Abstract
Twelve years after the first transcatheter aortic valve implantation (TAVI) for severe aortic stenosis (AS), European and American guidelines, as well as the FDA, indicated that TAVI is the treatment of choice in “inoperable” patients and an alternative option to SAVR in high-risk patients. Recently, there has been a trend in clinical practice and trials to treat “lower” risk patients, and data in this subset of patients suggest better outcomes with TAVI, equivalent to surgery, using propensity matching analysis. The awaited results of the randomised PARTNER II trial with the Edwards XT valve and of the SURTAVI trial with the Medtronic CoreValve will bring an evidence-based comparison of TAVI versus SAVR in this patient population. Refinement in patient selection, new devices and long-term assessment of valve durability should also contribute to an extension of the indication of TAVI to “lower” risk patients. It is likely that the next five to ten years will see this technology become the dominant therapy for AS. At the present time, one might already consider TAVI as an alternative to SAVR in a select subset of very old and otherwise healthy AS patients.
Introduction
The emergence of transcatheter aortic valve implantation (TAVI) in 2002 profoundly altered the landscape of cardiovascular medicine1. Thanks to numerous clinical trials and evidence-based investigations, TAVI is now increasingly accepted by the medical community as a viable and established option in patients with severe aortic stenosis (AS) without surgical option and in patients at high risk for surgical aortic valve replacement (SAVR). In the last decade, TAVI has been performed in about 150,000 patients worldwide and indications keep growing at a rate of 40% annually. Most of our knowledge on TAVI is based on an extensive experience acquired with two devices, the balloon-expandable Edwards prosthesis (Edwards Lifesciences, Irvine, CA, USA) and the self-expanding Medtronic CoreValve (Medtronic, Minneapolis, MN, USA). FDA approval was obtained for the Edwards device based on the results of the pivotal “Placement of Aortic Transcatheter Valve (PARTNER)” trial, a randomised US trial published in 2011 and 2012 (for non-operable and high-risk patients, respectively) and for the Medtronic CoreValve device after the results of the US pivotal trial for non-operable and high-risk patients2-5.
Recommended indications for TAVI were recently specified in the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery guidelines and by the American College of Cardiology/American Heart Association Task Force on Practice Guidelines in 2012 and 2014, respectively6,7. Briefly, TAVI can be performed in patients with severe AS without surgical option, and as an alternative to surgery in high-risk patients in whom TAVI is favoured by a multidisciplinary Heart Team based on the individual risk profile and anatomic suitability. Based on these guidelines, TAVI is currently limited to about 20% of all AS patients, with two thirds of the patient population being sent for SAVR, and the remaining maintained on medical treatment.
It has been a twelve-year-long journey since the first-in-man (FIM) TAVI case to reach this level of recognition1. What a cautious and progressive pathway from the initial feasibility studies in moribund patients, to critically ill patients, and then to patients at very high risk for SAVR (elderly patients with multiple comorbidities)! Evolutions of most cardiac interventions (e.g., percutaneous coronary interventions) demonstrate a gradual progression from use in the simplest (low risk) to the more complex (high risk) scenarios. The fact that it demonstrated improved survival, quality of life, and functional status in this high-risk patient population is therefore particularly remarkable.
Over the last couple of years, the field of TAVI has been rapidly evolving, with major refinements in technology, procedural techniques, patient selection and biomedical engineering making TAVI simpler and safer, and the question of its expansion to lower-risk patients is already being raised. This wish is being expressed by many a cardiologist and patient alike.
Indications for TAVI: where are we today?
The current indications and recommendations for TAVI are essentially based on the US randomised PARTNER trial and numerous registries (covering several thousands of patients)2,3,8-18.
What did we learn from the randomised trials? Briefly, in the PARTNER IB study, patients with severe AS and considered inoperable were randomly assigned to standard therapy or TAVI with the first-generation Edwards SAPIEN prosthesis. On the basis of a 40% decrease in all-cause mortality, sustained at three years, TAVI is now considered the standard of care for patients who are not suitable for SAVR. In the PARTNER IA study, patients at high risk for SAVR were randomised to TAVI or SAVR. All-cause mortality at 30 days was slightly lower with TAVI (3.4% versus 6.5%, p=0.07), but was similar at one-year (24.2% vs. 26.8%) (Table 1), two-year (33.9% vs. 35%) and three-year (44.2% vs. 44.8%) follow-up3,8. Although the rates of all neurologic events were higher after TAVI at 30 days and one year (5.5% vs. 2.4% and 8.3% vs. 4.3%, p<0.05), rates of major stroke were not significantly different between TAVI and SAVR at 30 days (3.8% vs. 2.1%, p=0.2) or at one year (5.1% vs. 2.4%, p=0.07). There were other important differences in periprocedural risks between the two groups, with more major vascular complications at 30 days after TAVI (11.0% vs. 3.2%, p<0.001), and more major bleeding (19.5% vs. 9.3%, p<0.001) and new-onset atrial fibrillation (16.0% vs. 8.6%, p=0.006) after SAVR. Marked improvement of symptoms was similar after TAVI and SAVR, and was sustained at three years in both groups3,8. From these results, TAVI emerged as a viable alternative to SAVR in high-risk patients, the choice being guided by the decision of the interdisciplinary Heart Team.
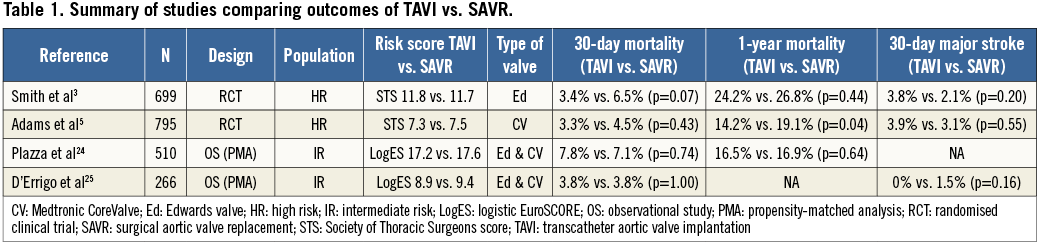
More recently, the results of the Medtronic CoreValve US Pivotal Trial were reported4,5. The “extreme risk” arm of this study included a non-randomised comparison of TAVI versus standard therapy. As in the PARTNER IB trial, this study demonstrated a 40% reduction in a combined endpoint of all-cause mortality and major stroke at one year. In the “high risk” arm of this pivotal trial reported early this year5, patients were randomly assigned to either TAVI or SAVR. Importantly, in comparison to SAVR, TAVI was shown for the first time to be associated with a significantly lower mortality rate at one year (14.2% versus 19.1%, p<0.05) (Table 1). Rates of major stroke were similar in the TAVI and SAVR groups at 30 days (3.9% vs. 3.1%) and one year (5.8% vs. 7.0%). Rates of major adverse cardiovascular and cerebrovascular events at one year were significantly lower with TAVI than with SAVR (20.4% vs. 27.3%, p=0.03). In addition, rates of moderate or severe paravalvular leak (PVL) for TAVI were acceptably low with 7.8% at discharge and 6.1% at one year. Interestingly, in this trial, patients were at lower risk than in the PARTNER IA trial (STS scores 7.3% vs. 11.8% in the TAVI group, and 7.5% vs. 11.7% in the SAVR group, and logistic EuroSCOREs 17.6% vs. 29.3% in the TAVI group, and 18.4% vs. 29.2% in the SAVR group, respectively, in the two studies). The question arises as to whether the lower-risk population included in the Medtronic CoreValve trial influenced the better results of both TAVI and SAVR compared to those in the PARTNER trial.
Trend in both clinical practice and in clinical trials to treat “lower” risk patients and related changes in clinical outcome
In spite of the recognised poor calibration of the two prognostic scoring systems, patients are usually classified as high risk for surgery when the logistic EuroSCORE and the STS score are greater than 20% and 10%, respectively, or when the Heart Team considers that patients have significant comorbidities or significant weakness/frailty not reflected in these scores. Although TAVI is only recommended in inoperable and high-risk patients, there is obviously a trend in clinical registries to treat lower-risk patients with both Edwards and CoreValve prostheses. In the “French” FRANCE and FRANCE 2 TAVI registries, using the two models of valve, mean logistic EuroSCORE decreased from 25.6±11.4% in 2009 to 21.9±14.3% in 2010-201114,17. Similarly, in the “European” SOURCE11 and SOURCE-XT (unpublished data) registries using exclusively the Edwards prosthesis, the mean logistic EuroSCORE decreased from 25.8±14.4% (2007-2009, SOURCE registry) to 20.5±12.6% (2010-2011, SOURCE-XT registry)11. Moreover, the mean logistic EuroSCORE also decreased in large series using exclusively the CoreValve, e.g., from 23.0±13.7% (2007-2009, n=663)15 to 19.2±12.4% (2010-2011, n=1,015) in the Advance registry (unpublished data). A similar trend to treat lower-risk patients has been observed in the USA. The mean logistic EuroSCORE and STS scores were 29.3±16.5% and 11.8±3.3%, respectively, in the PARTNER IA study (2007-2009, n=699) but decreased to a median STS score of seven (IQR: 5-11) in the post-market US registry using exclusively the Edwards SAPIEN prosthesis (2011-2013, n=7,710)3,19. Finally, in the most recent clinical trials, there was also a clear trend to include lower-risk patients with a mean STS score close to 6.0% and 7.4% in the CHOICE and US CoreValve studies, respectively5,20.
Several studies tend to confirm the positive impact of lower risk scores on the clinical outcome after TAVI. Lange et al21 investigated the evolution of patient selection criteria for TAVI and its impact on clinical outcomes. Patients enrolled in a single-centre study between 2007 and 2010 were subcategorised into quartiles (Q1 to Q4) defined by enrolment date. These subgroups were subsequently examined for differences in baseline characteristics and 30-day and six-month mortality rate. Each quartile included 105 patients. Compared with Q4, Q1 patients had higher logistic EuroSCORE (25.4±16.1% vs. 17.8±12.0%, p<0.001), and higher STS scores (7.1±5.5% vs. 4.8±2.6%, p<0.001). From Q1 to Q4, the 30-day and six-month mortality rates decreased significantly from 11.4% to 3.8% (p=0.05) and from 23.5% to 12.4% (p=0.07), respectively. The impact of logistic EuroSCORE on mortality at 30 days and one year was similarly evaluated in a prospective single-centre study performed in Germany between 2008 and 201022. Mortality was shown to depend on the logistic EuroSCORE, with odds ratio (OR) at 30 days of 1.92 (95% CI: 1.41-2.62, p<0.001), and at one year of 1.67 (95% CI: 1.34 to 2.08, p<0.001). Thirty-day mortality in patients with a logistic EuroSCORE <15% or ≥15% was 0.9% versus 9.1% and at one year 7.1% versus 23.5%, demonstrating significantly less mortality (p<0.001) in patients with lower logistic EuroSCOREs. Furthermore, Wenaweser et al23 categorised 389 consecutive patients who underwent TAVI between 2007 and 2011 according to the STS score into low (STS <3%, n=41, 10.5%), intermediate (STS ≥3% and ≤8%, n=254, 65.3%), and high-risk (STS >8%; n=94, 24.2%) groups. Again, lower mortality at 30 days (2.4 vs. 3.9 vs. 14.9%, p=0.001) and one year (10.1 vs. 16.1 vs. 34.5%, p=0.0003) was observed in lower-risk patients.
Recent data in lower-risk patients suggest comparable outcomes with TAVI and SAVR
Using propensity score analysis, two recent studies have compared 30-day and one-year outcomes between TAVI and SAVR in intermediate-risk patients (Table 1). In the first study24, propensity-score matched pairs of TAVI and SAVR patients with STS scores between 3% and 8% made up the study population. The primary endpoint was all-cause mortality at one year. Between November 2006 and January 2010, 3,666 patients underwent TAVI or SAVR. Four hundred and five TAVI patients were matched to 405 SAVR patients. Of the matched TAVI patients, 99 (24%) had STS scores <3%, 255 (63%) had scores between 3% and 8%, and 51 (13%) had a score >8%. Among “intermediate-risk patients” (STS scores between 3% and 8%), the mortality rates at 30 days and one year were similar after TAVI and SAVR. The second study, the OBSERVANT observational prospective multicentre cohort study25, reported similar results. Pairs of patients with the same probability score were matched. Within an unadjusted population of 2,108 patients, the matched population comprised 133 patients each for TAVI and SAVR with a mean logistic EuroSCORE of 9.4±10.4% (SAVR) vs. 8.9±9.5% (TAVI; p=0.65). Thirty-day mortality was similar in both groups (3.8%), as was the incidence of stroke (1.5% vs. 0%) and myocardial infarction (0.8%). In comparison to the TAVI group, a higher requirement for blood transfusion was reported after SAVR (49.6% vs. 36.1%; p=0.026). A higher incidence of major vascular complication (5.3% vs. 0%, p=0.007), pacemaker implantation (12% vs. 0.8%, p=0.001), and PVL (39.2% vs. 10.3%, p<0.0001) was reported in the TAVI group.
Patients at intermediate surgical risk, therefore, seem to have similar overall mortality at 30 days and one year after TAVI or SAVR. However, in these studies, although a propensity score adjustment analysis was performed in order to compensate partly for the baseline and angiographic imbalance between groups, each treatment was not assigned randomly but by specific criteria in each case, generating an unavoidable risk of bias regarding treatment selection.
Ongoing evidence-based studies in intermediate-risk patients
Ongoing randomised studies have been designed to provide an evidence-based comparison of TAVI and SAVR in intermediate-risk patients (PARTNER II cohort A trial with the Edwards SAPIEN XT valve in the USA, and SURTAVI trial with the Medtronic CoreValve in Europe). The PARTNER II study is a multicentre randomised non-inferiority study comparing TAVI versus SAVR in operable AS patients with an STS score >4%. The primary endpoint is combined all-cause mortality and disabling stroke at two years. The SURTAVI trial is a multicentre randomised non-inferiority study comparing TAVI versus SAVR in operable AS patients with an STS score between 4% and 10%, eligible for both techniques. Interestingly, in both arms, the benefit of coronary artery revascularisation by either concomitant PCI or CABG will also be assessed. The combined primary endpoint is all-cause mortality and major stroke at two years.
The results of these two studies might have enormous consequences on the future expansion of TAVI to lower-risk patients. However, it must be pointed out at this point that the results will reflect those of a second generation of transcatheter heart valve prostheses. With the recent launch of third-generation prostheses (SAPIEN 3, CoreValve Evolut, and other prostheses) which have already been shown in clinical practice to reduce the severity and incidence of most major complications, PARTNER IIA and SURTAVI results may not reflect those of contemporary technology.
What information is needed that will help extend TAVI to intermediate- or low-risk patients in 2014?
Based on many reports and their growing personal experiences, most cardiologists and surgeons are convinced that the best results of TAVI are obtained in lower-risk patients. With the widespread dissemination of information regarding TAVI amongst the general population, TAVI is requested emphatically by patients and their relatives, and recommending SAVR has become a difficult task for the physicians. Arguments are sometimes missing, more particularly in otherwise healthy elderly patients. As a matter of fact, it is a regular observation that “very old low-risk patients” are increasingly being offered TAVI to avoid the discomfort of burdensome surgery and the subsequent long period of recovery. As is the practice in our institution, TAVI can be performed, using the percutaneous transfemoral approach and local anaesthesia in 80% of cases, and turns into a minimalist “stent-like” procedure with early discharge home in the vast majority of patients, a very beneficial strategy in the elderly population26.
In “younger/low-risk” patients, however, advances in two areas will determine a broader acceptance of TAVI: the procedural safety and the long-term durability of the valve cusps and platform.
Addressing the issue of procedural safety remains a priority. Advanced technologies for patient screening and procedural steps have dramatically improved the rate and severity of complications. Moderate to severe paravalvular leak and major vascular complication are the two leading complications impacting negatively on the clinical outcome27,28. New models of valve and ultra-small-size delivery systems have been specially designed to reduce the incidence of these complications. New-generation valves, such as SAPIEN 3 and CoreValve Evolut R, have already demonstrated their great efficacy29,30. At the present time, the incidence of stroke and myocardial infarction is considered low and similar after TAVI and SAVR and should not be considered a limiting factor to an expansion of indications to younger/lower-risk patients. However, ongoing studies on embolic protection devices and changes in anticoagulant strategy are awaited to determine their safety and usefulness in selected patients. The incidence of complete heart block requiring permanent pacemakers (PPM) is remarkably device-related. Even though the impact of PPM on mortality has not been demonstrated, lowering this risk would be preferable in a younger population.
Information regarding long-term durability of valve cusp and platform is still missing and should be obtained before offering TAVI to younger/lower-risk patients. That said, should we mandatorily wait five to 10 years to obtain this information? Valve failure or deterioration is recognised as a slow process in case of surgical biologic valves, and has so far been reported very rarely in the elderly TAVI population. In our personal series beginning in 2002, we have seen no case of valve failure or deterioration. The potentially higher incidence of valvular calcification in younger patients remains questionable, as does the difference in comparative outcomes of transcatheter and surgical bioprostheses. Despite the absence of long-term data on durability, we already know that, in the event of accelerated valve failure, low-risk patients would remain good candidates for SAVR; in the event of late valve deterioration, valve-in-valve TAVI could always be an option.
Final thoughts
Currently, any uncontrolled application of this disruptive technology to younger/lower-risk patients should be avoided in the absence of evidence-based data. However, in view of the latest reported results and with the considerable benefits of advanced technologies, we believe that TAVI might already be justified in select subsets of lower-risk patients such as otherwise healthy elderly patients (>80 years) presenting with anatomy favourable for minimalist transfemoral procedures. The patient and relatives should be increasingly involved in the decision-making process of the Heart Team.
One can be quite optimistic about the future of TAVI, and it is likely that the next ten years will see this technology become the dominant therapy for AS. Is it too far-fetched to speculate that surgical valve replacement will eventually be reserved for patients with contraindications to TAVI?
Conflict of interest statement
A. Cribier is consultant and proctor for Edwards Lifesciences. H. Eltchaninoff has received lecture fees from and is proctor for Edwards Lifesciences. E. Durand has no conflicts of interest to declare.

