Introduction
Since the first-in-human transcatheter aortic valve implantation (TAVI) –performed with a balloon-expandable transcatheter heart valve (THV) ten years ago– this procedure has become a widely accepted treatment option for high-risk patients with symptomatic aortic valve disease. Improvement of survival and quality of life by TAVI over balloon valvuloplasty or best medical treatment has been demonstrated in randomised trials as well as in European registries1-3.
The balloon-expandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) and the self-expanding CoreValve (Medtronic, Minneapolis, MN, USA) are currently the most implanted devices. Despite favourable clinical outcomes there are still many device-related issues which need to be addressed to increase the safety of the procedure and to reduce typical complications like paravalvular leakage, stroke, conduction blocks requiring permanent pacemaker implantation and compromise of coronary blood flow. Various THVs with individual design have been developed to try to overcome these limitations.
The JenaValve system (JenaValve Technology GmbH, Munich, Germany) is a next-generation self-expandable transcatheter valve which, due to its specific design, may mitigate some of these challenges. The transapical system held promise of high safety and efficacy in a series of first-in-man implantations as well as in a pivotal study for CE mark approval.
The transfemoral JenaValve TAVI Plus system has been successfully implanted in animal experiments. The device is currently only available for investigational use and first-in-man implantation is scheduled for later this year.
Description - technical specifications
The JenaValve Plus prosthesis consists of three leaflets and a skirt which are manufactured from porcine pericardial tissue (Figure 1). The prosthesis is attached to a low-profile self-expanding crown-shaped nitinol stent (Niti-Stent) via polyester sutures. Three positional or guiding feelers are – in analogy to the TA device – again main components of the prosthesis. During deployment of the valve these feelers spread apart and are placed into the sinuses at the base of the native leaflets, which allows for anatomically correct positioning. The positional feelers along with the nitinol stent arms at the base of the prosthesis allow for “clipping” the prosthesis to the native leaflets. This active clip fixation is intended to reduce radial forces on aortic and cardiac structures as compared to other devices that expand in the aortic annulus. Secure anchoring of the valve in position to the native annulus and sealing in order to minimise paravalvular leakage is achieved by 24 diamond-shaped struts forming a ring at the inflow of the valve.
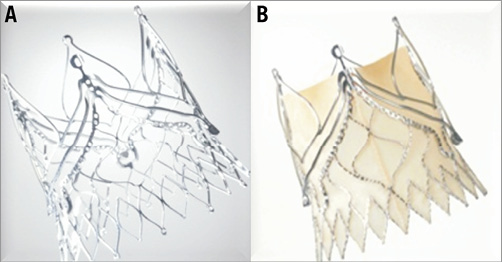
Figure 1. Nitinol stent and porcine JenaValve Plus prosthesis.
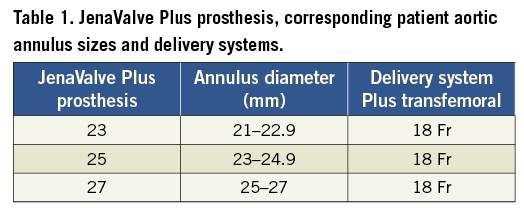
The valve is available in three sizes to fit the annulus from 21 mm to 27 mm (Table 1).
Delivery system Plus – transfemoral
Similar to the transapical device the delivery system is composed of three coaxial catheters combined in one but arranged in reverse order. The inner catheter consists of a soft distal tip and a funnel which houses the rhombus ring of the prosthesis mounted to a hydrotube made out of nitinol. The stent holder crown and a second nitinol hydrotube are the main components of the middle catheter. The outer catheter (18 Fr) consists of a sleeve which houses the prosthesis and a shaft that releases initially the feelers and finally the prosthesis during valve deployment.
Procedural and deployment characteristics
The transfemoral over-the-wire delivery system can be advanced through an 18 Fr femoral sheath. After balloon aortic valvuloplasty, which is recommended in native aortic stenosis prior to valve implantation, the delivery system with the loaded prosthesis is inserted transfemorally in the aorta and advanced in the native aortic valve. As a first step of the valve deployment, the positioning feelers of the stents are released from the delivery system via a handle. By careful manoeuvring the feelers are advanced into the native cusps to allow an anatomically correct circumferential and axial placement. In a second step of deployment the lower part of the prosthesis (inflow area) is released by further turning the handle. During this step the prosthesis is actively clipped to the native cusps. In a third and final step the upper part of the prosthesis is released by again turning the handle, which removes the delivery system resulting in complete deployment of the valve (Figure 2). The entire procedure is performed under fluoroscopic control without rapid pacing.
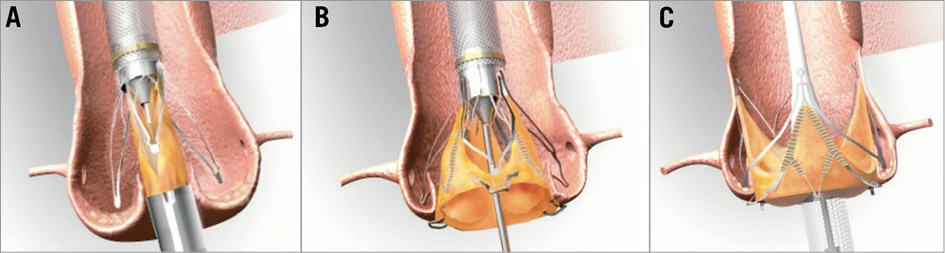
Figure 2. Deployment of the JenaValve Plus prosthesis. A) Release of the positioning feelers and positioning into the native cusps. B) Release of the lower part of the prosthesis accomplishes active clipping to the native cusps. C) Full release of the prosthesis.
Preclinical and clinical data of the JenaValve system
The transfemoral JenaValve Plus prosthesis has so far only been investigated in animal experiments. First-in-man implantation is planned: so far clinical data are awaited for late 2013. Since the basic design and deployment mechanism of the prosthesis are similar to the transapical JenaValve prosthesis, comparable results appear possible. The transapical device revealed a low rate of post-procedural paravalvular leakage and valvular aortic regurgitation, which is known to be a predictor of worse outcome in TAVI patients. The low rate of aortic regurgitation is mainly attributed to anatomically correct positioning of the valve and the sealing mechanism. There is also first evidence that the clipping fixation allows for implantation of this valve in patients with isolated aortic regurgitation4. This promises to convey an additional treatment option in high-risk patients with aortic regurgitation.
As compared to other self-expandable valves, the need for permanent pacemaker rate implantation was acceptably low (9.1%)5; this is potentially related to reduced radial force exerted on the annulus and left ventricular outflow tract, thereby eliminating conduction disorders.
Whether these favourable results of the transapical JenaValve device will be revisited using the transfemoral prosthesis needs to be tested in the first series of first-in-man implantations.
Conclusion
The self-expanding JenaValve Plus prosthesis is anticipated to be a next-generation transcatheter heart valve that allows a secure positioning and that will potentially reduce some of the current TAVI inherent caveats.
Conflict of interest statement
The authors have no conflicts of interest to declare.

