstraZeneca, Biosensors, Biotronic, Boehringer Ingelheim, Eisai, Eli Lilly, Exelixis, Geron, Gilead Sciences, Nestlé, Novartis, Novo Nordisc, Padma, Roche, Schering-Plough, St. Jude Medical, and Swiss Cardio Technologies. S. Windecker has received research contracts to the institution from Abbott, Boston Scientific, Biosensors, Cordis, Medtronic and St. Jude. C. Huber is a proctor for Symetis and Edwards Lifesciences, serves as consultant for Medtronic and has shares in Endoheart. All the other authors have no conflicts of interest to declare.
Appendix. Collaborators and Swiss TAVI Investigators
University Hospital Basel
Department of Cardiology: Raban Jeger, MD; Christoph Kaiser, MD
Department of Cardiothoracic Surgery: Oliver Reuthebuch, MD
University Hospital Bern
Department of Cardiology: Peter Wenaweser, MD; Stefan Stortecky, MD; Lorenz Räber, MD; Stephan Windecker, MD; Saskia Dunkel de-Raad, PhD
Department of Cardiothoracic Surgery: Christoph Huber, MD; Thierry Carrel, MD
Department of Clinical Research
Clinical Trials Unit, University of Bern: Peter Jüni, MD; Dik Heg, PhD; Nico Pfäffli; Serge Zaugg
University Hospital Geneva
Department of Cardiology: Marco Roffi, MD; Stephane Noble, MD
Department of Cardiothoracic Surgery: Mustafa Cikirikcioglu, MD
University Hospital Lausanne
Department of Cardiology: Didier Locca, MD
Department of Cardiothoracic Surgery: Enrico Ferrari, MD
Cantonal Hospital Lucerne
Department of Cardiology: Stefan Toggweiler, MD
Department of Cardiothoracic Surgery: Xavier Mueller, MD
Cardiocentro Ticino, Lugano
Department of Cardiology: Giovanni Pedrazzini, MD
Department of Cardiothoracic Surgery: Stefano Demertzis, MD
Triemli Hospital Zurich
Department of Cardiology: David Tüller, MD; Franz Eberli, MD
Department of Cardiothoracic Surgery: Michele Genoni, MD; Omer Dzemali, MD
Hirslanden Clinic Zurich
Klinik im Park
Department of Cardiology: Franz W. Amann, MD
Department of Cardiothoracic Surgery: Pascal A. Berdat, MD
Hirslanden Cardiac Centre Zurich
Department of Cardiology: Gabor Sütsch, MD
Department of Cardiothoracic Surgery: Franziska Bernet, MD
Heart Clinic Hirslanden
Department of Cardiology: Roberto Corti, MD
Department of Cardiothoracic Surgery: Jürg Grünenfelder, MD
University Hospital Zurich
Department of Cardiology: Fabian Nietlispach, MD; Ronald Binder, MD
Department of Cardiothoracic Surgery: Volkmar Falk, MD; Francesco Maisano, MD
Department of Anaesthesiology: Dominique Bettex, MD
St Clara’s Hospital Basel
Department of Cardiology: Lukas Altwegg, MD (prior member of the SC)