
Abstract
Transcatheter aortic valve implantation (TAVI) has, in less than 20 years, become the dominant interventional treatment for aortic stenosis in developed countries. Its development has been characterised by the growth of procedural expertise and device improvement. Every aspect of this therapy has been investigated, increasing clinical evidence and leading to continued optimisation. The purpose of this review article is to provide an overview of the rapidly changing field of TAVI therapy, briefly describing key past achievements and discussing residual challenges.
Introduction
In less than 20 years, transcatheter aortic valve implantation (TAVI) has taken an incredible journey, characterised by an extraordinary growth of procedural expertise, device improvement, and increasing clinical evidence1,2. This innovative therapy is now established as the dominant interventional treatment for aortic stenosis in many developed countries3,4,5,6. Many researchers and operators have investigated every aspect of this therapy, providing fundamental elements for further optimisation and development1. The cultural ferment is evident from the extremely high number of scientific articles on the field of TAVI that have crowded first-tier cardiology journals in the last two decades.
The purpose of this review article is to provide an overview of the rapidly changing field of TAVI therapy, briefly describing key past achievements and discussing residual challenges. In the interest of simplicity, the discussion has been subdivided into three areas: preprocedural, procedural and post-procedural challenges.
Preprocedural challenges
WHO SHOULD PERFORM TAVI?
Everyone wants to perform TAVI! Since severe aortic stenosis is the most common valvular heart disease affecting elderly patients in developed countries7, providing access to this procedure is a key issue8. When discussing TAVI penetration rates, there are several aspects that should be understood. National economic indices (i.e., healthcare expenditure per capita, sources of healthcare funding, and reimbursement strategies) are a major factor but will not be discussed in this article. However, at least two important questions have raised the interest of the clinical community. 1) Should TAVI be performed in centres without on-site cardiac surgery? 2) What is the minimum number of TAVI procedures that should be performed in a centre to ensure optimal outcomes?
The 2017 Valvular Heart Disease Guidelines of the European Society of Cardiology (ESC) mandate that TAVI should be restricted to hospitals with both cardiology and cardiac surgery on-site4. The permanent accessibility of both specialties in the same institution is considered optimal to ensure appropriate patient selection by the Heart Team as well as to prompt management of potential severe complications during TAVI, many of which require emergency cardiac surgery9 (Figure 1). Interestingly, recent data extracted from the Society of Thoracic Surgeons/American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) Registry demonstrated that the rate of surgical bail-out during TAVI was fairly stable over a period of four years (1.17% for the period 01/12-02/13, and 1.04% for the period 06/14-09/14)10. The lack of endorsement for TAVI at hospitals without on-site cardiac surgery stems from perceptions of inappropriate patient selection and poor outcomes at such sites, even in the absence of supportive data. However, it is undeniable that this represents the only practical way to avoid uncontrolled and indiscriminate adoption of the procedure in low-volume and low-experience centres. Of course, the lack of a cardiac surgery department is not synonymous with low levels of expertise. Here lies the “bug”. At some hospitals without on-site cardiac surgery, the Heart Team approach (a key prerequisite for TAVI) has been adopted by in-house cardiologists and visiting cardiac surgical teams from external, collaborating hospitals11. Preliminary data on the experience with this Heart Team approach in small numbers of patients undergoing TAVI at sites without on-site cardiac surgery have suggested favourable outcomes, supporting its feasibility and safety12,13. The German Quality Assurance Registry on Aortic Valve Replacement (AQUA), a prospective registry of all TAVI and surgical aortic valve replacement (SAVR) procedures performed in Germany, compared patient characteristics, complications and outcomes of patients undergoing TAVI in hospitals with and without an on-site cardiac surgery department14. Among 17,919 patients undergoing TAVI from 2013 to 2014, 1,332 (7.4%) underwent the procedure at hospitals without an on-site cardiac surgery department. Although these patients were older (82.1±5.8 vs 81.1±6.1 years, p<0.001) and at higher procedural risk (logistic EuroSCORE I 23.2±15.8 vs 21.0±15.4%, p<0.001), major complication rates (including strokes [2.6 vs 2.3%, p=0.452], vascular complications [10.1 vs 8.9%, p=0.161] and in-hospital mortality [3.8 vs 4.2%, p=0.396]) were not statistically different14.
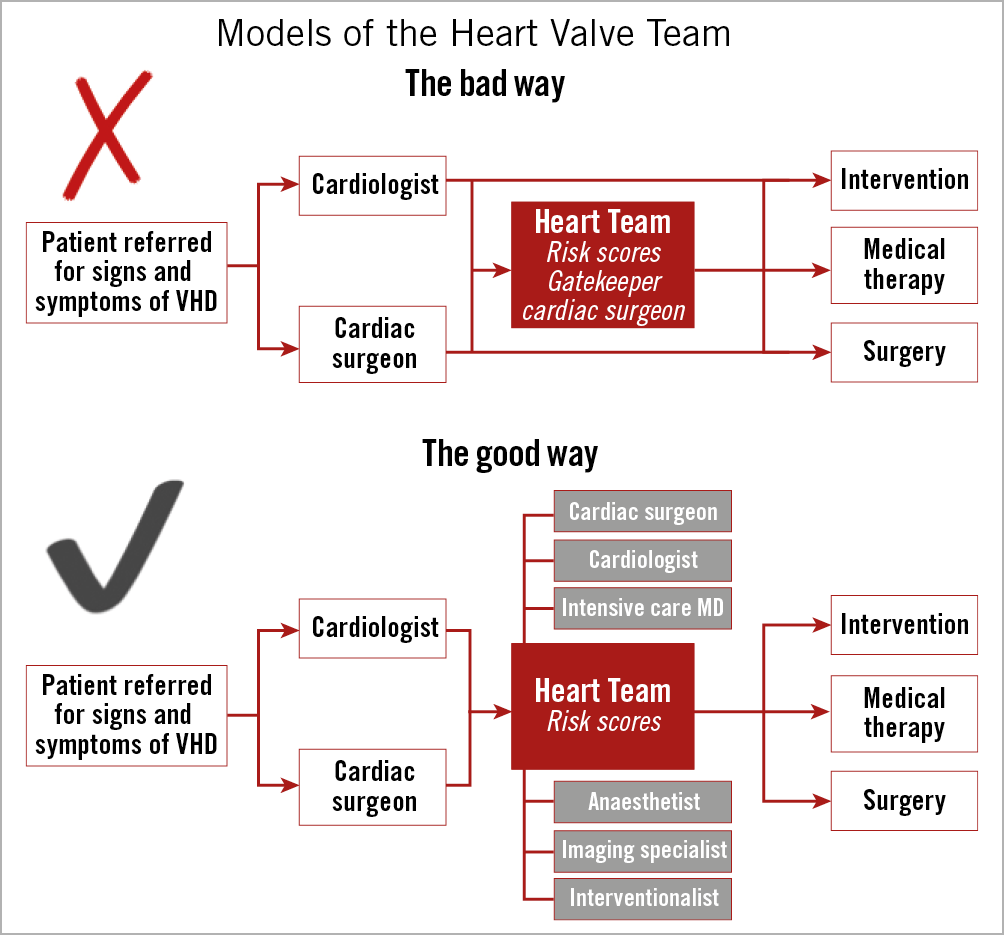
Figure 1. Examples of heart valve team dynamics. The upper panel shows a bad model, in which patients are referred to either cardiologists or cardiac surgeons and then treated without collegial assessment. In the context of the Heart Team discussion, when this is involved, the cardiac surgeon acts as a gatekeeper. In the lower algorithm, a good example of a Heart Team model is shown, in which every patient is discussed by the Heart Team, composed of a cardiologist, a cardiac surgeon and other specialists (if needed), and therefore undergoes the best treatment according to clinical and anatomical features.
More recently, an analysis of the prospective multicentre Austrian TAVI registry included consecutive high-risk and inoperable patients with severe symptomatic aortic stenosis undergoing transfemoral TAVI (n=1,822), of whom 15.9% were treated at hospitals without cardiac surgery departments, with no difference in propensity-matched periprocedural and post-procedural survival rates up to one year15. However, this study carries several important limitations. First, patients were not truly matched for all baseline factors. Second, there are issues other than survival such as pacemaker rates, vascular complications, stroke, appropriate device selection, paravalvular leak (PVL), duration of hospital stay, discharge home as opposed to another facility, resource use and cost, that should be considered. Third, there is also the issue of who did not undergo TAVI: the question is whether low-volume or non-surgical centres offered the procedure to all appropriate patients. These findings require validation in a randomised study given the small number of patients undergoing TAVI at hospitals without an on-site cardiac surgery department. However, besides the inherent limitations of self-reported registries (where underreporting and heterogeneity in outcomes among centres cannot be excluded), the AQUA and the Austrian registry findings raise the important question of whether there is a real and contemporary unmet clinical need that may justify extending TAVI to institutions without cardiac surgery departments (especially outside Germany). At this time, based on current guidelines and pending additional data, we believe that clinicians should strongly avoid performing TAVI procedures at hospitals without on-site cardiac surgery4,5,16. TAVI is more than just a procedure. It is part of a comprehensive treatment programme that embraces team-based care by experienced clinicians with shared decision making and access to all treatment options.
An expanding body of literature supports the observation that outcomes of interventional or surgical procedures improve with increasing centre volume – commonly referred to as the “volume–outcomes relationship”17,18,19,20. Although numerous studies (including some focused on TAVI) have attempted to define this relationship19,20,21,22,23,24, findings have been conflicting and much remains to be understood.
Recent data extracted from the Society of Thoracic Surgeons/ACC-TVT Registry support the need for careful continued examination of TAVI volume requirements24. Vemulapalli and colleagues analysed 113,662 TAVI procedures performed at 555 hospitals by 2,960 operators from 2015-2017 in the USA. Procedural volume at hospital level (for both transfemoral and non-transfemoral TAVI) was inversely associated with 30-day risk-adjusted mortality (3.19% [lowest-volume quartile; 95% CI: 2.78-3.67] vs 2.66% [highest-volume quartile; 95% CI: 2.48-2.85]; OR 1.21; p=0.02), even after excluding a hospital’s first year of cases to account for a potential learning curve (3.10% vs 2.61%; OR 1.19; 95% CI: 1.01-1.40). These findings contrast with a previous study that only included cases performed with the SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA)25, but are consistent with prior analyses (including one examining earlier data from the TVT Registry)26.
Both operator and hospital volumes were taken into account in this interesting analysis, highlighting the fact that multiple skills and a functional team are needed to achieve optimal TAVI outcomes, as in many other areas of modern interventional cardiology. Importantly, the lowest 30-day mortality rates are associated with operator volumes as low as 40 TAVI procedures/year, a volume that should be achievable relatively easily within functioning TAVI networks25.
NEXT STEPS IN TRANSCATHETER HEART VALVE SIZING
Although some TAVI-related complications can be attributed to patient characteristics or operator expertise, some arise from specific device-host interactions. Amongst the latter, the most significant and impactful are: 1) incomplete and/or non-circular frame expansion due to the presence of aortic calcification leading to PVL, and 2) unexpected movement of calcified cusps leading to coronary obstruction or aortic root rupture despite appropriate valve size selection27,28,29. Although current three-dimensional computed tomography (CT) reconstructions are highly accurate, device-host interactions are difficult to predict owing to wide variation in the geometry and dimensions of the aortic root and differing volume and distribution of calcium among patients30. The increasing number and spectrum of TAVI patients (and increasing variety of available valve types) will necessitate patient-specific tools to predict device-host interaction for case selection and procedural planning (i.e., selection of the valve that best fits the individual patient). Finite element computer simulation of a TAVI procedure (based upon integration of patient-specific anatomy, physical and mechanical valve properties, and biomechanical characteristics of the aortic root) may help to define in vivo device-host interactions, thereby enhancing the safety of TAVI31. To date, this extremely interesting field remains poorly explored. Schultz et al attempted to predict the in vivo morphology of two TAVI devices (CoreValve® [Medtronic, Minneapolis, MN, USA] and SAPIEN XT [Edwards Lifesciences]) and displacement of the calcified aortic leaflets immediately after implantation, by comparing the findings derived from a patient-specific cardiovascular computer model with those from CT performed after TAVI32. Although they were able to demonstrate high agreement between the predicted and observed dimensions of the valve frame, the model slightly overestimated the dimensions at all levels (except the commissures). These observations are promising, but at the same time demonstrate that these tools need significant improvement.
Procedural challenges
BICUSPID AORTIC VALVE
Bicuspid aortic valve (BAV) is a complex disease spectrum including several anatomical variants, ranging from true type 0 bicuspid valve (with no raphe) to forms in which three cusps can be identified (with variably located raphes [type 1 and type 2]) (Figure 2). Approximately 2-6% of TAVI patients have a BAV33,34 and give rise to several major concerns (Figure 3).
– Asymmetric shape and calcification may impair adequate prosthesis expansion, function and durability
– Associated aortopathy may increase the risk of aortic dissection and annular rupture
– Device sizing techniques have yet to be refined.
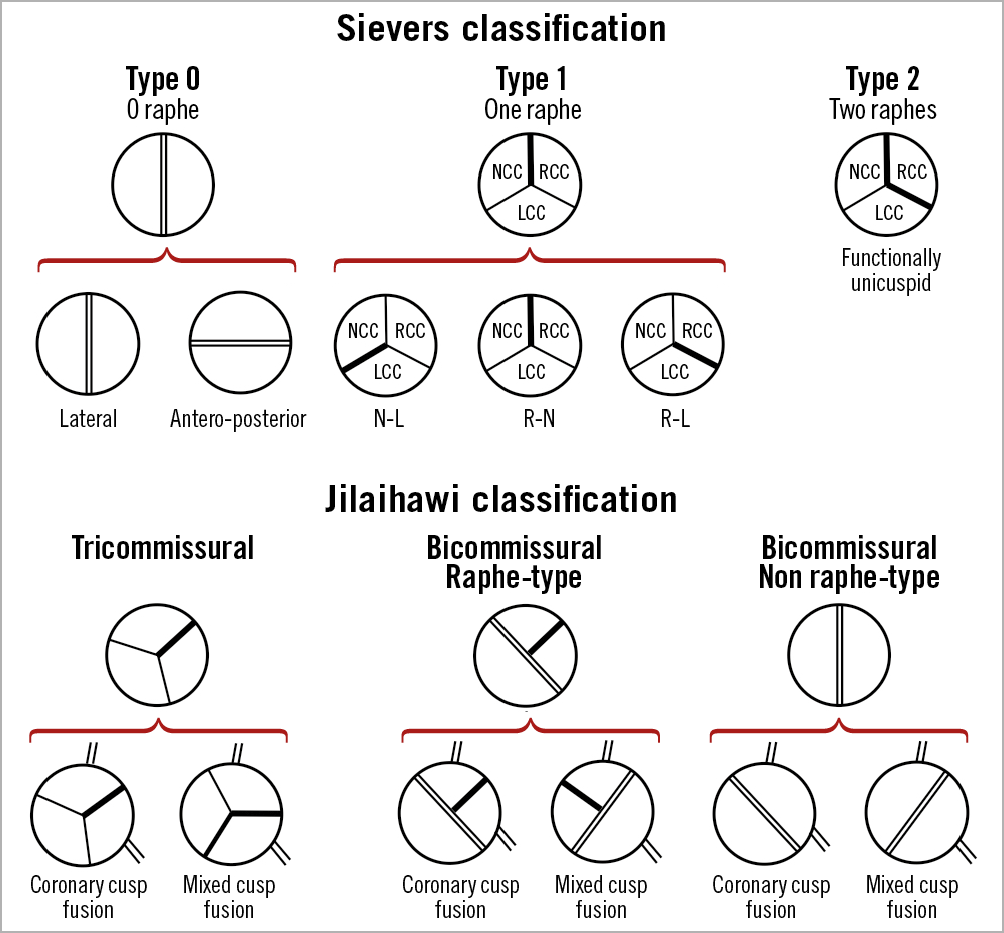
Figure 2. Schematic representation of bicuspid aortic valve morphologies according to Sievers’ and Jilaihawi’s classifications. LCC: left coronary cusp; NCC: non-coronary cusp; RCC: right coronary cusp
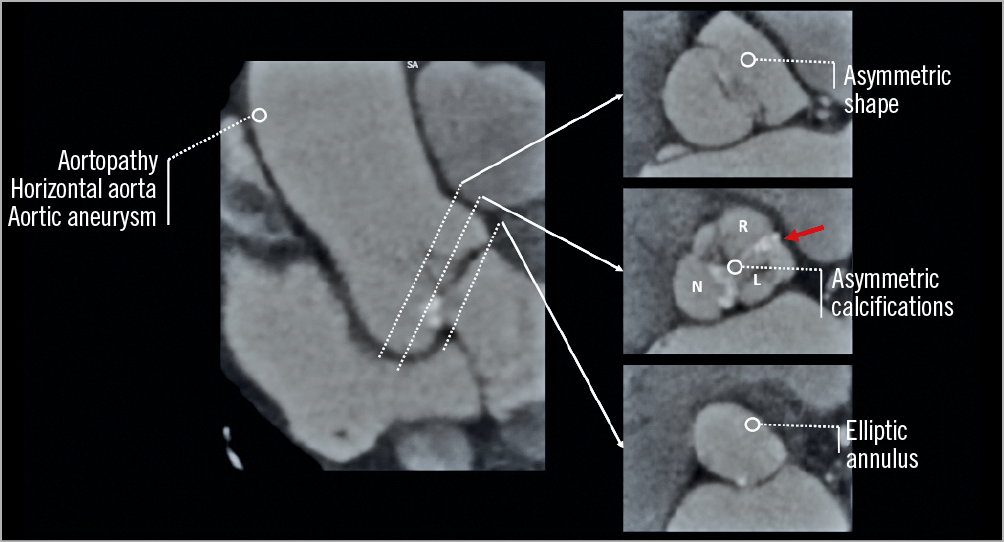
Figure 3. Computed tomography images of a type 1 R-L bicuspid aortic stenosis. The image summarises the current issues of TAVI therapy in bicuspid aortic valves. The red arrow indicates the raphe. L: left coronary cusp; N: non-coronary cusp; R: right coronary cusp
Despite these concerns, many patients affected by BAV have already been treated with TAVI over the years. In a large multicentre study, outcomes of 546 matched pairs of patients with BAV and tricuspid aortic stenosis were compared35. Overall, BAV was associated with a higher rate of conversion to surgery (2.0% vs 0.2%, p=0.006) (mainly due to aortic root injury), the need for a second valve (4.8% vs 1.5%, p=0.002) and ≥moderate PVL (10.4% vs 6.8%, p=0.04). However, in spite of these procedural pitfalls, no difference in survival was observed at 30-day or two-year follow-up. Of note, the increased rate of procedural complications in BAV patients was only observed in those who received older-generation devices36. Specifically, aortic rupture was observed more frequently with the SAPIEN XT, whereas second valve need and PVL were more frequent with the CoreValve. Conversely, results were markedly better in patients who received new-generation devices (in particular, the SAPIEN 3 and Lotus™ [Boston Scientific, Marlborough, MA, USA]), even though there was a numerical trend highlighting the problems related to TAVI in BAV (conversion to surgery 1.3% vs 0%, second valve need 1.3% vs 0.4%, PVL 2.7% vs 1.8%, all p>0.05)35.
More recently, Makkar et al reported a sub-analysis of the ACC/TVT Registry including 2,691 propensity score-matched pairs of bicuspid and tricuspid aortic stenosis. They found that patients with bicuspid versus tricuspid aortic stenosis had no significant difference in 30-day (2.6% vs 2.5%; HR 1.04 [95% CI: 0.74-1.47]) or one-year (10.5% vs 12.0%; HR 0.90 [95% CI: 0.73-1.10]) mortality and moderate or severe PVL at 30 days (2.0% vs 2.4%; absolute risk difference, 0.3% [95% CI: -1.3% to 0.7%]), but had increased 30-day risk for stroke (2.5% vs 1.6%; HR 1.57 [95% CI: 1.06-2.33])36.
The number of treated cases is still limited; more experience is needed to clarify fully the role of TAVI in BAV disease, particularly in younger and lower-risk patients. Furthermore, there are no randomised controlled trials comparing TAVI and SAVR in this specific anatomical subset. However, results with new-generation devices demonstrate promisingly low complication rates, similar to conventional tricuspid aortic stenosis35,36. As a general rule, cautious preprocedural screening and procedural performance remain necessary in BAV patients, in order to minimise aortic root trauma and avoid excessive prosthesis oversizing (even allowing some downsizing). In conclusion, BAV in itself is not an absolute contraindication to TAVI. Instead, the anatomy of each single case should be carefully assessed prior to the procedure to rule out possible issues (concomitant root dilatation, valve size, location of calcification, horizontal aorta) and choose the best type of prosthesis for the specific anatomy.
VALVE-IN-VALVE PROCEDURES
All surgical bioprostheses have limited durability and usually fail within 10-15 years37,38. Similarly, transcatheter heart valve (THV) durability up to six years is already a well-established reality, with low rates of structural valve dysfunction (SVD) demonstrated in large rigorous studies39,40. However, data concerning clinical outcomes and THV integrity beyond six years remain scarce41. Patients with failed surgical or transcatheter bioprosthetic valves are commonly at high surgical risk because of comorbidities, old age, and the need for repeat thoracotomy in patients with surgical valves42. In this context, implantation of a THV inside the failed aortic bioprosthetic valve (valve-in-valve [ViV]) has emerged as an effective and less invasive treatment for degenerated aortic bioprostheses43.
Successful ViV procedures require a detailed understanding of the anatomical and fluoroscopic appearances of the surgical heart valve, and knowledge of THV design and sizing for ideal implantation position of the chosen THV within the existing bioprosthesis. Although procedural success is achieved in the vast majority of patients, these TAVI-ViV procedures are associated with several potential complications, including THV-surgical heart valve mismatch, immediate or delayed THV migration and embolisation, high residual gradients, and coronary occlusion (especially amongst particular types of aortic bioprosthesis)43.
Coronary occlusion has poor prognosis and remains one of the major concerns of TAVI-ViV procedures43,44,45,46,47 despite its low frequency (TVT Registry 0.5%; Valve-in-Valve International Data [VIVID] registry 2.3%)45,46. Avoidance requires meticulous procedural planning to choose the correct prosthesis type and size. However, the main predisposing factor in ViV procedures is the proximity of a coronary ostium to the anticipated final position of the displaced bioprosthetic leaflets after THV implantation48. The virtual THV to coronary distance (VTC) is a CT-obtained predictor of the proximity of the coronary ostia to the anticipated final position of the displaced bioprosthetic leaflets after THV implantation48. The VTC combines the height of the coronary ostia, sinus width and THV size, and also simulates THV tilt in the annulus (coronary occlusion risk: maximum <4 mm, borderline 4-6 mm, low >6 mm)48. Predictors of coronary occlusion after ViV implantation include anatomical factors (low coronary ostia, narrow sinuses of Valsalva and sinotubular junction, bulky leaflets), bioprosthetic valve factors (supra-annular position, high leaflet profile, internal stent frame [e.g., Mitroflow (Sorin Group, Milan, Italy), Trifecta™ (St. Jude Medical, St. Paul, MN, USA)] or no stent frame [homograft, stentless valve]), and THV factors (extended sealing cuff, high implantation)49 (Figure 4). Coronary protection should be used in high-risk cases to allow swift restoration of coronary flow and improve clinical outcome49. Furthermore, intentional laceration of the bioprosthetic aortic scallop to prevent iatrogenic coronary artery obstruction –the BASILICA procedure– is emerging as an effective method to prevent coronary occlusion50.
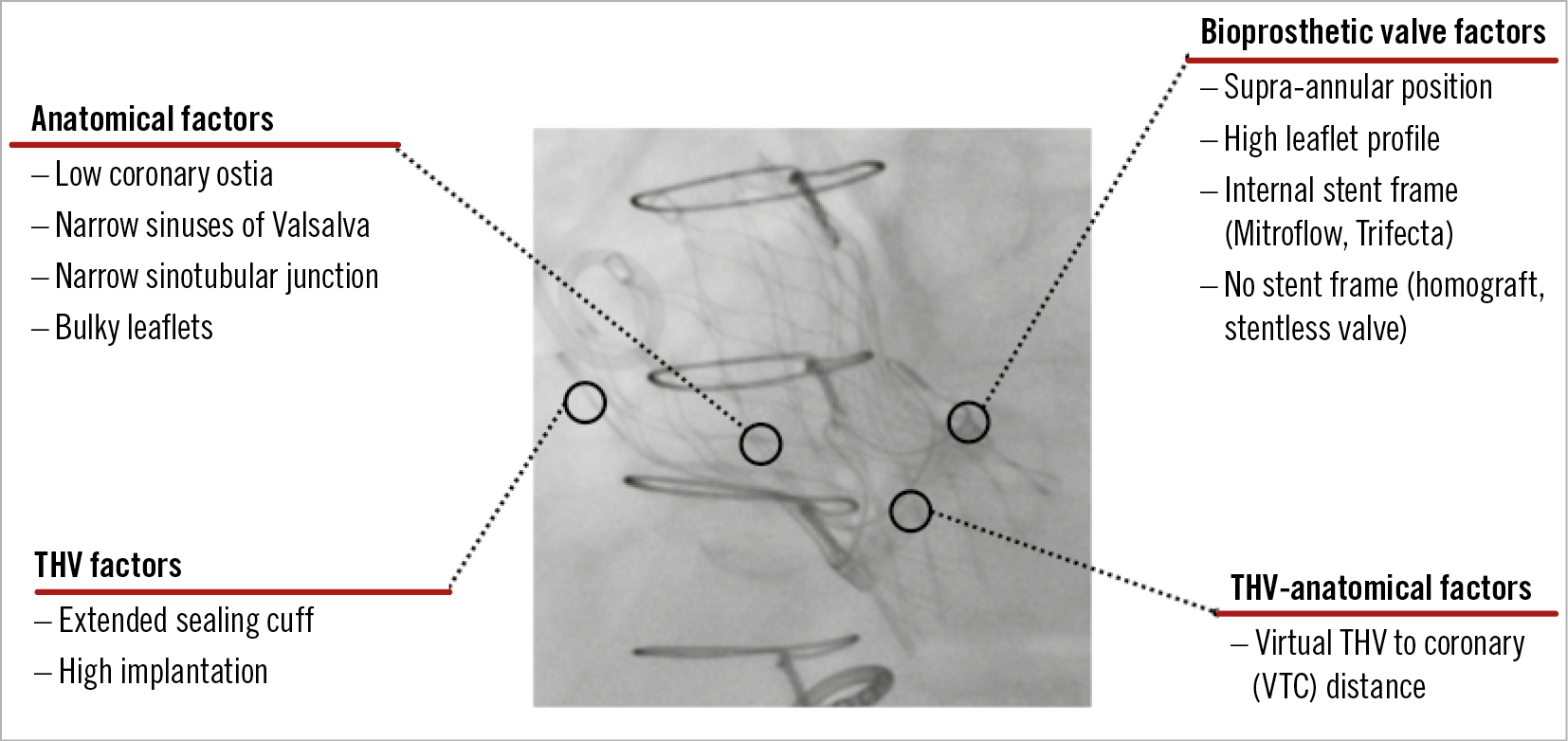
Figure 4. Predictors of coronary occlusion after valve-in-valve TAVI procedures.
Residual aortic stenosis is a major drawback of TAVI-ViV procedures. In the VIVID Registry, elevated mean gradients (≥20 mmHg) were recorded in 27% of patients45 and are especially common in patients receiving balloon-expandable THVs in small surgical valves (label size ≤21 mm)51. In such cases, expansion of the THV frame is constrained by the bioprosthetic surgical valve ring, with consequent underexpansion of the THV leaflets, greater obstruction to flow and suboptimal haemodynamics50. Potential solutions include (a) optimal positioning of the THV within the surgical bioprosthesis52, (b) implantation of the THV in a supra-annular position52, or (c) bioprosthetic valve ring fracture (BVF) using high-pressure non-compliant balloons (Atlas® GOLD or TRUE® balloons; Bard Peripheral Vascular Inc., Tempe, AZ, USA) before or after THV implantation53. Surgical valves with a metallic frame (Hancock® II [Medtronic], Trifecta and older-generation PERIMOUNT or Carpentier-Edwards [Edwards Lifesciences]) cannot be fractured using currently available balloons50. The procedural and haemodynamic outcomes of patients treated with ViV and BVF have been reported in two case series encompassing 30 cases53. In 15 cases in which BVF was performed after TAVI-ViV, mean gradient was reduced from 41.9 mmHg to 20.5 mmHg with ViV, and further reduced to 6.7 mmHg following BVF (p<0.001)53.
In contrast with redo valve surgery, preliminary data suggest that redo TAVI (so called THV-in-THV) procedures are safe, with low periprocedural complication rates and midterm survival and prosthesis performance comparable to recent TAVI series54. However, this concept is associated with several potential concerns. First, it is unknown whether the presence of two THVs could affect long-term durability. Second, it could be argued that patients with double THVs may be more prone to leaflet thrombosis, although this was not apparent in one small series (albeit without systematic CT assessment). Third, coronary access may be challenging, particularly after implantation of two THVs that extend into the ascending aorta (e.g., CoreValve/Evolut™ [Medtronic])54. Finally, it can be argued that a number of patients would not be able to have repeat TAVI due to the risk of coronary “occlusion” due to supra-annular THV-in-THV (Figure 5). This has implications for THV selection and positioning in younger low-risk patients.
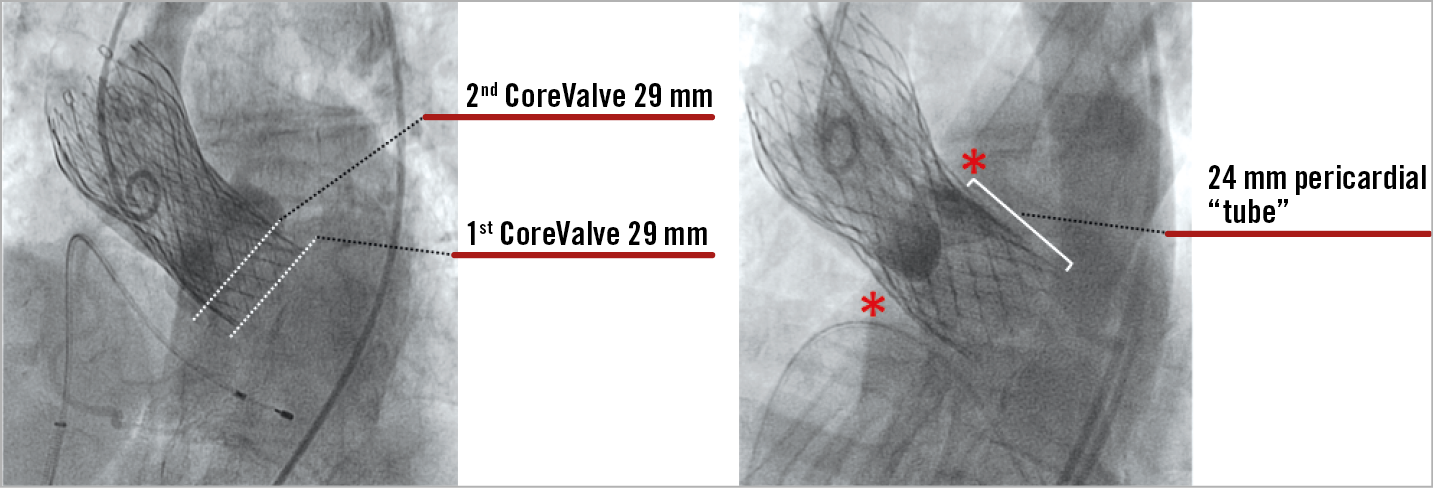
Figure 5. Case example of a THV-in-THV procedure. A degenerated 29 mm CoreValve treated with redo TAVI using a 29 mm CoreValve. The second THV was implanted approximately 6 mm higher than the first one. After 20 minutes, the progressive expansion of the second CoreValve created a covered cylinder of 24 mm that occluded the sinuses of Valsalva with a complete obliteration of the coronary ostia.
Post-procedural challenges
TRANSCATHETER HEART VALVE DURABILITY
TAVI is now being offered to younger and lower-risk patients. In this cohort, life expectancy will exceed that of the initial TAVI candidates, which makes the question of long-term prosthesis durability crucially important. Several limitations prevent robust evaluation of TAVI durability. First, TAVI is a relatively young technology given that its wider adoption started following CE mark approval in 2007 and US Food and Drug Administration approval in 2011. Thus, there are few data concerning valve durability beyond 10 years. Second, current data on long-term outcomes beyond five years relate to first-generation TAVI devices. Finally, the major limitation of long-term durability evaluation is the older age of the TAVI population, most of whom are affected by multiple comorbidities with high-risk profiles and limited life expectancy. A paucity of patients (usually <50% of the initial population) therefore remain alive at very long-term follow-up. In the past few years, results of several TAVI studies with more than five-year follow-up data have been published. The Placement of AoRTic TraNscathetER Valve Trial (PARTNER-1 trial) showed no evidence of SVD at five-year follow-up55,56. Moreover, the PARTNER-1A substudy demonstrated similar echocardiographic performance of both transcatheter and surgical valves, with a mean transvalvular gradient of 10.7 and 10.6 mmHg, and aortic valve area of 1.6 and 1.5 cm2, respectively55,56. This evaluation confirmed the satisfactory haemodynamic profile of transcatheter aortic valves up to five years post implantation, even if moderate or severe aortic regurgitation caused by PVL (excluded in the SVD definition) was more common in the TAVI group.
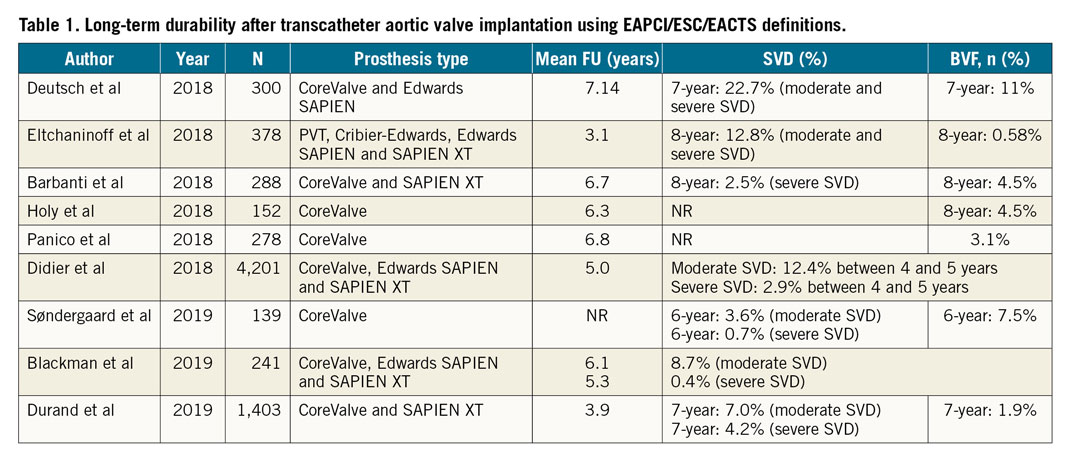
Following introduction of EAPCI/ESC/EACTS standardised criteria of SVD in 201757, an increasing number of studies have reported outcomes after TAVI with either SAPIEN or CoreValve THVs up to seven and eight years41,58,59,60,61,62 (Table 1). Three single-centre studies demonstrated stable transprosthetic gradients over time and rates of severe THV dysfunction of 2.4%, 3.2% and 3.6%41,58,59. Holy et al analysed long-term outcomes of 152 consecutive patients who had undergone TAVI with the self-expanding CoreValve between 2007 and 201160. Echocardiographic follow-up was undertaken at 6.3±1.0 years (5.0-8.9 years) and was 88% complete (60/68 participants who survived beyond five years). No case of SVD was reported and five patients (3.3%) had undergone redo TAVI or surgery due to PVL60. A similar analysis by Deutsch et al demonstrated an overall crude cumulative incidence of SVD of 14.9% seven years after TAVI (CoreValve 11.8% vs SAPIEN 22.6%; p=0.01)61. Further reports from national registries confirm low rates of THV dysfunction at long-term follow-up. Data from the French Aortic National CoreValve and Edwards (FRANCE-2) registry showed an incidence of severe and moderate/severe SVD of 2.5% and 13.3%, respectively, in patients surviving five years beyond the procedure63, while Blackman et al reported a 10-year incidence of severe and moderate SVD of 0.4% and 8.7%, respectively, in the UK TAVI Registry64. Finally, a recent analysis from the Nordic Aortic Valve Intervention (NOTION) trial reported lower rates of moderate-to-severe SVD after TAVI compared with surgery (4.8% vs 24% for TAVI and surgical aortic valve replacement, respectively), with no difference in bioprosthetic valve failure (6.7% vs 7.5%)39.
CORONARY ACCESS
As TAVI edges towards consideration in younger, lower-risk patients, it is important to address not only the clinical outcomes following initial implantation, but also longer-term considerations for future interventions such as percutaneous coronary intervention (PCI). Furthermore, the prevalence of coronary artery disease (CAD) in patients with severe aortic stenosis is high and there is no doubt that the presence of a THV in the aortic position may present an important challenge for coronary re-access (particularly those devices that extend into the ascending aorta). Whilst this has been particularly relevant for the CoreValve prosthesis for many years, the SAPIEN 3 valve (with a higher frame) has introduced potential issues with coronary re-engagement after balloon-expandable THV. In this context, the ACURATE neo™ bioprosthesis (Symetis SA/Boston Scientific, Ecublens, Switzerland) carries the important feature of a high frame, but with very large cells (stabilisation arches) that potentially minimise interference with coronary ostia re-access after THV deployment65. However, with limited published studies, it is nearly impossible to estimate the incidence, feasibility, and success rates of coronary angiography and PCI in this patient population. In the largest observational study investigating this issue, Zivelonghi et al66 reported the feasibility of angiography in 66 patients immediately after TAVI (CoreValve Evolut, n=25; SAPIEN 3, n=41). Selective angiography required guidewire positioning in 4% of vessels; only one artery could not be engaged due to high Evolut™ R implantation (possibly because the leaflet base of the supra-annular valve was located at the level of origin of the coronary ostium). PCI was successful in all 17 patients (including six with Evolut R). In conclusion, factors that could theoretically make coronary re-access challenging after TAVI are: 1) THV with closer cells landing above the origin of the coronary ostia, 2) high THV implant placing the base of the prosthetic leaflets in front of the coronary ostia; 3) narrow sinus of Valsalva and sinotubular junction; 4) commissure of the THV directly in front of the coronary ostia.
This last possibility has raised the attention of researchers. To date, there has been no reliable and consistent way to assess the position of the THV commissures in relation to those of the native aortic valve. In a pilot study, Tang et al reported techniques to reduce the severity of overlap of the THV commissures with one or both coronary ostia, suggesting that it may be possible to achieve a more ideal final position of the self-expanding Evolut R and Evolut™ PRO devices67. Using the C-tab as a marker, they co-registered valve positioning to the pre-TAVI CT scan using imaging software to orientate the valve commissures and their proximity to the left main and right coronary arteries. When the black capsule “hat” marker on the valve catheter was positioned at the inner curve/centre back of the aortic root during initial deployment, severe overlap of the neo-commissures with the left main and right coronary artery was common (63.9% and 52.8%, respectively). Conversely, when the capsule hat marker was positioned on the outer curve/centre front of the aortic root, overlap occurred in only 19.5% and 6.1% of cases, respectively67.
Conclusion
TAVI has revolutionised the management of patients with symptomatic severe aortic stenosis and indications are expanding towards younger patients with lower-risk profiles. Although many aspects of this innovative therapy have been deeply studied and clarified, several issues and challenges remain. Rigorous studies and ongoing technological development will help in providing patients with severe aortic stenosis with the most reliable and effective solution for their pathology.
Conflict of interest statement
M. Barbanti is a consultant for Edwards Lifesciences and an advisory board member for Biotronik. J.G. Webb is a consultant to, and has received research funding from Edwards Lifesciences, Abbott Vascular, Boston Scientific and ViVitro Labs. D. Dvir is a consultant for Edwards Lifesciences, Medtronic, and St. Jude Medical. B. Prendergast has received unrestricted educational and research grants from Edwards Lifesciences and speaker fees from Edwards Lifesciences.

