
Abstract
Transcatheter aortic valve implantation (TAVI) is evolving, with a dramatic increase in the number of procedures all over the world and a progressive shift to lower-risk patients. Valvular heart centres are accordingly adapting to the new needs and targets of the treated population. The purpose of this review is to provide an overview of the evolution of heart valve centres following changes in TAVI over time. In particular, we will discuss: 1) new challenges for the Heart Team in patient profiling and procedural tailoring; 2) the continued need for TAVI to be performed in high-volume centres with cardiac surgery on site; and 3) the importance of integrated imaging tools in modern heart valve centres.
Abbreviations
AVB: atrioventricular block
CAD: coronary artery disease
CT: computed tomography
LBBB: left bundle branch block
LVOT: left ventricular outflow tract
PCI: percutaneous coronary intervention(s)
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
TOE: transoesophageal echocardiography
Introduction (the known past…)
Transcatheter aortic valve implantation (TAVI) was introduced into clinical practice in 2002, when Alain Cribier performed his first transseptal case in a prohibitive-risk patient1. The introduction of the transfemoral approach was the key for wider penetration in interventional practice, with the first cases tackled being complex and expensive procedures due to prolonged hospital admissions that usually required multiple examinations for adequate procedural planning, and the exclusion of post-procedural complications in this context. It cannot be denied that many people questioned whether TAVI could be sustainable, affordable and applicable on a large scale. However, with increasing clinical experience and use of modern transcatheter heart valves, TAVI procedures have become more and more standardised, and outcomes have improved continuously. Consequently, scientific evidence has confirmed the efficacy of TAVI in inoperable2 and high surgical risk3 patients. The strict relation between the assessment of operability and indication for TAVI made it necessary to extend the remit and competence of the Heart Team to avoid region-dependent referral patterns4 and disagreement between specialties, both of which might have led to undertreatment of the disease5. Over time, the Heart Team gained the ability to select “high-risk” patients using a multidisciplinary approach including not only cardiologists with expertise in valvular heart disease, cardiac surgeons and structural interventionists, but also imaging specialists, cardiovascular anaesthesiologists, geriatricians and cardiovascular nursing professionals6. By putting together such a wide and interconnected range of expertise, it became easier to evaluate the single patient - not only on the basis of a surgical score, but also according to a shared decision-making approach based upon a comprehensive understanding of the risk-benefit profile. Tailoring the therapeutic strategy to individual patients was actually the key for the evolution of TAVI.
The picture in 2018 (the uncertain present...)
Evolving clinical experience definitely simplified the TAVI procedure. Improved valve and delivery catheter technologies made valve implantation via transfemoral access achievable in approximately 90% of patients using expandable sheaths and/or atraumatic small-bore delivery catheters7. Preprocedural planning and valve selection are now standardised, with computed tomography (CT) imaging serving as the optimal modality for assessing vascular access, annular dimensions and valve morphology, and predicting potential complications. The widespread adoption of similar protocols definitely increased procedural success, thus leading to a progressive lowering of the risk profile of treated patients. In the PARTNER 2A trial enrolling a population of patients with a mean age of 82 years and STS score of 5.8%, there was no significant difference in the primary endpoint between TAVI and surgery8. Similarly, but in a large population of all-comer patients, the NOTION trial found no significant difference between TAVI and surgery for the composite rate of death from any cause, stroke, or MI after one year9. Finally, and more recently, the SURTAVI trial found no significant difference in the primary endpoint (composite of death from any cause or disabling stroke) between TAVI and surgical aortic valve replacement at two years10. These three trials marked a definite change in clinical practice, with a potential boom in patients referred for TAVI. In the meantime, this required each valve centre to have safer optimised protocols to reduce complications and increase the cost-effectiveness of the procedure. Recently published ESC/EACTS guidelines11 incorporated this new concept and emphasised the central role of the modern Heart Team. With widening knowledge and expertise, the Heart Team plays a central role in every decision-making process12. The ESC/EACTS guidelines advocate centralised care, with a Class I recommendation that TAVI is performed in a heart valve centre with on-site surgery and within an environment that provides comprehensive diagnostic and treatment options. These heart valve centres, in which a multidisciplinary team works together on a regular basis with established communication structures, seem to be ideally placed to become high-volume centres of excellence for the treatment of heart valve disease. It is paramount that these centres provide a comprehensive diagnostic armamentarium of the highest quality, as well as being embedded at the hub of a network within the community and other referring hospitals. Links between the various organisations and levels of care that build a cardiovascular network need to be supported by communication structures that allow appropriate information exchange between interventional/non-interventional cardiologists, cardiac surgeons and referring physicians from primary and secondary care. Finally, clinical outcomes need to be periodically reviewed and submitted to national and European databases for further analysis.
Once the defining characteristics of a modern Heart Team have been established, it becomes essential to understand how the Heart Team should work. Discussions concerning patients with aortic stenosis should focus on clinical characteristics, anatomical/technical aspects and cardiac comorbidities. Results from different specialist areas should be taken into account and weighed against each other according to their importance in an individual patient. In this context, Heart Teams also need to reflect on the specific strengths of surgery and TAVI. While TAVI offers particular benefits in terms of reducing trauma and improving post-interventional mobilisation, surgical aortic valve replacement has particular strengths when it comes to dealing with certain aortic valve/root anatomies (asymmetric calcification, low coronary ostia or bicuspid aortic valve), concomitant aortic regurgitation and endocarditis. In addition, the already proven long-term durability of surgical prostheses should always be considered when treating younger patients with aortic stenosis.
New challenges for the Heart Team
In 2018 the Heart Team is the central decision-making organ for the treatment of aortic stenosis. While the first Heart Team experiences showed frequent antagonism between the cardiac surgeon and the interventional cardiologist, the modern concept of a multidisciplinary “Team” seems to have overcome this issue. Although time-consuming and potentially difficult to organise, the combined expertise of a Heart Team provides several advantages: balanced appraisal of specific cases (especially when clear supportive evidence [e.g., risk scores] is limited), better allocation of resources according to the anticipated benefit of treatment in different patient subsets, standardised diagnostic and operative procedures and, finally, a significant contribution to the education of medical students and trainees in one of the most difficult and rapidly evolving areas of medicine. In this context, three different models can be considered: the first, where a cardiologist acts as gatekeeper and every patient with severe aortic stenosis is discussed in a Heart Team; the second, where a cardiologist from a TAVI clinic assesses the possibility of aortic stenosis and can refer to the Heart Team; the third, where a cardiologist from the heart valve clinic refers patients with severe aortic stenosis to a dedicated heart valve centre13. Independent of the mode of referral, the presence of a TAVI clinic is essential to allow preliminary discussion with the patient (and their family) concerning treatment options and preferences.
With a wider range of patients being treated, Heart Teams need to follow specific and tailored pathways according to the global TAVI risk (which often differs from the surgical risk as calculated by standard scores) (Figure 1). Lower-risk populations require more efficient systems for patient assessment and screening, optimisation of procedural outcomes (including a lower rate of paravalvular leak and new permanent pacemaker implantation) without compromising safety. Indeed, concerted efforts have been made to simplify the TAVI procedure in lower-risk groups14,15. In this subset, patient recovery needs to be accelerated by use of fully percutaneous transfemoral vascular access using conscious sedation instead of general anaesthesia16-18, and early mobilisation after the procedure. A number of studies have demonstrated that transthoracic echocardiography is associated with similar TAVI outcomes to transoesophageal echocardiography (TOE)-guided procedures19-21.
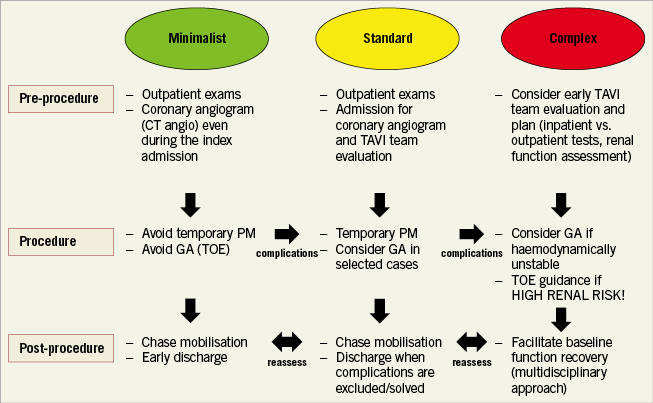
Figure 1. TAVI patient profiling. A proposed algorithm to tailor and optimise TAVI treatment according to the patient’s anatomical and clinical characteristics and anticipated procedural complexity. The key to the algorithm is to reassess TAVI procedural risk at each step (before, during and after the procedure) in order to switch to a lower- or higher-risk pathway according to circumstances. GA: general anaesthesia; PM: pacemaker; TOE: transoesophageal echocardiography
However, even in patients treated with a minimalist approach, global risk should be reassessed at baseline, during the procedure and in the post-procedural phase; when complications arise, the patient should be reassigned to a higher risk category and treated according to a pre-specified protocol. The most common application of this algorithm is the presence of new conduction disturbances after TAVI – most commonly left bundle branch block (LBBB) and complete atrioventricular block (AVB) that may require pacemaker implantation22,23, with about 33% and 50% of pacemakers being implanted within the first 24 and 48 hours following TAVI, respectively24,25.
On the other hand, TAVI still represents the only chance of treatment for high-risk frail patients with severe aortic stenosis. In this subset, the main objective is to provide a safe and uncomplicated procedure whilst avoiding futile interventions in patients who are unlikely to benefit. A considerable proportion of patients undergoing TAVI demonstrate a lack of improvement in functional status or mortality during the first year26,27. Understanding which patients will not derive any benefit from the procedure is still one of the most challenging tasks for the Heart Team. Numerous frailty scores have been proposed to improve risk stratification28. Among these, ACC/AHA guidelines recommend the Katz ADL index, measurement of gait speed, grip strength, and muscle mass for evaluation of surgical and interventional risk, in spite of a lack of specificity for TAVI procedures29. Consequently, the presence of a geriatric specialist within the Heart Team is crucial to avoid partial (and often misleading) patient evaluation. Finally, patients with concomitant coronary artery disease (CAD) are often the subject of debate. Overall, current management of CAD in TAVI patients is largely based on observational evidence, with the decision to pursue coronary revascularisation in TAVI patients usually tailored case-by-case according to clinical and anatomical variables. Although the presence of proximal vessel disease and/or high ischaemic burden usually requires preliminary revascularisation, optimal timing should be determined on an individual basis according to individual clinical and anatomical characteristics. Of note, while available evidence in support of different revascularisation strategies is mostly based on retrospective data, the ongoing percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) study is the first randomised controlled trial to compare preprocedural PCI and medical therapy for the treatment of CAD in patients undergoing TAVI30.
Do we still need on-site surgery?
TAVI figures are increasing exponentially, thus generating significant debate concerning the performance of TAVI in centres without on-site cardiac surgery, consistent with the history of percutaneous coronary interventions (PCI), that were progressively relocated from cardiac surgical centres to smaller “unprotected” institutions. The AQUA registry31 suggested that the incidence of TAVI complications was not statistically different between 75 hospitals with and 22 hospitals without on-site cardiac surgery, which has been advocated as crucial in securing “equal access” to TAVI for every patient in every local hospital32. However, such comparisons raise numerous doubts. The frequency of emergency cardiac surgery following PCI (0.2-0.6%)33,34 is not much lower than that for TAVI35, but this scenario is much less dangerous. Emergency cardiac surgery after PCI, even in the setting of acute coronary syndromes, is associated with a much lower mortality (1-20%)33,34 than that observed in the recently published large EuRECS registry35 that demonstrated a need for emergency surgery in only 0.76% of 27,760 TAVI patients, but with a mortality of 34.6%, 46% and 78% at 72 hours, within the hospital stay, and at one year, respectively. In addition, even though a number of measures (including the use of mechanical support devices and interventional bail-out strategies) can be undertaken to gain time before performing emergency surgical intervention in coronary patients, the most frequent TAVI complications requiring surgery are difficult to predict and can occur rapidly (Table 1), with success being strongly related to the immediate availability of a rapid and skilled cardiac surgeon. Are we willing to accept this risk while moving to lower-risk populations?
Tailored procedures and integrated multimodality imaging
Tailoring TAVI procedures to individual patient characteristics requires appropriate use of a wide armamentarium of diagnostic tools, mostly available in high-volume heart valve centres. While operators should rely on the most frequently used diagnostic tool in an individual heart valve centre (and/or the one with which they are most familiar), adjunctive tools should be available for selected patients if large volumes are desired. Angio-CT is the gold standard for annular measurement: it allows an orthogonal view of the centre axis of the left ventricular outflow tract (LVOT), careful measurement of the size of the sinuses of Valsalva, the distance of the coronary ostia from the annulus (which identifies patients at risk of coronary occlusion), the size of the aorta at the sinotubular junction, the extent and distribution of aortic calcification, and information concerning vascular access36. However, use of contrast may present a risk in the subgroup of patients with pre-existing (or risk of) renal dysfunction; modern heart valve centres should consider an alternative strategy in this setting. A wide body of literature confirms the utility of TOE (especially 3D imaging) in annular sizing (Figure 2): several studies have demonstrated good correlation with angio-CT in predicting moderate or severe paravalvular regurgitation after TAVI37-39. In addition, TOE can identify the extent and location of annular and LVOT calcification which may interfere with valve deployment.
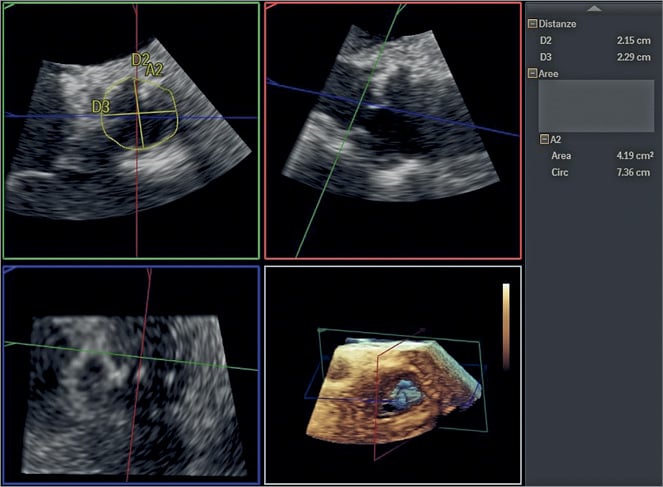
Figure 2. Aortic valve annular sizing at TOE. Aortic annular diameters and perimeter are calculated using 3D TOE. The subsequent TAVI procedure was performed without using contrast dye.
There has been some concern that 3D TOE undersizes the annulus compared to angio-CT, with figures between 9-12% quoted for the degree of discrepancy40,41. However, this has not translated into clinically significant undersizing36-38 and centres using TOE have not reported relevant differences from angio-CT. The opportunity to use TOE guidance is particularly relevant whenever the use of contrast medium should be avoided. The use of specific protocols aimed at avoiding the use of contrast in patients with pre-existing severe renal dysfunction should be encouraged according to local preference and availability. TOE guidance combined with assessment of peripheral vascular access using carbon dioxide angiography (Figure 3) and no-contrast CT (to localise and quantify vascular calcification better) could be implemented on a larger scale to allow performance of TAVI without use of contrast dye from the planning to the execution of the procedure42. Although TOE may be associated with the need for general anaesthesia, the clinical benefits of such an approach in very high-risk patients are potentially significant.
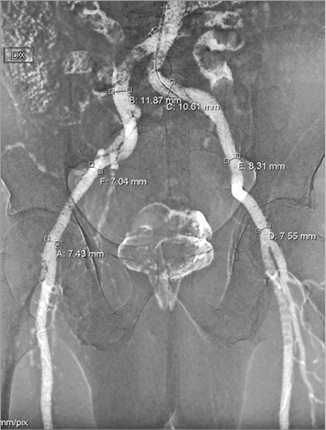
Figure 3. Carbon dioxide iliofemoral angiography. Transradial iliac and femoral angiography using carbon dioxide in a patient with severe renal dysfunction providing reliable vessel sizing. The same technology could be considered a back-up tool to investigate possible vascular complications at the end of the procedure without need for contrast injection.
Finally, the opportunity to integrate TOE or CT images alongside fluoroscopy is crucial when more detailed anatomical information is required (e.g., bicuspid anatomy, mechanical mitral valve prosthesis, horizontal aorta). Fluoroscopic imaging limitations often relate to the difficulty of finding a comfortable working view and acquiring sufficient anatomical detail to guide the intervention. Although data concerning such “fusion imaging” are limited, application of this technology may increase the safety and accuracy of transcatheter valve procedures, employ less contrast material, and reduce overall radiation dose43.
What to expect (the optimistic future…)
In the context of an expanding ageing population, the number of TAVI procedures is set to grow exponentially over the next decade. Nowadays, the main limiting factors to TAVI implementation are the availability of resources and procedural costs. The desired reduction of device costs has yet to happen, so the procedure is still not considered entirely cost-effective when performed in low-volume centres.
Of note, the opportunity of using a simplified transfemoral procedure is strongly related to the maintenance of high-quality decision making, excellent outcomes and training of a sufficient number of specialists in every heart valve centre. Moving to low-risk patients will require maintenance of strict levels of care and rigorous decision-making processes, with each centre tailoring protocols and strategies according to their internal logistics and requirements to reduce the duration of preprocedural and post-procedural in-hospital stay without compromising safety44. To achieve this goal, every procedure should still take place in a centre with on-site cardiac surgery to ensure availability of the best emergency option for every subset of patients. In addition, caring for more patients will definitely require an effective cardiac surgical team to treat the growing number of patients with aortic root dilatation, coronary artery disease, and concomitant mitral or tricuspid valve disease.
The immediate future will clarify whether TAVI becomes the standard of care for most patients with severe aortic stenosis, including those at low surgical risk. Remaining technical issues, including the efficacy of the procedure in selected populations (e.g., bicuspid valves), the rate of pacemaker implantation and the highly debated long-term durability of the devices45 will determine this progress. Movement to lower-risk patients will require a dramatic reduction in the rate of new permanent pacemaker implantation, with specific devices preferred in younger patients8,46. An improved understanding of the clinical course of conduction disturbances after TAVI will be crucial to identify the most relevant predictors of new permanent pacemaker requirement and avoid unnecessary device implantation. Although evidence is growing, many questions remain and more studies are needed, particularly with newer-generation transcatheter valve platforms47.
The availability of less expensive and more versatile devices will increase operator confidence and experience and facilitate access to TAVI, even in lower-volume or economically deprived countries.
Meanwhile, greater awareness and preparation for the wide variety of different patient profiles will require operators to tailor procedures and protocols to the most relevant issues associated with each clinical case, and to shape each TAVI procedure according to the clinical and anatomical features of each individual patient.
Conclusions
In 2018, TAVI is a definitive and simplified procedure ready to be brought to a lower-risk population. This will require the presence of strict standardised pathways in each heart valve centre in order to tailor procedural and in-hospital decisions to an individual patient’s clinical and anatomical conditions. Optimisation of TAVI requires careful planning of each step –from the diagnosis of aortic stenosis to full post-procedural rehabilitation– in a multidisciplinary Heart Team environment.
Conflict of interest statement
B. Prendergast has received unrestricted research grants from Edwards Lifesciences and lecture fees from Edwards Lifesciences and Boston Scientific. F. Castriota has received consultant fees from Boston Scientific, Abbott Vascular and Medtronic. R. Nerla has no conflicts of interest to declare.

