Abstract
In the last 15 years, transcatheter aortic valve implantation (TAVI) technology has met with rapid uptake, with >350,000 procedures performed in >70 countries. Over the same period, this technique has evolved from a challenging intervention to a standardised, simple, and streamlined procedure. The purpose of this review article is to provide an overview on the rapidly changing field of TAVI therapy, exploring past achievements, current issues and future perspectives. The discussion has been subdivided into three sections: 1) the evolution of clinical and anatomical indications for TAVI, 2) the description of procedural advances and complication rate trends over time, and 3) the progressive adoption of a minimalist approach.
Introduction
Since the first-in-human transcatheter aortic valve implantation (TAVI) performed by Alain Cribier in 20021, this innovative procedure has gained widespread recognition as the treatment of choice for severe aortic stenosis in inoperable patients and as a reasonable alternative to conventional surgical aortic valve replacement (SAVR) in patients with intermediate and high surgical risk2 (Figure 1). Over the last 15 years, TAVI technology has had impressive uptake, with >350,000 procedures performed in >70 countries3. Over the same period, TAVI has evolved from a challenging intervention to a standardised, simple, and streamlined procedure3.
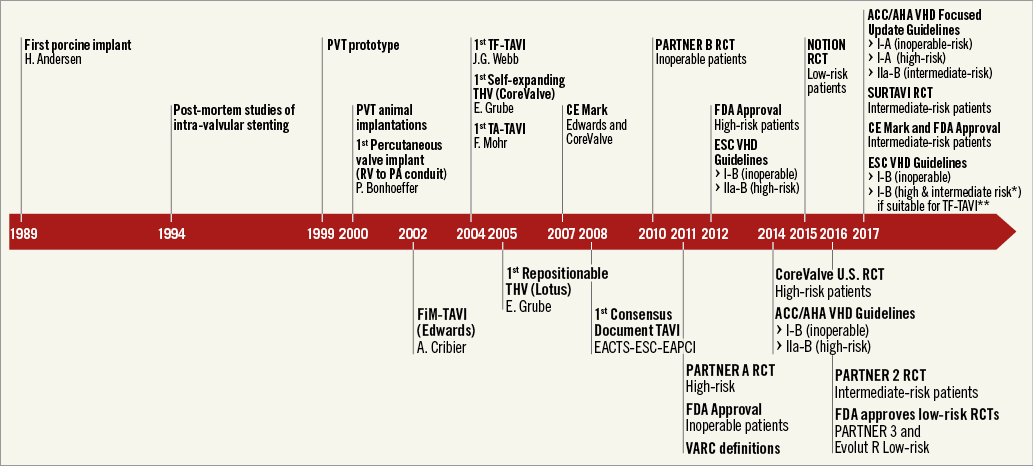
Figure 1. The most representative moments of TAVI history. *Defined as STS or EuroSCORE II ≥4%. **The decision between SAVR and TAVI should be made by the heart team, with TAVI “being favoured” in elderly patients suitable for transfemoral (TF) access.
The purpose of this review article is to provide an overview on the rapidly changing field of TAVI therapy, exploring past achievements, current issues and future perspectives. In the interest of clarity, given the complexity and the vastness of the topic, the discussion has been subdivided into three areas that will cover: 1) the evolution of clinical and anatomical indications for TAVI, 2) the description of procedural advances and complication rate trends over time, and 3) the progressive adoption of a minimalist approach.
Evolution of TAVI indications
For many years SAVR has been the treatment of choice for the majority of patients with severe aortic stenosis, with relief of symptoms and improved survival2. However, in 2005, the Euro Heart Survey demonstrated that, in a substantial number of patients, mainly the very elderly and those with severe comorbidities, the risk of SAVR was often considered to be excessive. Therefore, these patients were often not offered surgery4. TAVI has evolved as a response to this important unmet clinical need.
Over the past 15 years, the indications for TAVI have changed dramatically, shifting quickly from compassionate cases, to inoperable and high-risk patients, and more recently towards intermediate-risk populations (Figure 1). Favourable outcomes of TAVI in observational registries5 and in randomised comparisons with surgery3 (Figure 2) led, in the 2017 American Heart Association (AHA)/American College of Cardiology (ACC)/Society of Thoracic Surgery (STS) Guidelines, to upgrading the indication for TAVI in inoperable and high-risk patients (I-A) and to including, for the first time, TAVI as an option also in intermediate-risk patients (IIa-B)2 (Figure 1). More recently, the European guidelines included the SURTAVI intermediate-risk trial, along with PARTNER A (high risk), PARTNER B (inoperable), PARTNER 2 (intermediate risk), CoreValve (high risk), and NOTION (intermediate/low risk).
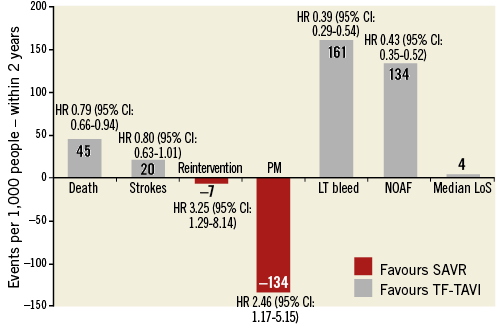
Figure 2. Comparison of major clinical outcomes between transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR). Original figure created from data reported by Siemieniuk RA et al39. CI: confidence interval; HR: hazard ratio; LT: life-threatening; LoS: length of stay; NOAF: new onset atrial fibrillation; PM: pacemaker
On the basis of this evidence, the new guidelines give a clear indication for SAVR for symptomatic AS in low-risk patients (I-B), while TAVI is recommended for patients deemed not suitable for surgery, as assessed by the Heart Team (I-B). In patients at increased surgical risk (STS or EuroSCORE II ≥4%, a cut point which also included intermediate-risk patients), guidelines recommend that the decision between SAVR and TAVI should be made by the Heart Team, with TAVI “being favoured” in elderly patients suitable for transfemoral access (I-B)6.
Indications for TAVI in specific subsets
An area of particular interest is the expansion of TAVI to many other patient populations, in which outcomes are still suboptimal or simply unexplored.
BICUSPID AORTIC VALVE
Bicuspid aortic valves have a high prevalence in younger patients with AS; however, even in the elderly (>80 years of age), bicuspid valves comprise approximately 20% of surgical cases7. Early experience with TAVI in bicuspid valves demonstrated that this anatomical entity has several features that more often make the outcomes of TAVI suboptimal and less predictable – a more oval annulus shape, unequal leaflet size, heavy and uneven calcification of the leaflets, and the presence of calcified raphes.
One study on early-generation TAVI devices showed a higher incidence of transcatheter heart valve (THV) malposition requiring multiple THV implantation (7.2%), and moderate or severe paravalvular regurgitation (PVR) in 15.9% of patients7. In a recent large multicentre propensity-based analysis comparing TAVI outcomes in bicuspid versus tricuspid aortic valves, Yoon et al7 showed that, within the group receiving the early-generation devices, patients with bicuspid AS more frequently had aortic root injury, second valve implantation and moderate or severe paravalvular leak (PVL). According to the authors of the study, these complications occurred more frequently in patients with a heavily calcified raphe in a Type 1 bicuspid valve8.
In conclusion, according to the current evidence, bicuspid anatomy should not be excluded from TAVI. However, it is undeniable that outcomes are not as favourable as with tricuspid valves, and indications for TAVI must be carefully discussed based on patient-specific aortic root anatomy and calcium distribution.
DEGENERATED SURGICAL BIOPROSTHESES
Transcatheter valve-in-valve (ViV) implantation has emerged as a novel, less invasive therapy for failed bioprosthetic surgical valves8. On the basis of clinical registry data, the self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA) and the balloon-expandable SAPIEN XT and SAPIEN 3 valves (Edwards Lifesciences, Irvine, CA, USA) have been approved for use in high-risk patients with aortic bioprosthetic valve failure. Of note, in this multicentre report, stenotic degeneration of the surgical bioprosthesis and small valve implant size were associated with worse clinical outcomes9. From a technical standpoint, compared with native valve TAVI, transcatheter ViV therapy resulted in less frequent leaks and fewer new pacemakers but more frequent coronary occlusion and residual patient-prosthesis mismatch.
Recently, the results of the PARTNER 2 Valve-in-Valve registry and the CoreValve U.S. Expanded Use Study were released10,11. In contrast to the VIVID registry, these were prospective studies that incorporated a clinical events committee and core lab adjudication. The PARTNER 2 Valve-in-Valve registry included the 96 patients from the initial nested ViV registry for the original PARTNER 2 trial plus an additional 265 patients treated as part of a continued access protocol. For the cohort as a whole, 30-day and one-year mortality rates were 2.7% and 12.4%, respectively. Similarly, when the first 100 patients were compared with those who followed, the results pointed to significant improvements in parallel with operator experience: mortality at one year was nearly 20% among the first 96 patients treated as compared with 9.8% among the patients who followed11. The CoreValve U.S. Expanded Use Study enrolled 233 patients with symptomatic surgical valve dysfunction. The all-cause mortality rate was 2.2% at 30 days and 14.6% at one year. Moderate or severe aortic regurgitation occurred in 3.5% of patients. Factors significantly associated with higher discharge mean aortic gradients were surgical valve size, stenosis as modality of valve failure, and presence of surgical valve prosthesis-patient mismatch10,11.
Growing experience has shown that ViV TAVI-specific complications can often be prevented with accurate screening and some technical expedients12. In particular, patients with small surgical bioprostheses represent an important challenge, as they seem to have higher residual gradients and higher late mortality than other patients undergoing ViV TAVI. High implantation of either annular or supra-annular THVs has been shown to partially mitigate this phenomenon12. More recently, some investigators described a technique of bioprosthetic valve ring fracturing using a high-pressure balloon to facilitate ViV TAVI. This strategy seems to allow further expansion of the transcatheter valve with reduced residual transvalvular gradients and increased valve effective orifice area13.
Given these favourable results, in the future, it is likely that TAVI may become the preferred treatment for surgical bioprosthesis valve failure and bicuspid valves, especially if customised devices are developed.
PURE AORTIC REGURGITATION
To date, the role of TAVI for native aortic regurgitation treatment is marginal and has consisted mainly of “off-label” application in very high-risk patients. The currently approved TAVI devices are specifically designed for the treatment of calcific aortic stenosis. Limited data are available describing the safety and efficacy of TAVI for the treatment of patients with pure severe aortic regurgitation. The JenaValve (JenaValve Technology, Munich, Germany) is the only commercially available THV which has obtained Conformité Européenne (CE) mark approval for the treatment of inoperable or high-risk patients with severe native aortic regurgitation using a transapical approach14. Moreover, a few other reports have described successful THV implantation using the less invasive transfemoral approach14. Compared with TAVI for aortic stenosis, TAVI for pure native aortic regurgitation has proved to be more challenging and is associated with lower device success, safety, and clinical efficacy rates14. The absence of aortic annular and aortic valve leaflet calcification in patients with pure aortic regurgitation is a known risk factor for THV embolisation and migration. In fact, real-world results with old-generation THV have been disappointing, with high rates of THV embolisation, migration, and PVL. However, in this anatomical subset, Sawaya et al recently demonstrated improved periprocedural outcomes using newer-generation devices (device success: 54% vs. 85%, p=0.011)15. New device technologies (J-Valve™; JieCheng Medical Technology Co., Ltd., Suzhou, China), specifically designed to be implanted in non-calcific aortic valves, are under preclinical and clinical investigation and will probably extend the indications for TAVI in this population16.
LOW-RISK POPULATIONS
Our knowledge of the implications of TAVI in low-risk patients is limited. However, considerable information will soon be available from the results of ongoing trials (PARTNER 3 and Low-Risk Evolut R). To date, the only published randomised trial of TAVI vs. SAVR in low-risk patients is the NOTION study17. In this Nordic study, 280 patients, 82% of whom were low-risk, were randomised 1:1 to TAVI or SAVR. There was no significant difference in the primary endpoint of all-cause death, stroke or myocardial infarction at two years (15.8% vs. 18.8%, p=0.43)17. Similarly to previous trials on high-risk and intermediate-risk populations, TAVI patients had a higher rate of pacemaker implantation and aortic valve regurgitation. SAVR patients had more major or life-threatening bleeding, cardiogenic shock, acute kidney injury, and periprocedural atrial fibrillation17.
Despite the paucity of data, there is an inevitable desire by policy makers, manufacturers and the medical community to take our past experience with TAVI in high- and intermediate-risk18,19 patients and apply it to lower-risk populations. In future, specific technical factors that favour either TAVI or SAVR may become more relevant in choosing the best therapy for low-risk patients. These factors include the availability of minimally invasive access surgical approaches, the differing risks of pacemaker and new atrial fibrillation, valve performance and durability, and the effectiveness of THVs in bicuspid valves and complex anatomies. Finally, the dynamics of the Heart Team will need to be re-discussed. In particular, it is reasonable to believe that the cardiac surgeon will not act as a gatekeeper determining the destination therapy of each patient. Relationships between cardiac surgeon and interventional cardiologist will be more balanced and, in addition, patient preference will influence decision making between TAVI and SAVR.
Procedural advances and complication trends
PROCEDURE AND VALVE ADVANCES
The latest generation of TAVI devices has incorporated features to reduce the delivery catheter profile (down to 14 Fr)20, facilitate deployment, and enable repositioning and retrieval capability, with the aim of obtaining the desired position and reducing TAVI-related complications.
TAVI devices currently approved for clinical use are: the SAPIEN 3 (Edwards Lifesciences), Evolut™ R (Medtronic), LOTUS™ valve (Boston Scientific, Marlborough, MA, USA), ACURATE neo™ (Symetis, Ecublens, Switzerland), Portico™ (St. Jude Medical, St. Paul, MN, USA), and Allegra (New Valve Technology, Hechingen, Germany). According to the mechanism of deployment, these can be divided into the categories of balloon-expandable (SAPIEN 3), self-expanding (Evolut R, Portico, ACURATE neo, Allegra), and mechanically expandable (LOTUS)21.
The SAPIEN 3 valve is designed with a cobalt-chromium frame and three bovine pericardial tissue leaflets. As compared with the previous generation of SAPIEN XT, the design of the SAPIEN 3 frame has been modified to enhance the geometry (open upper cells and closed lower cells) for an ultra-low delivery profile (14 Fr) and has incorporated a skirt at its inflow portion and an outer sealing skirt to reduce PVL.
The Evolut R device consists of a tricuspid porcine pericardial valve sutured inside a self-expanding nitinol frame. As compared with the previous generation of CoreValve devices, cell geometry has been redesigned to achieve optimised radial force during release and to enable recapturability and repositionability. The latest-generation Evolut PRO, which recently obtained approval from the FDA, consists of the same platform as the Evolut R, incorporating an outer porcine pericardial tissue wrap intended to improve sealing further between the device and the native aortic annulus and to minimise PVL.
The LOTUS valve system consists of a trileaflet bovine pericardial valve supported on a braided nitinol frame. The frame is covered with a seal. This is the only new-generation TAVI device that is fully recapturable and repositionable even after the valve has been fully deployed. Among the approved TAVI devices, the LOTUS valve was associated with the lowest rate of PVL21 (Figure 3). However, the high rate of conduction disturbances requiring pacemaker implantation with this valve (approximately 30%) remains a concern (Figure 3). The newer-generation LOTUS Edge™ THV (Boston Scientific) and the new release mechanism (Depth Guard™; Boston Scientific) were developed with the hope of reducing the frequency of conduction disturbances. However, no data on their efficacy are available yet. Boston Scientific recently announced a voluntary removal of all LOTUS TAVI devices from global commercial and clinical sites. The company expects to bring their platform back to Europe and other international markets by Q4 of 2017.
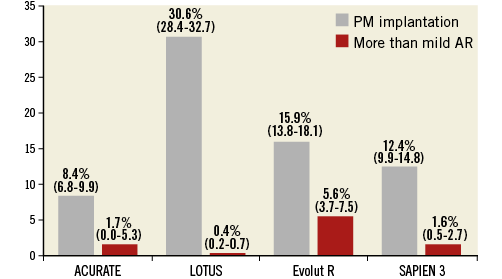
Figure 3. Comparison of the incidence of pacemaker (PM) implantation and more than mild aortic regurgitation (AR) across patients receiving new-generation TAVI devices. Reported are cumulative rates and 95% confidence intervals (CI). Results of new-generation devices were described by Barbanti et al21.
The ACURATE neo aortic bioprosthesis is composed of porcine pericardium sewn onto a self-expanding nitinol stent, covered both externally and internally by a porcine pericardium skirt. The device includes three stabilisation arches for axial alignment to the aortic annulus, a top crown for capping the aortic annulus, and a bottom that is open to the full distribution on the native valve. The valve does not allow recapturability, but it has been demonstrated to be extremely stable during deployment.
The Portico valve is composed of a self-expanding stent, bovine pericardial leaflets, and a porcine pericardial sealing cuff. The key characteristics of this THV are the repositionability and the large cell area and the annular positioning that allow easy engagement of the coronary ostia after implantation.
ACCESS ROUTES
The very early TAVI procedures were accomplished using an anterograde transseptal approach. After a few years, this original route was abandoned in favour of the more reproducible transfemoral and other approaches (transapical, transaortic and trans-subclavian). While the transfemoral approach is today the preferred access route, additional alternative routes have been developed (transcarotid and transcaval). Although these newer procedures have been shown to be feasible and reliable22, they need to be better evaluated in larger series. In the absence of additional studies, operator preference, local expertise, and case discussion will be the main drivers of access choice where there are contraindications to the transfemoral approach.
COMPLICATION TRENDS
Despite important improvements in current device technology and implantation techniques, specific complications remain and warrant consideration. Over the years, the clinical community has shifted its focus towards different complications, in response to their frequency and clinical impact.
Vascular complications, periprocedural neurological events, conduction disturbances, and paravalvular regurgitation were the first concerns to emerge with TAVI3. More recently, aortic rupture and coronary occlusion, despite the low incidence, have been a source of increased interest due to their potentially catastrophic impact11. Also, as TAVI is progressively being offered to younger and lower-risk patients, the questions of long-term prosthesis durability and thrombosis have gained importance23-25.
VASCULAR COMPLICATIONS
Historically, vascular complications, with the attendant blood loss, transfusion requirements, and haemodynamic instability, have been a major limitation of TAVI26. However, improvements in delivery system profile, patient screening and selection, and experience have made life-threatening vascular complications very rare events26. Today, vascular complications are mainly represented by minor bleeding or vascular injuries (i.e., dissection, occlusion) that can be effectively managed percutaneously or with limited surgical intervention26 (Figure 4).
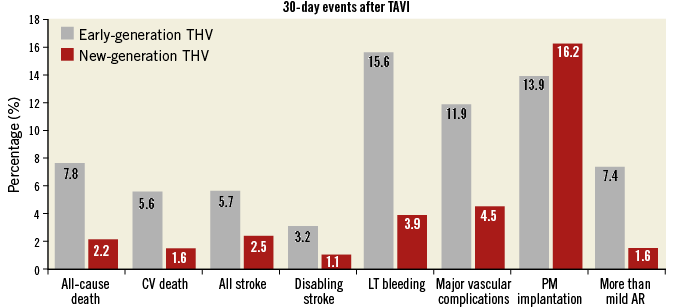
Figure 4. Thirty-day major outcomes of early-generation (Medtronic CoreValve, Edwards SAPIEN and SAPIEN XT) vs. new-generation transcatheter heart valves (SAPIEN 3, Evolut R, Portico, LOTUS, ACURATE, ACURATE neo). Reported are cumulative rates for each outcome. Results of early-generation devices were described by Généreux et al31. Results of new-generation devices were described by Barbanti et al21. AR: aortic regurgitation; CV: cardiovascular; LT: life-threatening; PM: pacemaker
CEREBROVASCULAR EVENTS
Cerebrovascular events (CVE) can be associated with significant morbidity and mortality and were a major concern in the early experience3. In a large meta-analysis of 64 studies involving 72,318 patients, the overall rate of CVE was 3.3% at 30 days27. Numerous variables have been described as potential predictors of CVE after TAVI. It is known that most of the procedural cerebral embolic events during TAVI occur during balloon valvuloplasty, manipulation of catheters across the aortic valve, and valve deployment3. However, it has been shown that about 50% of CVE may occur more than 24 hours after TAVI28. These neurological events probably have a thrombogenic origin (e.g., new onset of atrial fibrillation), and may reflect the highly comorbid profile of TAVI candidates. Although stroke clearly manifests in clinical symptoms, many studies have also demonstrated silent new cerebral ischaemia on diffusion-weighted magnetic resonance imaging in the majority of patients3.
However, recent studies have suggested a decrease in CVE over time, down to rates of around 2.5% compared with earlier TAVI series20 (Figure 4). This favourable trend can be explained by the development of lower-profile delivery systems and increased operator experience. In addition, several cerebral protection devices have been developed with the objective of reducing CVE which occur primarily during or immediately after TAVI29. These protection devices work by filtering (Sentinel™; Claret Medical, Inc., Santa Rosa, CA, USA) or deflecting (TriGuard™; Keystone Heart, Tampa, FL, USA) the debris away from the cerebral circulation, while allowing ongoing cerebral perfusion. These devices seem to reduce the number and size of silent cerebral embolisations during TAVI29. One device has been approved by the FDA (Sentinel), but clinical trials could not demonstrate a clinically relevant reduction of strokes.
CONDUCTION DISTURBANCES
Since the aortic valve has close spatial proximity to the conduction system, conduction abnormalities are frequently observed in TAVI. The most prevalent conduction disturbances following TAVI are left bundle branch block and complete atrioventricular block that lead to permanent pacemaker implantation30. The incidence of new pacemaker following TAVI differs between the types of prosthesis used. To date, there is much evidence to support efforts to avoid this complication, which may result in left ventricular dyssynchrony, poorer recovery of left ventricular ejection fraction, increased duration of hospitalisation, increased early and late requirement for pacemakers, and more frequent repeat hospitalisation30. The evaluation of valve anatomy and the selection of the most appropriate prosthesis have been proposed as valuable options to reduce its incidence30. However, the development of second-generation devices (particularly Evolut R and LOTUS), with their retrievability and repositioning capabilities, has failed to solve the problem21 (Figure 4).
PARAVALVULAR AORTIC REGURGITATION
Paravalvular leak (PVL) is a complication of aortic valve prostheses and is seen more frequently after TAVI than after SAVR3,31. The incidence of moderate or severe PVL after TAVI with first-generation devices has been reported to be 12-21%31. Residual moderate-to-severe PVL is clinically relevant, and has been associated with an increased risk of all-cause mortality32. The three main mechanisms of PVL following TAVI are incomplete apposition of the THV against the native annulus due to severe calcification, undersizing of the valve, and malpositioning of the device. In the most recent series, the rate of significant PVL has fallen significantly21 (Figure 4), as a direct consequence of the integration of more accurate preoperative annular sizing protocols and growing penetration in clinical practice of new-generation TAVI devices that have incorporated key features for a more predictable deployment including repositionability and retrievability of the valve, and an external sealing cuff to fill gaps between the THV and the aortic wall. Although globally reduced, the rate of more-than-mild PVL differed across different new-generation THVs (Figure 3).
CORONARY OCCLUSION AND AORTIC RUPTURE
In contemporary series, the incidence of coronary occlusion and rupture of the aortic root or annulus has usually been less than 1%12. In native aortic valves, coronary ostia obstruction mainly occurs due to the displacement of a bulky calcified native valve (or externally sealed valve leaflets of surgical bioprostheses) over a coronary ostium12. Narrow sinus of Valsalva, bulky leaflet calcifications and low-lying coronary ostia have been identified as the main predictors of coronary occlusion during TAVI12. A pre-implant balloon valvuloplasty with simultaneous associated aortography may be useful to ensure the patency of the coronary ostia during balloon inflation. According to this technique, when the balloon is fully inflated, the coronary ostium could be occluded by the calcified cusps crushed against the wall of the sinuses of Valsalva33. Coronary protection by placing a wire and a balloon or stent in the coronary vasculature before TAVI is the most popular strategy adopted in case of high risk of coronary ostia occlusion.
Aortic root rupture (particularly uncontained rupture) is often associated with an extremely poor acute prognosis12. At least two important features are associated with annular rupture/periaortic haematoma: 1) moderate or severe LVOT/subannular calcification, and 2) significantly oversized prostheses12. Prosthesis type and size selection is part of the patient selection process that allows the operator to prevent these complications.
THV FAILURE
TAVI is now progressively being offered to younger and lower-risk patients. In this particular subset, life expectancy is expected to exceed that of the initial candidates for TAVI, which makes the question of long-term prosthesis durability of crucial importance. Failure of THVs may present as stenosis (as a consequence of calcification, pannus, or thrombosis), or as intraprosthetic regurgitation (as a consequence of reduced leaflet mobility, tears, or endocarditis)23. To date, no significant increases in mean THV gradient or cases of structural valve deterioration have been reported during the five-year follow-up of the PARTNER (Placement of Aortic Transcatheter Valves) I trial23. Three studies have also reported outcomes after TAVI with either the Edwards SAPIEN or CoreValve bioprostheses up to five years23: two of them did not suggest any major concerns regarding durability of THVs, with stable transprosthetic gradients over time and a rate of THV dysfunction of 3.4% and 4.2%, respectively, using different definitions23.
Among the types of THV failure, thrombosis has raised the interest of the clinical community, even though clinically evident thrombosis remains a rare event24,25.
More recently, reduced leaflet motion of bioprosthetic aortic valves associated with normal transvalvular gradients (probably related to subclinical leaflet thrombosis) has been reported as affecting both transcatheter and surgical aortic valves25. Of 890 patients who underwent computed tomography imaging and had interpretable scans in the RESOLVE and SAVORY registries, 12% had subclinical leaflet thrombosis, including five of 138 patients (3.6%) with thrombosis of surgically implanted valves and 101 of 752 patients (13.4%) who underwent TAVI25. Overall, the rate of stroke was not significantly different among patients with and without reduced leaflet motion, but subclinical leaflet thrombosis was associated with a significantly increased risk of transient ischaemic attack compared with no CT-identified leaflet thrombosis (6% vs. 1%; p<0.001)25. These data should challenge the current standard of care with dual antiplatelet therapy (DAPT) prescribed after TAVI, given that anticoagulation, not DAPT, was protective. The prevalence of reduced leaflet motion was significantly lower among patients receiving oral anticoagulation than in patients who received DAPT (3.6% vs. 14.9%; p<0.001), with the non-vitamin K oral anticoagulants as effective as warfarin for the prevention of leaflet thrombosis25.
Two trials, GALILEO and ATLANTIS, are studying the use of non-vitamin K oral anticoagulants (rivaroxaban and apixaban, respectively) to prevent valve thrombosis and other events in patients who have undergone TAVI. While waiting for the results of these trials, the new 2017 ACC/AHA guidelines recommend three months of warfarin after TAVI2.
Infective endocarditis is also a rare (0.5% to 3.1%)33 but serious complication after TAVI. In a recent multicentre registry that included 250 cases of definite infective endocarditis after TAVI, the in-hospital mortality was 36% and the two-year mortality rate was 66.7%34. Younger age, male sex, history of diabetes mellitus, and moderate to severe residual aortic regurgitation were significantly associated with an increased risk of infective endocarditis34.
Although the possibility of late THV failure is perceived as a major concern, preliminary observations have demonstrated that, in contrast with redo valve surgery, which is technically challenging with a significant risk of mortality and morbidity, redo TAVI seems to be safe and effective35.
Shift to minimalist approach
Today, TAVI is a relatively standardised and reproducible procedure. One of the most debated issues is whether TAVI should be simplified. In recent years many groups have been working on local programmes, which incorporate specific pre-, peri- and post-procedural pathways aimed at simplification of the TAVI pathway36-38.
PREPROCEDURAL OPTIMISATION
The TAVI procedure requires a number of preparatory evaluations and exams aimed at confirming the clinical and anatomical indication for TAVI (echocardiogram, computed tomography). Ideally, these investigations should be performed without admitting the patient to hospital. In addition, the local TAVI team should assess whether a patient can be eligible for a minimalist protocol by looking beyond the traditional clinical and anatomical criteria and incorporating additional clinical, non-clinical and psychosocial factors that could affect the patient’s post-procedural recovery. These include patient engagement, willingness to participate in cardiac rehab, and possible presence of dementia. During pre-TAVI assessment, physicians should assess family dynamics to determine whether patients will have the support needed at home for a successful recovery. Also, given the age and complexity of the majority of TAVI patients, geriatricians should be involved in the patient selection process from the outset.
PROCEDURE OPTIMISATION
There are some procedural expedients that can be applied to optimise the TAVI procedure without compromising its safety.
Many operators still perform TAVI in a hybrid room. However, TAVI can be safely performed in a regular catheterisation laboratory. If the procedure is accomplished through the transfemoral approach, the presence of a cardiac surgeon in the room is not mandatory, but it is key that the cardiac surgeon is involved in the screening process and is available to manage life-threatening complications.
Performing transfemoral TAVI under local anaesthesia, using a fully percutaneous approach, and eliminating transoesophageal echocardiographic guidance (maintaining back-up transthoracic echocardiography) and balloon predilation are strategies to reduce the invasiveness and costs of the procedure. Ideally, the temporary pacemaker should be removed at the end of the procedure in the absence of concerns about conduction disturbances.
During transfemoral TAVI, the ideal team should include two operators, one clinical specialist/nurse to prepare the device, one circulating nurse and one X-ray technician. The presence of an echocardiographist, anaesthesiologist, cardiac surgeon, vascular surgeon and perfusionist in the catheterisation laboratory by default is not required; this should be reserved for selected cases in which specific procedural issues are anticipated (Table 1, Figure 5).
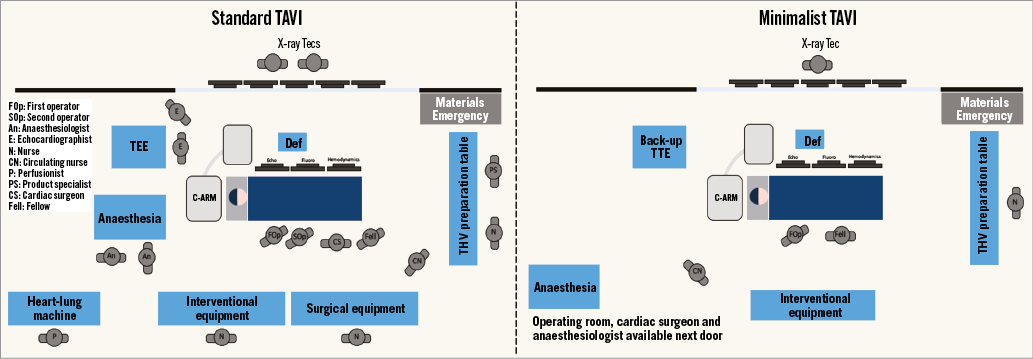
Figure 5. Procedural set-up for a standard TAVI and an optimised TAVI.
POST-PROCEDURAL OPTIMISATION
Immediately after the procedure, all patients must be monitored in the catheterisation laboratory or operating room for at least 10-15 minutes with special attention to haemodynamics and cardiac rhythm. Thereafter, patients should be transferred to a coronary care unit or a regular cardiology ward on telemetry, according to local protocols. The clinical status of the patient, with particular attention to procedural outcome, ECG, echocardiographic and laboratory findings, must be carefully evaluated. Mobilisation should be prescribed after a few hours, provided that there are no vascular access issues (haematoma, bleeding) and the temporary pacemaker has been removed. Patients not experiencing complications (or those in whom complications were resolved) can then be discharged as early as the following day.
The motivation to reduce the duration of hospitalisation after TAVI stems from the desire to accelerate recovery and mobilisation and minimise unnecessary use of resources. Early discharge (within 24-72 hours) after TAVI in most of the patients has been shown not to preclude the safety of the procedure, as demonstrated in several European and North American experiences34,35. Clinical experience has demonstrated that the most frequent issues that usually prolong hospitalisations after TAVI are conduction disturbances, bleeding and acute kidney injury, the need for prolonged monitoring for acute atrioventricular block being by far the most important one.
The incremental cost-effectiveness ratio of minimalist TAVI strategies is still unclear. In a small US series of 142 patients (n=70 undergoing minimalist transfemoral TAVI and n=72 undergoing standard transfemoral TAVI), Babaliaros et al demonstrated that the minimalist strategy decreases the cost of TAVI ($2,869 estimate) and can be used frequently to prevent the overheads associated with hybrid operating rooms and general anaesthesia35.
In conclusion, although TAVI is a complex procedure, important progress towards simplification of the procedure has already been made. In many centres, a simplified or minimalist approach to TAVI is already routine and has been shown to be as safe and effective as the more traditional approach. The multicentre 3MTAVR trial (NCT02287662) and the FAST-TAVI study (NCT02404467) will provide invaluable insight into the real effectiveness and reproducibility of this approach.
Conflict of interest statement
M. Barbanti and J. Webb are consultants for Edwards Lifesciences. C. Tamburino has received speaker honoraria from Medtronic, St. Jude Medical and Symetis. D. Capodanno has no conflicts of interest to declare. M. Gilard has no conflicts of interest to declare.

