
Abstract
Aims: Our aim was to identify current discrepancies among European countries, and provide a basis for a general agreement on decision making specifically related to TAVI procedures. The European Association of Percutaneous Coronary Interventions (EAPCI) therefore assessed the current status of transcatheter valve therapy (TAVI) in Europe through a web-based survey.
Methods and results: Three hundred and one European centres responded to the survey (61.4% of the invited centres). Fewer than 200 TAVI procedures per site had been performed up to the date of response in 47% of centres, while over 500 procedures had been performed in 21% of centres. The Heart Team consisted mostly of interventional cardiologists and cardiac surgeons. In 79% of the centres, specific TAVI protocols are in place. Of note, 45% of centres perform TAVI in intermediate-risk patients, while only 10% do so in low-risk patients. Valve selection was based principally on patient-specific variables (74%), followed by operators’ skills (55%), rates of valve-related complications (31%), and device cost (30%). Multislice computed tomography is the imaging modality most frequently performed prior to TAVI. Coronary revascularisation is usually performed before TAVI (86%), while no uniformity was observed in terms of antithrombotic therapy.
Conclusions: The EAPCI survey documents the current TAVI practice and penetration in Europe. Despite economic and regulatory difficulties, the procedure is increasingly performed and mostly according to specific protocols. The heterogeneity of the approach apparent in the survey suggests a call for an update in practice recommendations.
Introduction
Aortic and mitral valve diseases have a considerable impact on the elderly population and this is expected to increase in the forthcoming years. Transcatheter valve interventions are one of the most important innovations in cardiology, representing a major breakthrough in valve therapy across Europe. Since 2007, when transcatheter aortic valve implantation (TAVI) was introduced into clinical practice in Europe, there has been a remarkable increase both in the number of procedures and in the number of centres performing this treatment. Regarding mitral regurgitation, one percutaneous technique for mitral valve repair (PMVR) was introduced in 2008, and others have been developed more recently. Since 2012, when the Guidelines on Valvular Heart Disease of the European Society of Cardiology first recognised the effectiveness of TAVI for inoperable and high-risk patients and accepted the possibility of PMVR for specific subgroups of patients1,2, the application of these techniques in the clinical arena has been developed further in terms of indications3-5, consensus of the experts, and general beliefs6,7.
The European Association of Percutaneous Coronary Interventions (EAPCI) sought to assess the current status of percutaneous valve therapy in Europe through a web-based survey, in order to underline discrepancies among countries, and provide a basis for a general agreement on decision making specifically related to the procedures.
This paper will examine the results obtained from the TAVI section of the EAPCI survey, while a following one will discuss the section related to PMVR.
Methods
The survey was designed to investigate both TAVI and PMVR activity, focusing on the following topics in each field: 1) organisation and size of activity, 2) patient and device selection, and 3) procedural technique. The survey was divided into two sections for TAVI and PMVR, with a total of 39 questions.
The questionnaire was drafted by the EAPCI Database and Registry Committee and subsequently approved by the EAPCI Board. The survey was undertaken using a free web-based survey tool (Survey Monkey, Palo Alto, CA, USA) and comprised multiple-choice questions, including the possibility to add comments if required. It was mandatory to reply to the entire survey. The survey could be filled in anonymously, but each centre had the opportunity to provide its address in order to receive a summary of the information collected after completion of the survey.
The sample population comprised a list of European interventional centres performing structural valve procedures, obtained through merging information from each National Society and Working Group in interventional cardiology, from each national TAVI registry and from the mailing list of EuroIntervention, the official journal of the EAPCI. Overall, 490 centres were invited to participate. The survey was performed between November 2015 and January 2016. The present paper describes data obtained from the TAVI section. The results were reported as descriptive statistics.
Results
DESCRIPTION OF CENTRES
A total of 301 (61.4%) European centres participated in the survey. Individuals (n=301) who completed the survey were 274 (91%) interventional cardiologists, 18 (6%) cardiac surgeons, and nine (3%) clinical cardiologists. Numbers of centres across European countries are listed in Table 1. Among participating centres, 31 (10%) were private, 108 (36%) were community hospitals and 162 (54%) were university hospitals. Among 258 responding centres, 58% began the TAVI programme between 2007 and 2009, while 38% started between 2010 and 2015. Fewer than 200 TAVI procedures had been performed up to the date of response in 47% of responding centres (n=263), while more than 500 procedures had been performed in 21% of centres (Figure 1). The distribution across countries of centres (n=56) that have so far performed >500 TAVI procedures is shown in Figure 2. Out of these 56 centres, 43 (76.8%) began the TAVI programme before 2008.
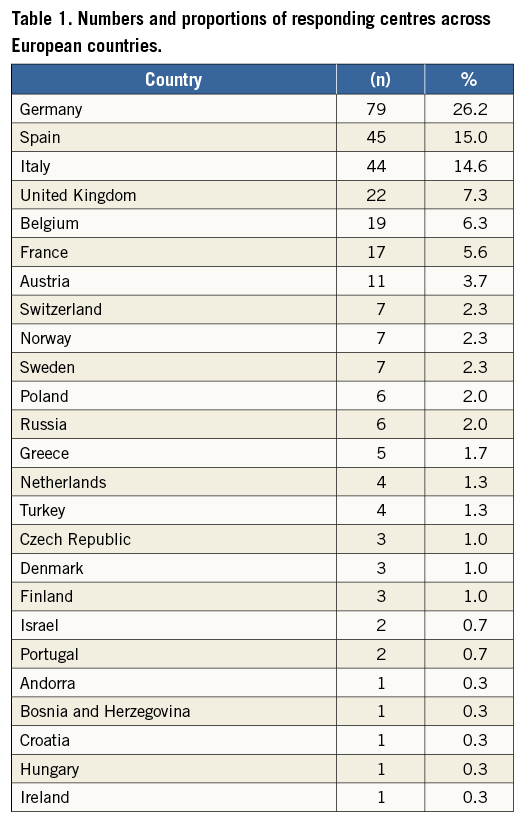
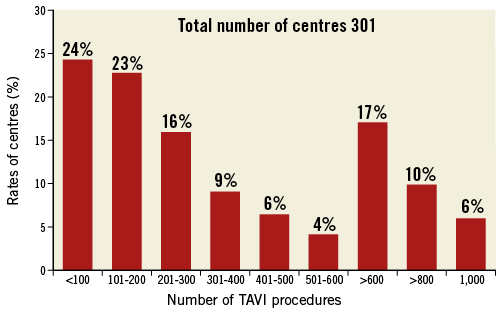
Figure 1. Rates of centres according to their TAVI experience. All 301 centres are distributed according to their accumulated experience.
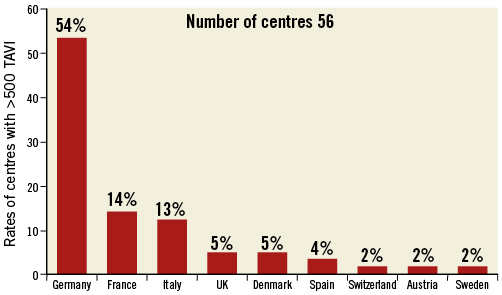
Figure 2. Distributions across countries of centres (n=56) that have performed more than a total of 500 TAVI procedures.
TAVI ORGANISATION PROGRAMME
Among the 301 centres, the head of the TAVI programme was the interventional cardiologist in 174 (58%), the cardiac surgeon in 11 (4%) and both in 116 (39%). Among responding centres (n=268), in most cases (60%) patients were referred by cardiologists within the centre or from other hospitals, while the cardiac surgeon, geriatrician and general practitioner were less frequently represented (19.5%, 6.9% and 12.9%, respectively). About half (52%) of the centres (n=268) have a dedicated ambulatory clinic for the selection and management of patients with heart valve disease. The frequency distribution of different medical professionals who form the Heart Team in centres (n=268) which responded to the specific question are shown in Figure 3. The most prevalent combinations of specialties making up the Heart Team include: interventional cardiologist and cardiac surgeon plus an echocardiographer (65%) or plus an anaesthesiologist (60%); all these latter specialists composed the Heart Team in 42% of the responding centres. The Heart Team has scheduled meetings in 81% of centres.
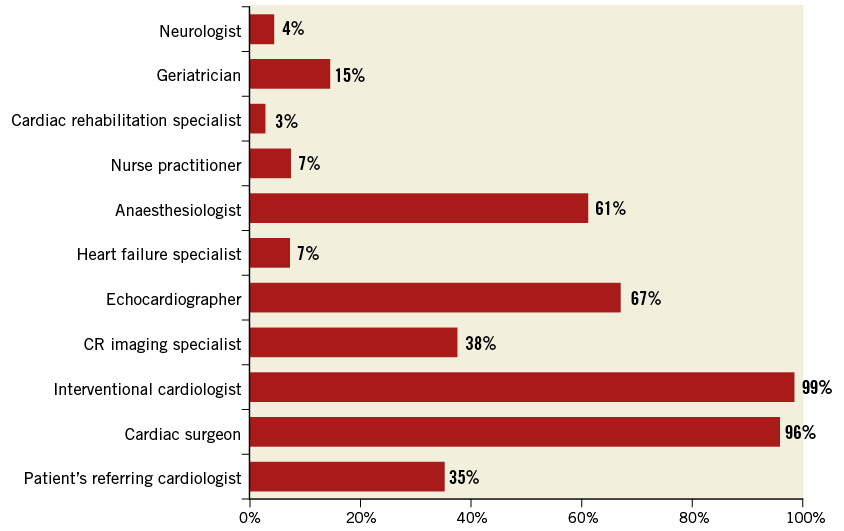
Figure 3. Heart Team structure. Proportions of different medical professionals comprising the Heart Team in different communities.
CRITERIA FOR TAVI PATIENT SELECTION
In 79% of responding centres (n=268), there are predefined internal protocols for TAVI patient selection. The majority (95%) of these centres use at least one risk score, including the logistic EuroSCORE (36%), the Society of Thoracic Surgeons (STS) score (33%) and EuroSCORE II (31%). Only 26% of centres routinely assess frailty scores in patients being evaluated for TAVI. As shown in Figure 4, centres mostly perform the TAVI procedure in inoperable patients (89%) and in those (95%) at high surgical risk (STS >8). TAVI was restricted only to inoperable patients in 5% of centres. Moreover, 45% of centres report performing TAVI also in intermediate surgical risk patients as estimated by an STS score ≥4 and <8 (Figure 4). Only 10% of centres perform TAVI in patients with an STS score <4 (Figure 4). The proportion of centres performing TAVI in patients with an STS score <8 was 44.5% among centres with at least 400 TAVI and 19.3% among those with fewer than 400 procedures performed.
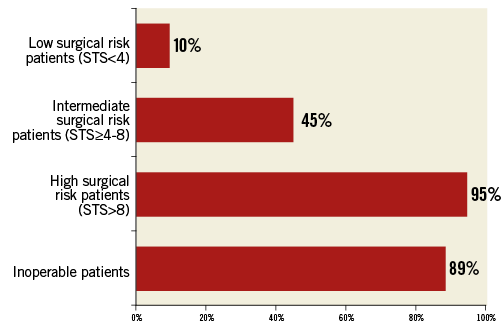
Figure 4. Patient risk distribution. Rates of patients in whom TAVI is performed across responding centres according to different risk levels described with STS score.
Finally, in most centres the TAVI procedure would be considered a possible option for bicuspid aortic valve (74%), failed surgical bioprosthesis (93%) and failed TAVI prosthesis (75%). Additionally, 49% of centres would consider TAVI for isolated aortic regurgitation.
CRITERIA FOR TAVI PROSTHESIS AND VASCULAR ACCESS SELECTION
In the vast majority of responding centres (96%), multislice computed tomography (MSCT) is the imaging modality performed to assess valve anatomy prior to TAVI. As shown in Figure 5, the most prevalent criteria used for TAVI valve selection were patient-specific variables (74%), followed by operators’ skills (55%), rate of valve-related complications described in the literature (31%), cost of the device (30%), company relationship (26%), and regulatory issues or reimbursement (16%).
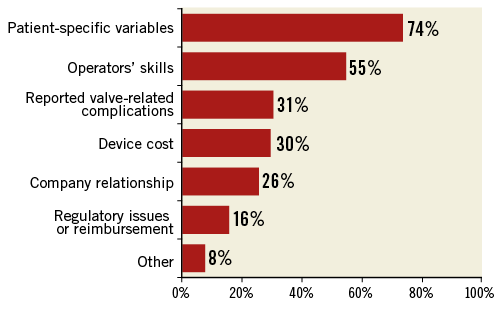
Figure 5. Valve choice. Proportions of criteria guiding the selection of a specific valve prosthesis responding to the question: which criteria are used for TAVI valve selection?
The most used imaging modality to assess the vascular access route for TAVI was MSCT (80%), followed by angiography alone (18%).
TAVI PROCEDURAL AND POST-PROCEDURAL MANAGEMENT
The TAVI procedure is performed in 61% and 39% of responding centres in the standard catheterisation laboratory or hybrid operating room, respectively. In 46% of centres, the cardiac surgeon routinely participates as an operator in transfemoral TAVI.
Overall, local and general anaesthesia are used at a mean percentage of 53% and 47%, respectively. The most commonly used vascular access route for TAVI is the percutaneous femoral (71%), followed by femoral surgical cut-down (15%), transapical (9%), percutaneous and surgical cut-down of the axillary/subclavian artery (4%). There was no association between the choice of vascular access route and centre volume. When the transfemoral access is not possible, the most frequently used accesses are transapical (37%), subclavian (32%) and transaortic (22%).
In the presence of concomitant significant coronary artery disease in patients undergoing TAVI, most centres (62%) perform percutaneous coronary intervention (PCI) of only major coronary artery branches (Figure 6). Moreover, in the majority of centres (86%), PCI is performed before the TAVI procedure (Figure 6).
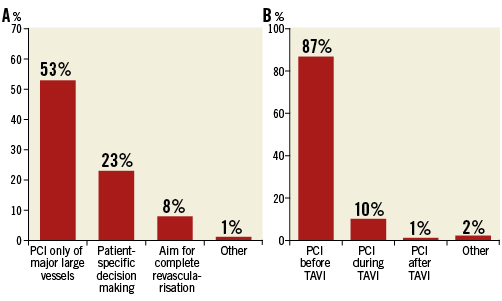
Figure 6. Management of coronary artery disease. Revascularisation strategy in (A) responding to the question - How do you manage patients with significant coronary artery disease prior to TAVI? Timing of PCI in (B) responding to the question - At which time do you perform usually coronary revascularisation in patients undergoing TAVI?
The antithrombotic regimens prescribed after TAVI include dual antiplatelet therapy (DAPT) in 75%, and single antiplatelet therapy in 10% of centres (Figure 7). When anticoagulation is indicated, only one antiplatelet agent is prescribed in combination. DAPT is recommended for up to six months in 88% of responding centres, with 53% and 16% stopping at three months and one month, respectively (Figure 7).
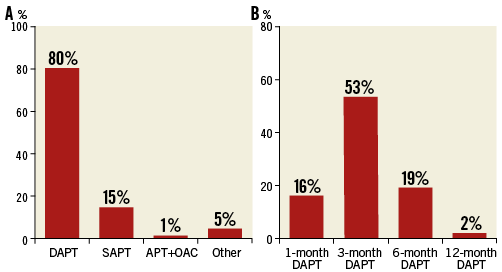
Figure 7. Antithrombotic management after TAVI. A) Type of antithrombotic therapy used. B) Timing of DAPT duration. APT: antiplatelet therapy; DAPT: dual antiplatelet therapy; OAC: oral anticoagulant; SAPT: single antiplatelet therapy
The average hospital stay for TAVI patients is <4 days in 11%, four to five days in 40% and six to seven days for 49% of responding centres. No association was found between length of stay and centre volume. At follow-up, the majority of TAVI patients are managed in a dedicated outpatient clinic in 74% of centres.
Discussion
GENERAL EXPERIENCE AND ORGANISATION
Since the introduction of TAVI, there has been a great expansion of the treatment; however, the range of distribution in different regions is considerable. In fact, the overall experience ranges from >1,000 (6%) to <100 (24%) TAVI procedures and, although more than 90% of the centres began their experience before 2010, 47% still perform fewer than 50 procedures/year (data from 2014).
The local organisation built around this therapy for the majority abides by the actual guidelines and recommendations1,2, and involves various experts among whom the principal ones are the interventional cardiologist and the cardiac surgeon. However, 21% of centres do not have an internal protocol or a routinely scheduled discussion amongst professionals.
PATIENT AND PROSTHESIS SELECTION
The results concerning patient selection point to some diversity in practice. While there is a universal acceptance of TAVI in patients with a high or extreme surgical risk in over 45%, this therapy is felt to be useful also in patients with intermediate risk and low risk (10%). It is also important to underline that the evaluation of frailty, which has been underlined and encouraged by the more recent ACC/AHA guideline2, is lacking. Moreover, more than 36% still utilise the logistic EuroSCORE, which is nowadays discouraged by recommendations2.
These findings underline the difficulties in utilising surgical risk scores and the necessity of establishing dedicated TAVI evaluations.
TAVI is widely accepted for valve-in-valve therapy in degenerated prostheses and is also used for treatment of bicuspid valves. The widespread use of TAVI in these off-label indications has to be interpreted in the light of its appraisal in the literature8-10.
The variety of devices now available allows addressing the choice of valve according to patient characteristics (74%), though it is important to underline that, after valve details and operator preferences, the economic burden for TAVI is still high, influencing decision making in over 46% (cost 30% and reimbursement 16%).
On the other hand, there is wide consensus on using MSCT as the imaging technique of choice for the selection of both valve dimensions and vascular access.
PROCEDURAL MANAGEMENT
In European centres, the majority of procedures reflect the imprint of interventional experience, as they are performed with a high use of the percutaneous approach, frequent use of local anaesthesia and quite frequently (61%) in the standard catheterisation laboratory. Of note is the coexisting participation of a surgeon (46%) during percutaneous procedures.
Coronary revascularisation procedures in patients with a TAVI indication are performed most frequently according to the actual European recommendations11 before the procedure, in 73% on a different day.
While more evidence is needed on antithrombotic therapy, at the moment DAPT is the most frequent therapy, with a wide range in duration of treatment and a rare use of anticoagulation if not needed for other reasons.
Limitations
As is usual in this kind of investigation, this survey has important limitations, which should be taken into account when interpreting the results. First of all, the questionnaire was answered by most of the centres (61.4%) but not by all. Therefore, the results are partly representative of the whole community. Moreover, although the survey was meant to address the practice of a whole centre we had to rely on the responder as the representative of the whole TAVI team. Moreover, the use of multiple-choice questions may be limiting and only partially representative of the real situation. To correct this bias, we asked the participants to add to their answers any specification they felt necessary.
Conclusions
The EAPCI survey highlights the diffusion of TAVI treatment which has shown an exponential increase, although uneven, through the whole community with an amazing lack of relation between length of experience and increase of TAVI treatment. It is probable that economic and regulatory difficulties in reimbursement are still factors in many countries and could hinder its expansion.
It is important to underline that a remarkable widening of TAVI indications has been observed, with extension to patients not included in the current guidelines but already described by contemporary literature. Moreover, not all procedural steps meet with general agreement, particularly regarding medical treatment following TAVI.
Finally, though TAVI has developed in most parts of Europe, important discrepancies are noted, and some differences and controversies point to the need for updates on recommendations that should probably be realised in the next guidelines expected in 2017.
| Impact on daily practice TAVI treatment in the European Community has demonstrated a wide but not uniform application, and the interventional community has already expanded its use to patients beyond current recommendations. These results provide a tangible demonstration of the need for available evidence to update practice guidelines on valve disease. |
Acknowledgements
The authors would like to express their gratitude to the staff of Europa Organisation/EuroPCR for the support given during survey planning and conduct. Moreover, the authors would like to thank European Presidents of National Societies and Working Groups of Interventional Cardiology for providing centre contacts.
Conflict of interest statement
A.S. Petronio is a proctor for Medtronic and Boston Scientific. N. Piazza is a consultant for Medtronic and MicroPort. The other authors have no conflicts of interest to declare.

