Abstract
Transcatheter aortic valve implantation (TAVI) is an established treatment for inoperable patients or patients at high risk for surgery. Despite growing experience, issues remain associated with first-generation TAVI devices, including valve malpositioning, vascular complications, paravalvular regurgitation and conduction disorders. Several second-generation TAVI devices, aimed at addressing these issues, are CE marked or under evaluation for CE marking. The objective of this overview is to describe and illustrate the key design features of the second-generation devices that are entering contemporary clinical practice.
Introduction
Transcatheter aortic valve implantation (TAVI) is an established treatment for patients with severe symptomatic aortic stenosis deemed inoperable or at high risk for conventional surgery1-3. Since the first-in-man case performed in 2002, more than 120,000 procedures have been performed worldwide with the chronologically first CE-marked devices: Edwards SAPIEN™/SAPIEN XT™ (Edwards Lifesciences, Irvine, CA, USA) and Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA). Despite highly favourable outcomes with these first-generation devices, several issues remain, including, non-exhaustively, paravalvular regurgitation, valve malpositioning, vascular complications and conduction disorders4. Various new TAVI devices, so-called “second-generation devices”, are now CE marked or under evaluation for CE mark. These new-generation devices incorporate features to address the limitations of the first-generation devices. The aim of this overview is to describe and illustrate the key design features of the second-generation devices that are entering contemporary clinical practice (Table 1).
Medtronic Evolut R
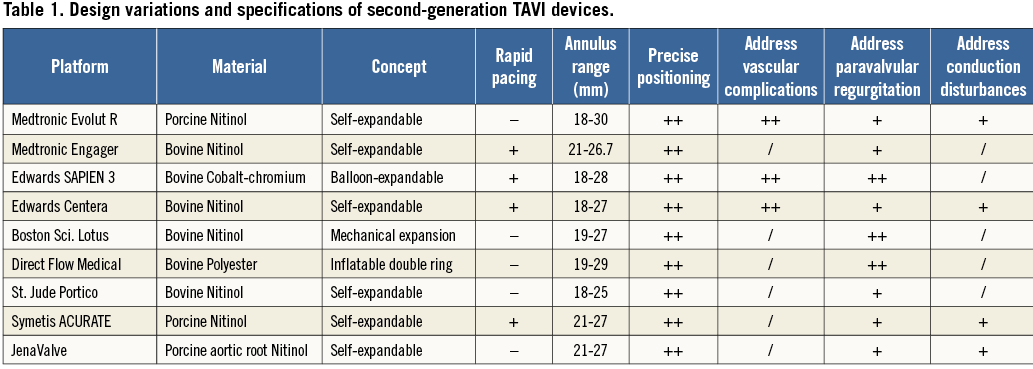
The Evolut R™ (Medtronic) is the new iteration of the Medtronic CoreValve® (MCV) (Figure 1). Several key features of the MCV have been preserved, namely the trileaflet porcine pericardial tissue, the supra-annular position of the valve leaflets, the self-expanding nitinol stent frame and the architecture in three levels of function: inflow portion with a high radial force for anchoring within the aortic annulus, mid portion with a constrained structure for preservation of the coronary flow, and outflow portion with a high hoop strength for coaxiality with the aortic root. The Evolut R presents several improvements as compared to the MCV. The outflow portion has been shortened by approximately 10 mm and redesigned to fit the aortic root better. The geometry of the distal diamond cell of the inflow portion has been modified with a slightly asymmetric configuration and extended length for better conformability and consistent radial force across a range of annulus sizes. As a consequence, the nadir of the valve leaflets is sutured 13 mm from the edge of the inflow portion. The distal skirt has been extended with a scalloped design for better sealing (Figure 2). The prosthesis is fully repositionable and retrievable before final detachment of the hooks and will be available in four sizes (23, 26, 29 and 32 mm) covering aortic annuli from 18 to 30 mm. The Enveo™ R delivery system and Enveo Inline™ sheath (both Medtronic) represent a 14 Fr design with a modified intuitive handle, a reinforced nitinol capsule for resheathing and an integrated sheath (Figure 3). The Enveo R delivery system is designed for transfemoral (TF) and subclavian access.
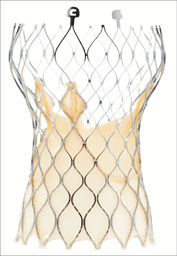
Figure 1. Evolut R™ TAVI bioprosthesis.
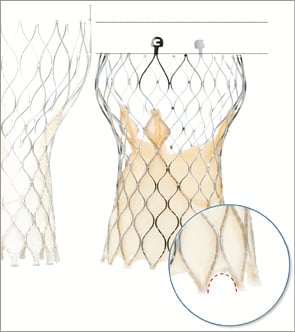
Figure 2. Visual comparison of Medtronic CoreValve® and Evolut R™. Evolut R™ is 10 mm shorter and its inflow portion has been redesigned with an extended skirt.
Figure 3. Enveo™ R and Enveo Inline™ sheath. Various components of the handle. An analysis of the technical features of the Evolut R helps to understand the potential of this prosthesis. Whereas positioning of the actual MCV can be challenging in some situations, such as horizontal aorta, the 1:1 ratio between revolutions applied to the handle and prosthesis unsheathing-resheathing response, combined with repositionability of the Evolut R, aims at achieving more controlled and accurate positioning even in difficult anatomies. The redesigned tubular inflow portion and the extended skirt should reduce the risk of paravalvular regurgitation, which has been a limitation of the MCV5. Subsequent to modifications applied to the inflow portion, the target depth of implantation of the Evolut R is 3 mm with a potentially favourable impact on conduction disturbances and the need for a pacemaker6. Finally, the 14 Fr profile of the delivery system (true outer 18 Fr), with the option of proceeding to sheathless procedures, will probably reduce the rate of vascular complications. In case of need for post-dilatation, the delivery system needs to be exchanged for a standard 14 Fr sheath.
Medtronic Engager
The Medtronic Engager™ TAVI prosthesis (Medtronic) combines a self-expanding nitinol stent frame and a bovine trileaflet valve. The architecture of the stent frame has two components: a central frame housing the leaflets and a support frame with control arms designed to stabilise the device in the sinuses of Valsalva (Figure 4). The Engager is designed to fit the aortic root anatomy respecting the coronary ostia, thus minimising the risk of coronary obstruction. A polyester sleeve is sutured to the main frame aimed at reducing the risk of paravalvular regurgitation. The leaflets are in a supra-annular position to maintain circular configuration and coaptation, irrespective of the shape of the aortic annulus. The Engager is available in two sizes, 23 and 26 mm, for an annulus range between 21 and 26.7 mm. Procedures can be performed via transapical or direct aortic routes. The delivery system combines a 29 Fr introducer and a flexible delivery catheter creating one integral unit (Figure 5). It allows for a three-step release of the prosthesis by first releasing the control arms and then releasing the commissural posts, through unlocking a safety button, when the optimal position has been achieved. The last step is the full release of the self-expanding valve. Repositioning can be performed until unlocking the safety button and releasing the commissural posts. The final deployment is ideally performed under rapid ventricular pacing for stability and accuracy of positioning.
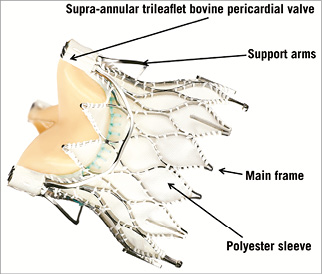
Figure 4. Components of the Engager™ TAVI prosthesis.
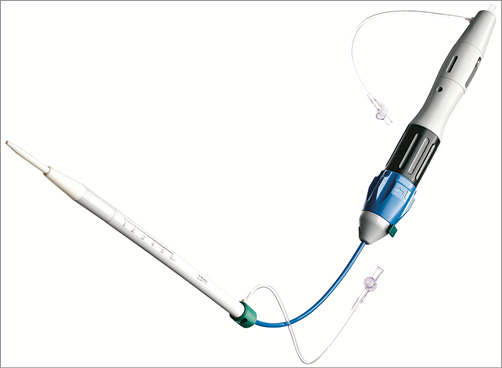
Figure 5. Engager™ transapical delivery system. Delivery catheter and integrated sheath (29 Fr). Boston Lotus
The Lotus™ valve system (Boston Scientific, Natick, MA, USA) is made of a single nitinol wire, braided according to a specific design and connected in the middle of the stent frame with a central radiopaque tantalum positioning marker. The stent frame houses a trileaflet bovine pericardial valve and is surrounded in its inflow half by the Adaptive Seal™ (Boston Scientific), an outer sleeve for improved sealing (Figure 6). The valve is elongated to approximately 70 mm within the delivery catheter and shortens to 19 mm while it radially expands in situ (Figure 7). The Lotus is available in three sizes (23, 25 and 27 mm) covering aortic annuli from 19 to 27 mm. The valve is connected to the inner catheter by three coupling fingers, ending with a connecting collar and buckles and interacting with posts related to the commissures of the tissue valve. The Lotus is mechanically expanded and locked in its final configuration by connecting the buckles to the posts (Figure 8). The device is fully repositionable and retrievable before detachment of the collars and coupling fingers. The delivery system of the Lotus (18-20 Fr) is precurved to accommodate bends in the peripheral vasculature and adapt to the angulation of the aortic arch. It is covered with a hydrophilic coating for better trackability. The handle of the delivery system is intuitive with two distinct parts: the unsheathing-resheathing knob and the release collar (Figure 9). Finally, to simplify preparation, the valve is pre-attached to the delivery system.
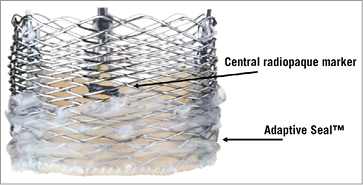
Figure 6. Lotus™ valve. Details of the central radiopaque marker and the Adaptive Seal™.
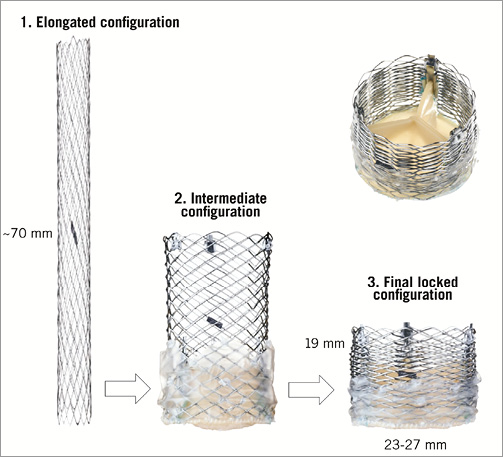
Figure 7. Various configurations of the Lotus™ TAVI system. The valve is mechanically expanded and shortens while it radially expands.
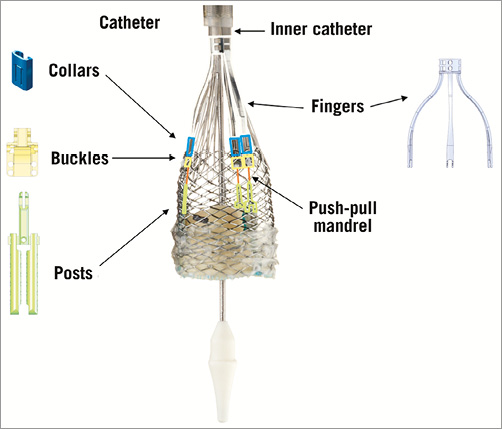
Figure 8. Lotus system. Elements attaching the prosthesis to the inner catheter of the delivery system.
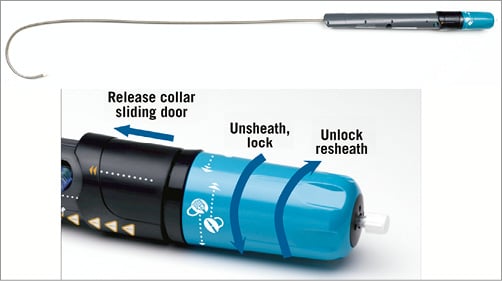
Figure 9. Delivery system and handle of the Lotus™ TAVI device. The central radiopaque tantalum marker and the fully repositionable design allow for very controlled and accurate positioning. Moreover, the valve functions early during the expansion phase ensuring haemodynamic stability throughout the valve deployment. Aortic root calcification at the landing zone is a predictor of paravalvular regurgitation post TAVI by preventing adequate contact of the stent frame with the annulus and subannular zone. The Adaptive Seal can fill in remaining gaps between the Lotus and surrounding structures, thus mitigating paravalvular leakage7.
Edwards SAPIEN 3
The transfemoral SAPIEN 3 (S3) (Edwards Lifesciences) is the fourth generation in the Edwards family of balloon-expandable transcatheter heart valves (THV)8 (Figure 10). It builds on the success of its predecessors and adds several iterations to reduce its profile, improve positioning and deployment, and reduce paravalvular regurgitation. The S3 has a modified bovine pericardial tissue leaflet and stent design which has further downsized the overall profile. Three sizes are available (23-29 mm) covering annulus sizes between 18 and 28 mm by TOE, and 20.5 to 29.5 mm by MSCT sizing, respectively. The entire assembly is now 14 Fr (S3 23-26 mm) to 16 Fr (S3 29 mm) sheath-compatible. The inflow portion has an additional outer polyethylene terephthalate (PET) sealing skirt on top of the previous inner PET skirt (Figure 11). The Commander delivery system (Edwards Lifesciences) varies from the pre-existing NovaFlex delivery catheter (Edwards Lifesciences). Similar to the previous-generation SAPIEN XT, the S3 is loaded onto the balloon in the abdominal aorta. A balloon lock knob has been added to secure the balloon catheter to the flex catheter. The outer deflectable flex catheter allows for additional flexing to facilitate navigation through extreme tortuosity. A complementary wheel has been incorporated into the delivery handle for fine alignment of the THV within the aortic root before deployment (Figure 12). The balloon has a central radiopaque marker to aid precise valve positioning. Practically, the improved profile opens up TF S3 TAVI feasibility to smaller peripheral anatomies requiring a minimum vessel diameter of 5.5 mm. The presence of the central radiopaque marker and the additional fine alignment wheel provide accurate and more reproducible S3 positioning. The S3 is implanted with its central marker onto the virtual aortic annulus. During the gradual balloon inflation the frame foreshortens predominantly at the level of the left ventricular outflow. The outer PET sealing skirt affects final valve results and leaves no or only trivial/mild paravalvular AR in the vast majority of cases. All of these design changes have turned S3 TAVI into a fast, safe and reproducible procedure in properly selected patients.
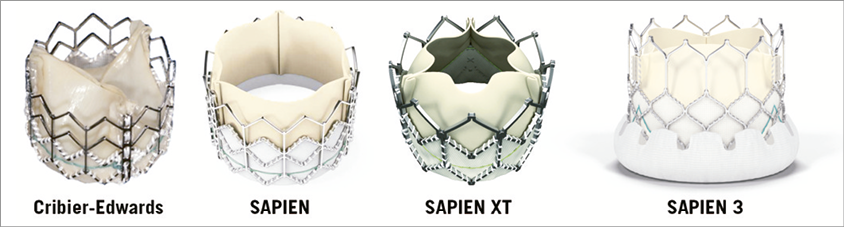
Figure 10. Four generations of balloon-expandable transcatheter heart valves. Note the additional outer PET skirt in the SAPIEN 3. 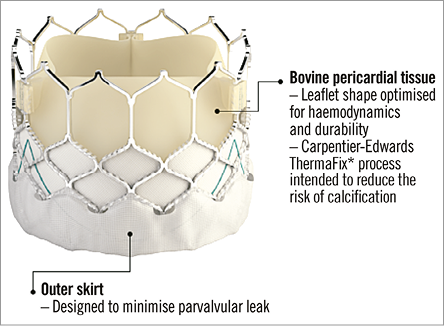
Figure 11. Close-up of the SAPIEN 3 transcatheter heart valve.
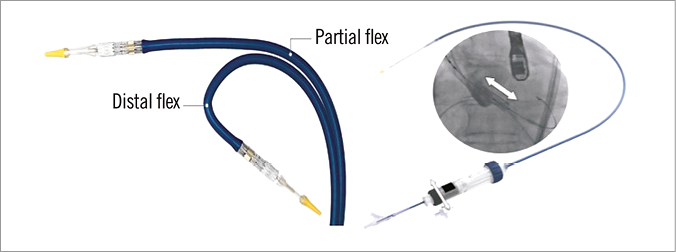
Figure 12. Commander delivery system (Edwards Lifesciences). Left panel: superior flexing ability. Right panel: the fine alignment wheel (white arrow). Transapical S3 implantation also underwent fundamental iterations from prior generations. The Edwards Certitude delivery system (Edwards Lifesciences) has been downsized from the previous 24 Fr Ascendra+ System (Edwards Lifesciences) to a smaller 18 Fr (for S3 23-26 mm) and 21 Fr (S3 29 mm) profile with a smaller nose cone. The smaller profile could reduce procedural bleeding and access-site complications.
Edwards Centera
The Edwards Centera™ THV (Edwards Lifesciences) is composed of an ultra-low-profile self-expanding nitinol frame housing a trileaflet bovine pericardial valve (Figure 13). The frame has been designed for an adequate fit within the sinuses of Valsalva and the aortic annulus, without extensive protrusion in the ascending aorta. It has a smoothly flaired inflow portion to accommodate the subannular region, minimising interaction with the conduction system. The frame height is relatively short as compared to current self-expanding TAVI devices: 17.5 mm for 20, 23 and 26 mm prostheses. A 29 mm device is also available. Compatible annuli range from 18 to 27 mm as measured by MSCT. A fabric cuff is sutured to the proximal stent struts. The delivery system of the Centera combines a low-profile deflectable catheter and a detachable battery-powered motorised handle (Figure 14). It is compatible with the 14 Fr Edwards eSheath (Edwards Lifesciences). The prosthesis is pre-attached to the delivery catheter. The inner catheter has a radiopaque marker which is maintained at the annulus during deployment to ensure a precise positioning. The Centera is repositionable and recapturable up to 70% of its expansion. So far, the Centera has been deployed using rapid pacing as it obstructs the outflow tract at about 50% of its deployment. Procedures without rapid pacing will be investigated.
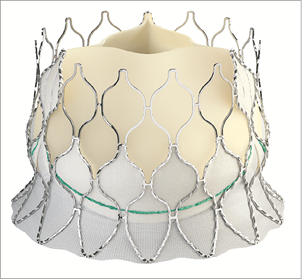
Figure 13. Edwards Centera™ TAVI device. Contoured self-expanding nitinol stent frame housing a trilealet bovine pericardial valve.

Figure 14. Motorised delivery system of the Edwards Centera™ device. Ultra-low-profile deflectable delivery catheter. The Centera is a self-centring prosthesis which allows for a single-operator implantation including the deployment and repositioning/recapture if needed. The contoured frame and fabric cuff are designed to obtain a perfect seal of the annulus with minimal risk of paravalvular regurgitation or conduction disturbances. The ultra-low profile of the delivery catheter should help to minimise vascular complications through the transfemoral and subclavian access.
Direct Flow Medical
The Direct Flow Medical® (DFM) Transcatheter Aortic Valve System (Direct Flow Medical, Inc., Santa Rosa, CA, USA) is a trileaflet bovine pericardial valve that is mounted over a tubular, non-metallic, inflatable structure covered with polyester fabric (Figure 15). The covered frame has two circular rings connected with vertical tubular supports. The upper aortic ring should be positioned above the aortic leaflets and below the coronary ostia, and the lower ventricular ring below the aortic annulus (Figure 16). The DFM is unsheathed in the left ventricle and the ventricular ring is inflated. The device is then progressively pulled back towards the aortic root. Before the inflation of the aortic ring, the position of the DFM is fine-tuned by controlled and millimetric manipulation of three positioning wires that are connected to the valve structure. Position, transprosthetic gradient and regurgitation can be regularly assessed. If the position is suboptimal and/or there is significant leakage, the aortic ring or both rings can be deflated to reposition the DFM. The valve functions early, allowing for control and safety during deployment. Once optimal positioning is reached, the radiopaque solution is exchanged for a quick curing polymer, which forms the permanent valve structure. The position wires are then detached and the valve is released. The DFM is available in four sizes (23, 25, 27 and 29 mm), for aortic annulus sizes between 19 and 28 mm. The system is fully repositionable and retrievable before polymer exchange. The absence of a metallic valve structure facilitates trackability and provides enhanced delivery system flexibility. The delivery system is fully sheathed and 18 Fr-compatible for all valve sizes (Figure 17).
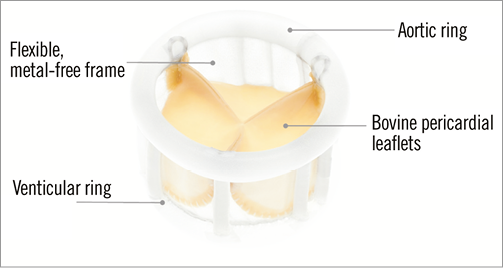
Figure 15. The structure of the Direct Flow Medical® TAVI device.
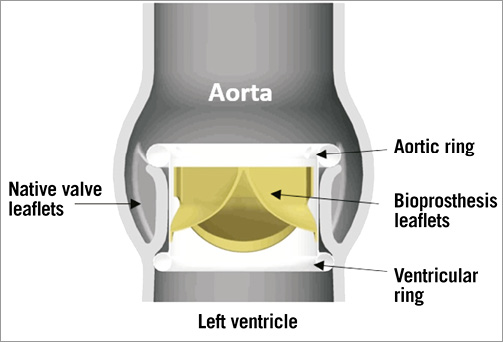
Figure 16. Direct Flow Medical® valve in situ.
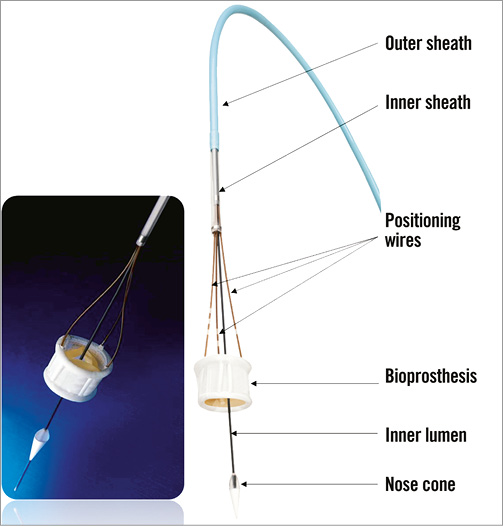
Figure 17. Flexible delivery system and positioning wires connected to the Direct Flow Medical® TAVI device. The technical features of the DFM allow for accurate and controlled deployment with perfect sealing and reduced risk of paravalvular regurgitation9. The flexible delivery catheter allows for smooth tracking through peripheral vasculature and adaptation to various aortic root angulations. The early valve function ensures haemodynamic stability during deployment.
St. Jude Portico
The St. Jude Portico™ prosthesis (St. Jude Medical, Inc., St. Paul, MN, USA) combines a self-expanding nitinol stent frame and a trileaflet bovine pericardial valve (Figure 18)10. The stent frame has a large open cell design, a tubular inflow portion and a porcine pericardial sealing cuff. Both leaflets and sealing cuff are treated with the Linx™ anticalcification technology (St. Jude Medical, Inc.). The Portico is currently available in 23 and 25 mm sizes, related to the diameter of the inflow portion; additional 27 and 29 mm devices are anticipated. The height of the device is 47 mm and the diameter of the outflow aortic portion is 38 mm. The open cell design aims to achieve better conformability with the aortic annulus and adaptation to calcium protrusion, preserves coronary flow and allows for coronary artery engagement post TAVI. The Portico is designed for subannular placement, 1-9 mm below the aortic annulus, in order to take advantage of the sealing properties of the cuff. Ideally the device should not extend more than 6 mm into the left ventricle outflow tract to minimise conduction disturbances (Figure 19). The Portico is fully resheathable, repositionable and retrievable up to 85% of its expansion. The valve is prepared and loaded onto the delivery system at room temperature. It is deployed without rapid pacing and functions early during deployment.
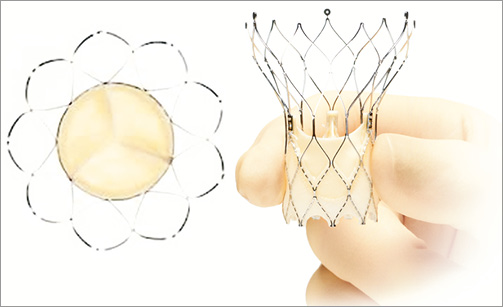
Figure 18. St. Jude Portico™. Self-expanding nitinol frame with a trileaflet bovine pericardial valve.
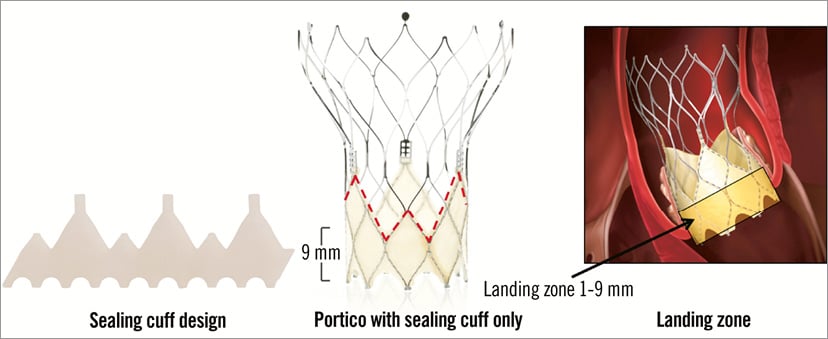
Figure 19. St. Jude Portico™ TAVI device. Details of the sealing cuff design and height determining a target landing zone between 1 and 9 mm below the aortic annulus. The transfemoral delivery system is a flexible 18 Fr catheter with a radiopaque marker in the inner shaft for precise positioning of the valve (Figure 20). A gradual and controlled deployment of the annular portion of the device is achieved with the possibility of resheathing the prosthesis in situ to fine-tune the position. The transapical delivery system is a 24 Fr sheathless system that also firstly deploys the annular section of the valve11.
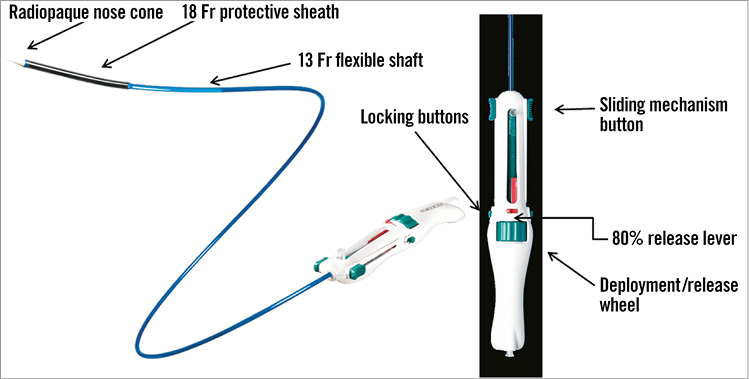
Figure 20. Details of the Portico™ transfemoral delivery catheter and handle. Symetis ACURATE
The ACURATE TA™ (Symetis SA, Ecublens, Switzerland) obtained a CE mark in September 201112. The initial transapical design consists of a porcine tissue valve mounted within a self-expanding nitinol stent frame (Figure 21). The distal/aortic edge of the frame contains three stabilisation arches to prevent tilting of the prosthesis and ensure proper alignment during final deployment. The upper-crown segment of the stent contains the valve leaflets (which are also placed supra-annularly) and provide supra-annular anchoring and tactile feedback during the deployment. The lower crown is the stent edge that minimally resides within the left ventricular outflow tract. A PET sealing skirt is mounted around the intra-annular part of the stent to mitigate paravalvular regurgitation. The ACURATE TA comes in three sizes (S, M, and L) to cover annulus sizes ranging from 21 to 27 mm. The transapical delivery system –a sheathless concept and so-called 28 Fr equivalent– contains a radiopaque valve-housing segment and has three markers to ease commissural alignment. The deployment starts by introducing the delivery system and subsequently releasing the stabilisation arms and the upper crown of the stent body. By pulling on the system the operator will get tactile feedback, the delivery system will embrace the native calcified leaflets and the upper crown will compress the tissue. Resheathing and repositioning are possible up to this point. Finally, the stent body is fully deployed and self-detaches from the delivery system (Figure 22).
The ACURATE neo™ aortic bioprosthesis (Symetis SA) is the device iteration for the transfemoral approach and basically shares the same features as the ACURATE TA13 (Figure 21). It comes with a dedicated 18 Fr ACURATE TF™ delivery system (Symetis SA). The “top-down” implantation starts by introducing the delivery system within 2 to 4 mm of the annular plane. The upper crown is unsheathed and subsequently pushed down to capture the native leaflets. The stabilisation arches are then released; up to this point the valve can still be repositioned and retrieved. After removing the safety button, the lower crown is unsheathed during a short run of rapid right ventricular pacing.
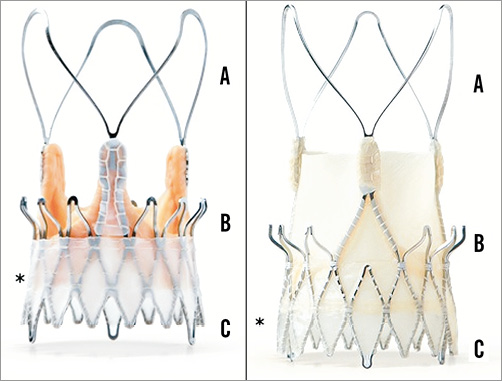
Figure 21. Symetis ACURATE TA (left) and neo TF (right) with the three stabilisation arches (A), upper crown (B) and lower crown (C). Note the PET sealing skirt (*). JenaValve
The JenaValve prosthesis (JenaValve Technology GmbH, Munich, Germany) obtained CE mark for treatment of severe degenerative aortic stenosis in September 2011 and is the only transcatheter heart valve design with a CE mark expansion for severe aortic insufficiency, awarded in September 201314. The JenaValve design consists of a natural porcine aortic root fitted within an outer porcine pericardial patch and sewn onto a self-expanding crown-shaped nitinol stent frame (the “Niti-Stent”) (Figure 23). The JenaValve was initially a transapical-only platform. It contains three so-called feelers that should be planted in the proper (left, right or non-coronary) cusp, in order to secure correct subcoronary alignment within the native valve. The JenaClip™ (JenaValve Technology GmbH) is an intrinsic mechanism to clip the JenaValve prosthesis onto the native valve leaflets for active fixation and to prevent migration. The rationale behind the feelers concept is to reduce radial forces on vital cardiac structures (e.g., conduction system). There are currently three sizes available (23, 25 and 27 mm) to cover annulus sizes from 21 to 27 mm. The JenaValve is partially repositionable and retrievable. The implantation requires a sheathless 32 Fr delivery catheter (Cathlete; JenaValve Technology GmbH) (Figure 24). After introduction of the delivery system with the crimped JenaValve into the ascending aorta, the positioning feelers are released. The correct anatomical orientation of the respective feelers relative to the native cusps is confirmed, and the catheter is pulled back up until a tactile feedback indicates the contact of the feelers with the proper cusps. The second step is to release the lower stent frame, thereby clipping and anchoring the bioprosthesis onto the native leaflets. At this point the bioprosthesis is competent and functioning but still repositionable and retrievable. The third and final step is the opening of the upper part of the nitinol frame (Figure 25).
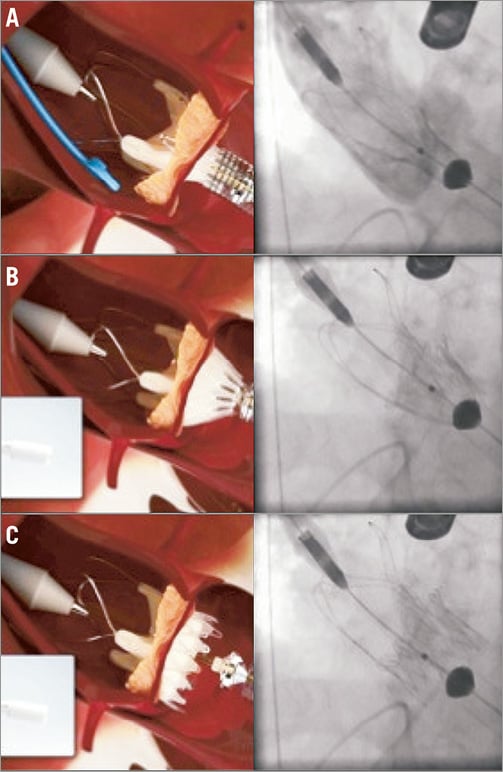
Figure 22. ACURATE TA™ implantation. A) Implantation step 1. The prosthesis is partially unsheathed releasing the stabilisation arms and the upper crown of the stent body. B) By pulling on the system the operator will get tactile feedback, the delivery system will embrace the native calcified leaflets and the upper crown will compress the tissue. C) The stent body is fully deployed and self-detaches from the delivery system.
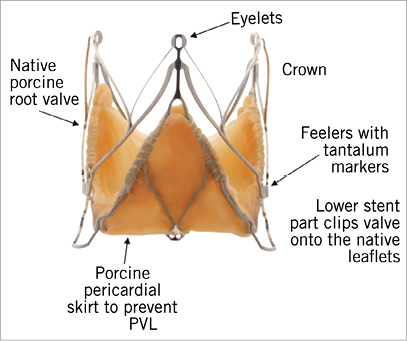
Figure 23. JenaValve demonstrating the native porcine root valve within the stent frame, including the feelers.
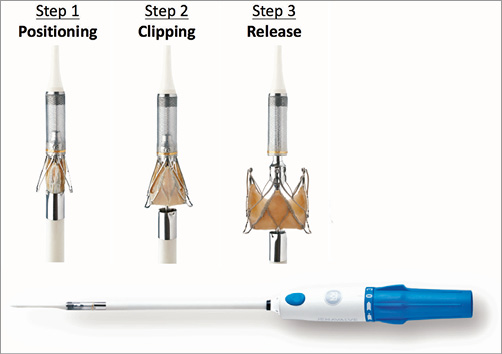
Figure 24. The transapical JenaValve delivery catheter - Cathlete. The three steps of JenaValve deployment (see text for details).
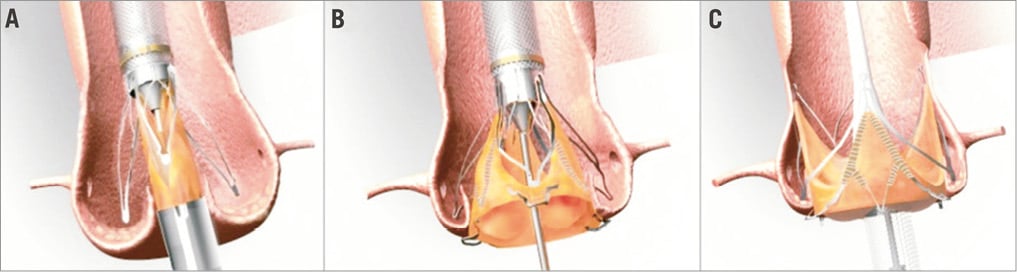
Figure 25. The transfemoral JenaValve with the three essential steps of deployment. A) Release of the feelers. B) Release of the lower part of the frame. C) Release of the upper part of the frame. In 2013 the transfemoral JenaValve concept was presented15. The intrinsic JenaValve features are similar to the transapical version. The 18 Fr delivery catheter houses the prosthesis and contains a shaft that releases initially the feelers and afterwards the entire prosthesis. The transfemoral bioprosthesis sizes and corresponding annulus ranges to be covered are similar to the transapical version. TF implantation varies somewhat. In the first step the positioning feelers are released and subsequently advanced (contrary to the transapical pushing manoeuvre) into the native cusps. The second step is the deployment of the lower inflow area of the stent frame during which the prosthesis is clipped to the respective cusps. In the third step the upper part of the prosthesis is released.
Conclusion
TAVI technology is rapidly evolving. New valve designs are addressing the main drawbacks and TAVI-associated complications that emerged from the experience with the first-generation transcatheter heart valves. Growing clinical experience and well-designed trials will determine whether these novel valve platforms will eventually have a clinical impact and extend the TAVI armamentarium. Tailoring the type of device to the patient could be possible with potentially favourable impact on the outcome post TAVI.
Conflict of interest statement
D. Tchetche is proctor for Edwards Lifesciences, Medtronic and Boston Scientific. N. Van Mieghem has received research grants form Medtronic, St. Jude Medical and Claret Medical.

