Abstract
Aims: To analyse patient characteristics, decision-making processes, and outcomes of TAVI performed in hospitals with versus those without on-site cardiac surgery (CS).
Methods and results: Current guidelines mandate transcatheter aortic valve implantation (TAVI) to be performed at hospitals with both cardiology and on-site CS departments. Some hospitals in Germany perform TAVI without CS departments in-house. We analysed the data of 1,432 patients enrolled in the German TAVI registry at 27 hospitals between January 2009 and June 2010. Nineteen of these had on-site CS (group 1), while eight centres performed TAVI with no CS department at their institution (group 2). Patients in group 2 (n=178, 12% of the overall study population) were older than in group 1 (mean age 82.6±6.3 years vs. 81.6±6.2 years) with similar logistic EuroSCORE (average: 21%). Patients in group 2 were haemodynamically more stable (higher blood pressures, better ejection fraction, less low-flow or low-gradient aortic stenosis, and less urgent procedures). Procedure times and use of contrast were higher in group 2. The procedural success rate was higher in group 1 (98% vs. 95%). Post-procedural complications were similar in the two groups with 30-day mortality of 6.2% in group 2 compared with 8.3% in group 1 patients.
Conclusions: Only 12% of patients enrolled in the German TAVI registry underwent TAVI at hospitals without an on-site CS department. Overall patient characteristics appeared to be similar, although patients in non-CS centres appeared to be haemodynamically more stable and more often had a history of previous heart surgery. Despite longer procedures, complication rates were similar. These preliminary data in a modest number of patients suggest the feasibility of performing TAVI in appropriately selected patients at hospitals without CS but this requires confirmation in future studies involving a large number of patients.
Introduction
Current guidelines –by expert consensus (level of evidence C)– strongly endorse transcatheter aortic valve implantation (TAVI) being performed only in hospitals with both cardiology and cardiac surgery (CS) departments on-site1,2. This is considered an optimal logistic environment for best patient outcomes mitigated through interdisciplinary decision making by the Heart Team (consisting of cardiologists, cardiac surgeons, anaesthesiologists and perfusionists) with regard to selection of appropriate patients and operative techniques, and also by allowing prompt management of potential severe procedural complications that may ultimately require emergent cardiac surgery (ECS)1,2.
According to the annual German Hospital Quality Report, a total of 81 centres throughout Germany performed 7,231 TAVI procedures in 2011. In 2012 this number increased to 9,355, while the number of open aortic valve replacements (AVR) slightly decreased from 10,289 to 9,949 during the same time period3,4. Of these 81 centres, 14 hospitals having a cardiology unit performed TAVI without having an in-house CS department. In 2012, the number of such hospitals had increased to 18 and has been stable since3. The organisational structures of these hospitals, to ensure interdisciplinary decision making by the Heart Team as well as cardiac surgical support for TAVI complications necessitating ECS, are not well defined. Similarly, demographics, clinical characteristics, treatments, complications, and outcomes of patients undergoing TAVI at these sites compared with those with on-site CS are unknown.
We analysed data of patients enrolled in the German TAVI registry4,5 to compare patient demographics, clinical features, treatments, procedural complications and outcomes in hospitals with on-site CS versus those without this capability.
Patients and methods
DESIGN OF THE REGISTRY
The German TAVI registry was a multicentre prospective national registry, which was designed to monitor the utilisation, safety and cost-effectiveness of TAVI in contemporary clinical practice4,5. The registry was performed without industry support, driven by the scientific interest of the participating hospitals, and financed by the Institut für Herzinfarktforschung (IHF, Institute of Myocardial Infarction Research) in Ludwigshafen, Germany. Details of the registry have been previously described4,5.
PATIENT POPULATION
We analysed 1,432 patients undergoing TAVI at 27 hospitals in Germany between January 2009 and June 2010. We categorised participating sites into two groups: hospitals with cardiology and CS departments on-site (group 1, n=19), and those with cardiology units but without an in-house CS department (group 2, n=8). Either group 2 hospitals performed TAVI in-house (five centres, 49 patients) with the support of an external, visiting heart surgery team (including cardiac surgeons and perfusionists) which was involved both in the TAVI decision-making process (Heart Team approach) and the procedures, or the cardiologists of hospitals without a CS department did the TAVI procedures at the surgeon’s (external) hospital (three centres, 129 patients)5.
TAVI PROCEDURES
TAVI was performed as previously described4,5, using standard transfemoral (TF) or transapical (TA) access, with or without aortic balloon valvuloplasty for predilatation and subsequent valve implantation using the CoreValve (Medtronic, Minneapolis, MN, USA) or the SAPIEN prosthesis (Edwards Lifesciences, Irvine, CA, USA).
Definitions
Similar to the updated Valve Academic Research Consortium (VARC) definitions6, technical success was defined as: (I) successful vascular access, delivery and deployment of the device with successful retrieval of the delivery system, (II) correct position of the device in the proper anatomical location with adequate performance of the prosthetic heart valve (mean pressure gradient ≤20 mmHg) and without use of multiple prostheses7. Stroke was defined as rapid onset of a focal or global neurological deficit without the presence of alternative causes, with a duration of >24 hours or available neuroimaging documenting a new haemorrhage or infarct7. Myocardial infarction was defined as new ischaemic symptoms or signs with elevated cardiac biomarkers (troponin and/or CK-MB)7. Major vascular complications were any thoracic aortic dissection, access-site or access-related vascular injury leading to either death, need for significant blood transfusions, unplanned percutaneous or surgical intervention, or irreversible end-organ damage7. Emergent conversion from TAVI to surgery was defined as a TAVI-related, life-threatening complication, occurring strictly peri-interventionally, and making immediate open heart/aortic surgery indispensable8. Post-procedural aortic regurgitation was assessed angiographically at the end of the TAVI procedure after final device deployment and removal of the catheter and guidewire, and graded using the Sellers criteria9 (absent [0], trace or mild [1/4], mild-to-moderate [2/4], moderate-to-severe [3/4], and severe [4/4]) by the treating physician4.
Data collection and statistical analysis
Data were collected via the internet by the Stiftung Institut für Herzinfarktforschung (IHF) in Ludwigshafen. Follow-up was obtained either by telephone interview (75% of patients), written communication (17%) or clinical visit (8%).
Statistical analysis was performed using the SAS statistical package, version 9.3 (SAS Institute Inc., Cary, NC, USA). Absolute numbers and percentages as well as mean (with standard deviation) were computed to describe the patient population. Categorical values were compared by chi-square test, and continuous variables were compared by two-tailed Wilcoxon rank-sum test. Thirty-day survival rates were reported using Kaplan-Meier curves.
Results
Between January 2009 and June 2010, a total of 1,432 patients underwent TAVI at 27 sites and were included in the German TAVI registry. Of these, 1,254 (88%) were treated in 19 hospitals with on-site CS (group 1, average annual volume 44), while the remaining 178 (12%, average annual volume 15) patients underwent TAVI in eight hospitals without a CS department (group 2).
Baseline data
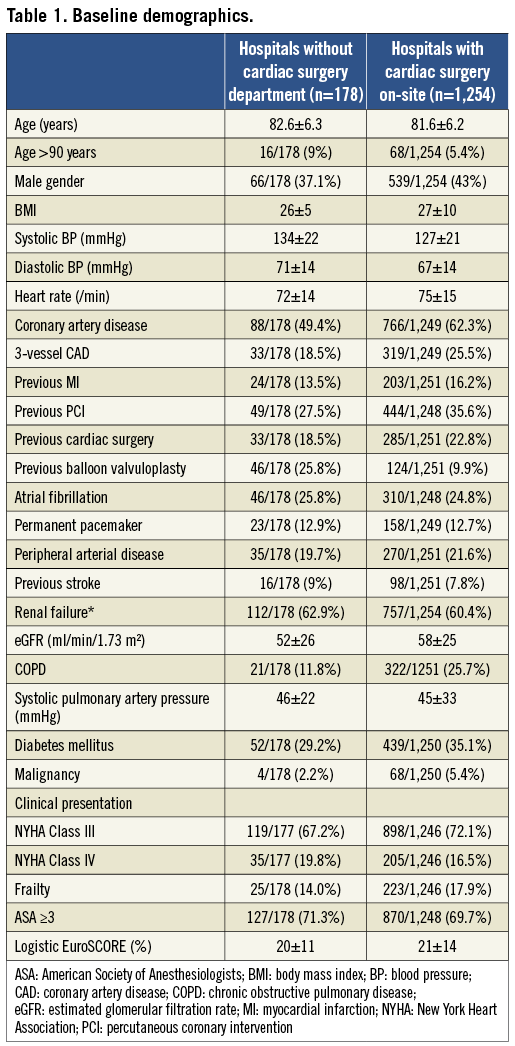
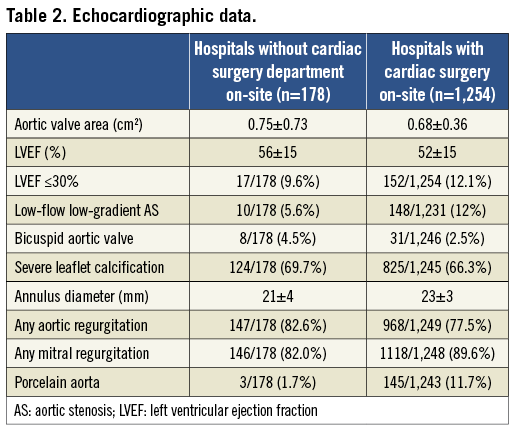
Baseline demographics are shown in Table 1. Patients in group 2 were older, were similarly symptomatic, had similar risk profiles with respect to log EuroSCORE and ASA score, but appeared to be haemodynamically more stable (higher blood pressures, lower heart rates) than patients in group 1. Echocardiographic data revealed a similar severity of aortic valve stenosis, but better left ventricular function and less frequent low-gradient low-flow aortic stenosis in group 2 (Table 2).
Procedural data
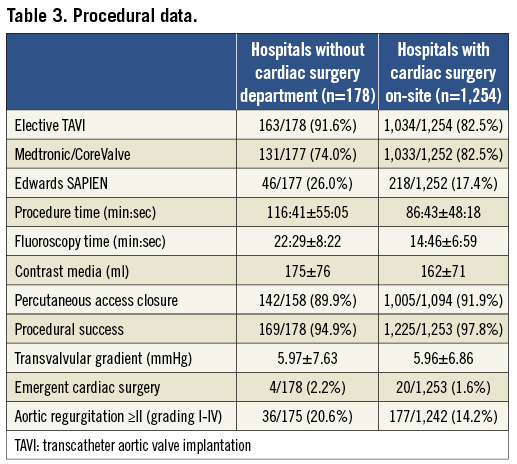
TAVI procedures were more often performed electively in group 2 as compared to group 1 (Table 3). Procedure duration in group 2 was longer, and was associated with longer fluoroscopy time and higher amounts of contrast use. Procedural success rates were ≥95% in both groups, but marginally higher in group 1 as compared to group 2 (98% vs. 95%). Four out of 178 (2.2%) group 2 patients required emergent cardiac surgery as compared to 20/1,253 (1.6%) patients in group 1. Indications for ECS in these four patients were: annular rupture (n=1), aortic perforation (n=1), coronary obstruction (n=1), and prosthesis embolisation (n=1). Three out of the four conversions in group 2 occurred during TA TAVI procedures performed at external surgical hospitals (3/129=2.3%), while a single ECS case occurred during in-house TF TAVI (1/49=2%). Two of the four patients died within 30 days after ECS (mortality: 50%), while in group 1 nine of 20 patients requiring ECS died postoperatively (mortality: 45%).
Outcomes after TAVI
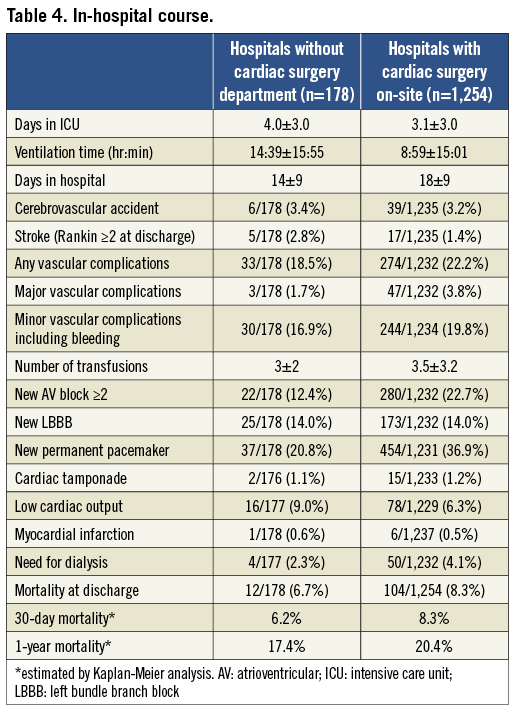
After TAVI, patients in group 1 had shorter ICU stay, but longer overall in-hospital stay as compared to group 2 (Table 4). There were no significant differences in major post-procedural complications, including cerebrovascular events, vascular access complications, and myocardial infarction. Observed mortality at 30 days and one year after TAVI was similar for patients in group 2 and those in group 1 (30 days: 6.2% vs. 8.3%; one year: 17.4% vs. 20.4%) (Table 4). Figure 1 shows Kaplan-Meier survival curves for group 2 compared with group 1 up to one year.
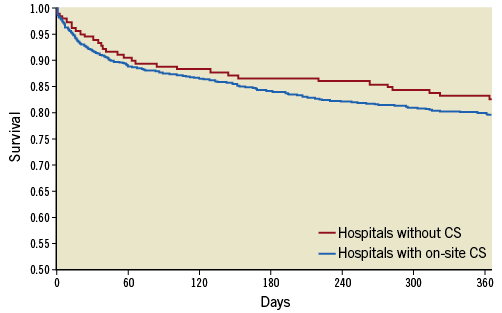
Figure 1. Kaplan-Meier survival curve of patients undergoing TAVI in centres with a cardiac surgery (CS) programme versus those without.
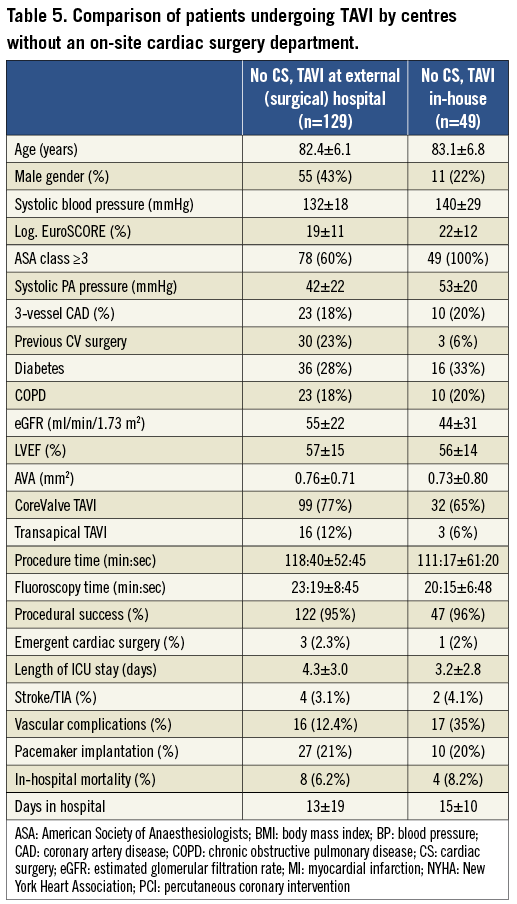
TAVI at centres without CS departments: comparison of in-house TAVI vs. TAVI at external hospitals
Patients’ age was similar in hospitals without CS performing TAVI in-house as compared to those performing TAVI at external hospitals (Table 5). Log EuroSCOREs of in-house TAVI patients were higher, while patients had less frequently undergone previous cardiovascular surgery. In-house procedures were shorter with respect to both procedure and fluoroscopy times. In-house patients had a shorter ICU stay after TAVI. Rates of major complications as well as mortality were similar.
Discussion
More recent data from the mandatory German quality assurance registry (AQUA) have suggested that the number of hospitals performing TAVI without an on-site CS department has increased in Germany to 18 such centres performing this procedure in 20123. This number is likely to increase further with improvements in technology and growing experience with this beneficial, less invasive treatment option, and perhaps to some extent because of economic considerations (gain of retaining “highly reimbursed” TAVI procedures locally). Performing TAVI at centres without an on-site CS facility is at great odds with recent guideline recommendations which mandate that these procedures should only be undertaken at hospitals with both cardiology and CS departments on-site and with a Heart Team approach which comprises cardiologists, cardiac surgeons, anaesthesiologists and perfusionists who are collectively involved in patient selection, procedural as well as post-procedural management1,2.
This approach is considered to be the safest for patients undergoing TAVI as it also allows for rapid treatment of procedural complications which may ultimately require ECS1,2. As a consequence, German public health insurance currently denies reimbursement for any TAVI procedures performed in hospitals without an on-site CS department, referring to the European Society of Cardiology guidelines on the management of valvular heart disease (“on-site” surgery)1. This explains the low number of patients undergoing TAVI by this approach (only 49 patients) during the time period 2009-2010 in the present analysis.
Hospitals without a CS department that perform TAVI argue that the success rate of this procedure is increasingly high (>95%) and the requirement of ECS is very low (≤1%, mostly based on data from sites with on-site cardiac surgery)8,10,11, and that such procedures can be safely performed at sites without CS on-site thus avoiding limited or delayed access to this technique for patients living remotely in rural areas12. Furthermore, as for other cardiac procedures, elderly patients, women, minority groups and frail patients may be more comfortable with hospitals closer to home where “their” cardiologists practise or when such procedures are made available to them locally rather than at remote major hospitals with on-site surgery13. However, data on the feasibility and safety of TAVI at these sites currently remain unknown12.
The present preliminary analysis of the German TAVI registry comprising a modest number of 1,432 patients undergoing TAVI between 2009 and 2010 suggests that only a minority (12%) of these patients underwent TAVI at a few hospitals (n=8) without a CS department, while the vast majority of patients were treated in hospitals with on-site CS departments (19 centres). Patients undergoing TAVI at hospitals without a CS department were older but had similar EuroSCOREs and appeared to be haemodynamically more stable (higher blood pressures, lower heart rates, better ejection fraction, and less frequent urgent procedures). Procedural times were longer at sites without an on-site CS department, but in-hospital outcomes and 30-day and one-year mortality after TAVI were similar in the two groups.
While these early data support the feasibility of TAVI for selected patients at sites without on-site cardiac surgery, many aspects of the current study need highlighting to prevent overinterpretation of these findings. First, clearly in agreement with the 2012 ESC guideline recommendations1, most German sites performing TAVI had on-site CS with few sites performing this procedure without on-site CS. However, sites without a CS department had the TAVI expertise and surgical back-up for ECS available (e.g., visiting surgical team at their site during the procedure). The influence of this approach (i.e., having a visiting surgical team) to the success of TAVI procedures and outcomes cannot be overemphasised. In particular, it is plausible that the operator, knowing the availability of surgical back-up, may have been more aggressive during a procedure, which he would otherwise have aborted for fear of complications in the absence of such a back-up. This together with guidance from an experienced visiting surgical team during the procedure, as well as the availability of a team for ECS if needed, could certainly have contributed to the high success rate and low complications of the TAVI procedures at these sites. Some patients who were thought to be at prohibitively high risk and likely to suffer complications at these sites may have been transferred to sites with CS without performance of TAVI, on the recommendations of the Heart Team. This may also have contributed to the high procedural success and low complication rates at sites without CS. Second, the majority of the sites that performed TAVI without on-site cardiac surgery were low-volume centres and had relatively less experience in performing this procedure compared with the on-site CS hospitals. This was evident in longer procedural times at these centres. Prior studies have shown that outcomes for the majority of invasive cardiovascular therapeutic procedures have been directly linked to the volume of these procedures at an institution. While in the small number of patients enrolled in the registry the procedural complications were not higher in patients undergoing TAVI at sites without CS, a larger number of patients need to be evaluated to confirm the safety of TAVI at low-volume centres. Third, the observed mortality among patients undergoing TAVI at sites without on-site surgery was similar, but the small sample size and relatively fewer deaths precluded robust risk adjustments to compare this event meaningfully in the two groups.
The advantages and disadvantages of performing TAVI at sites without CS could not be ascertained from this study, but can be speculated. As noted above, the availability and utilisation of TAVI at local centres is likely to improve access to this procedure, particularly for those living in remote rural areas and for elderly, women and minority group patients. However, the safety and outcomes of this approach remain to be proven in larger numbers of patients undergoing TAVI at such sites, given that these centres are likely to remain low-volu me sites. A potential disadvantage may be that ECS, if needed, may not be available in a timely fashion at sites without a CS department and this may have a negative impact on such patients. Data on the proportion of patients requiring ECS for failed TAVI are conflicting. While some investigators have suggested that ECS is required in ≤1% of TAVI patients and is associated with poor outcomes (mortality between 46% and 67%)8,10,11, a recent German single-centre analysis indicated a higher ECS rate of 4.9% (with four of the 20 patients being “converted” from transfemoral to transapical TAVI with peripheral cardiopulmonary bypass) with similar in-hospital mortality of 45% among 411 patients undergoing TAVI at their institution14. More prospective data in a large number of patients are clearly needed to identify the true incidence of ECS and its related outcomes among patients needing TAVI. Nevertheless, as stated above, for safety reasons a close cooperation with and the personal presence of a visiting heart surgery team during TAVI at a centre without an in-house CS department cannot be overemphasised.
A strategy of back-up at TAVI sites, with on-site CS by a visiting cardiac surgery team, as in the present analysis, may be able to expand the availability of these procedures to many in remote areas and may perhaps also improve patient safety. However, more information in a larger number of patients undergoing this procedure at such hospitals through a well-designed registry is needed before changing the guidelines or incorporating these findings into healthcare policy decisions.
Limitations
Our study was observational, retrospective, and with few patients. Thus, we are unable to account for the influence of missing or unmeasured confounders on the study findings. In addition, given the small number of patients (and few events), we are unable to adjust reliably for even measured confounders using multivariate analysis (Cox proportional hazard). Similarly, a propensity score adjustment was considered unfeasible because additional patients would be lost if an appropriate match were not available for them. Thus, we have refrained from multivariate analysis and provided as simple a descriptive interpretation of our data as possible. Therefore, we recommend caution when inferring causal relationships from our data. This information should be considered hypothesis-generating and should stimulate healthy discussions and future research. Finally, as for any registry, clinical events were not centrally adjudicated. Thus, there may have been a potential bias for under-reporting of these events by sites, although this bias was probably similar for sites with and without on-site CS.
Conclusions
Our analysis is the first to provide insights into TAVI performed by hospitals without on-site CS departments showing that outcomes were similar to those observed at sites with CS. These preliminary data in a modest number of patients suggest the feasibility of performing TAVI in appropriately selected patients at hospitals without CS. However, given the relatively small number of patients undergoing TAVI at sites without CS, the safety of performing this procedure at these sites compared to those with on-site CS needs confirmation in future studies involving a large number of patients.
| Impact on daily practice Transcatheter aortic valve implantation (TAVI) has evolved as an effective and safe procedure with success rates >95%. As a result, TAVI rates are rising throughout Europe, to more than 10,000 procedures in Germany in 2013. By guideline consensus, TAVI should be restricted to hospitals with both cardiology and cardiac surgery departments. The present study analysing preliminary data from the German TAVI registry (2008-2010) showed that TAVI may also be feasible in hospitals with cardiology departments closely cooperating with visiting cardiac surgical teams. This may improve patients’ access to this beneficial treatment option, without widening the indication to less sick patients. More studies are needed to prove this hypothesis. |
Conflict of interest statement
H. Eggebrecht has been proctor for and has received honoraria from both Medtronic/CoreValve and Edwards Lifesciences. R. Zahn has received research funding from Medtronic and Edwards Lifesciences. U. Gerckens is a proctor for Medtronic/CoreValve and has received honoraria from Medtronic, Edwards and Boston Scientific. All of the other authors have no conflicts of interest to declare.

