Abstract
Transcatheter aortic valve implantation (TAVI) has become a feasible and effective therapeutic option for patients with severe aortic stenosis and high operative risk or relative contraindications for surgical aortic valve replacement (SAVR). However, as with every new technology, a number of potential pitfalls and limitations have to be addressed and future opportunities have to be clarified in well-conducted clinical trials and well-monitored registries. Procedural issues are still controversial, and technical and procedural enhancements are required to reduce complications. Moreover, further studies are essential to evaluate second-generation devices in a broader patient population in order to understand whether the theoretical benefits of improved valve designs translate into fewer complications and a better long-term survival. Adjunctive devices, such as cerebrovascular protection devices, have to be further evaluated in clinical trials. This manuscript will focus on current procedural and post-procedural limitations of TAVI and future opportunities to overcome those challenges.
Abbreviations
LAHB: left anterior hemiblock
LBBB: left bundle branch block
PVR: paravalvular regurgitation
RBBB: right bundle branch block
SAVR: surgical aortic valve replacement
TAVI: transcatheter aortic valve implantation
Introduction
Transcatheter aortic valve implantation (TAVI) has become a feasible and effective therapeutic option for patients with severe aortic stenosis and high operative risk or contraindications for surgical aortic valve replacement (SAVR). Using a balloon-expandable device, TAVI has been shown to be non-inferior as compared to SAVR in high-risk patients with severe calcific aortic stenosis and to be superior to conservative management, including balloon valvuloplasty, in the PARTNER trial1,2. However, TAVI with a self-expandable device has recently been shown to be superior to SAVR after one year of follow-up3. These findings are most likely not due to the mechanism of valve deployment, but rather explained by the different risk profile of the included patients, e.g., STS score of 11.8% in the PARTNER trial vs. 7.3% in the U.S. CoreValve trial2,3. Numerous prospective registries using both balloon and self-expandable devices via different access sites4-10 support TAVI as an effective alternative to SAVR in real-world patients with high surgical risk, which has led to the rapid acceptance and widespread use of this new technology11.
However, as with every new technology, a number of potential pitfalls and limitations have to be addressed and future opportunities have to be clarified in well-conducted clinical trials and well-monitored registries. This manuscript will focus on current procedural and post-procedural limitations of TAVI and future opportunities to overcome those challenges.
Complications in TAVI and potential solutions
Access-site complications, major bleedings, cerebrovascular events, paravalvular regurgitation and conduction abnormalities remain the most frequent complications related to TAVI. Rare, but potentially fatal complications include annular rupture, aortic dissection, coronary occlusion, pericardial tamponade, and mitral valve injury. The precise scientific definitions of these intricacies have been published recently12. Complications can be avoided by rigorous patient evaluation and selection, including risk estimation and exact imaging of the anatomy. Identification of “futile” patients (“cohort C”) is of particular interest because these patients (e.g., STS score >15%, extreme frailty usually with a dependent social status, severe pulmonary or liver disease, severe dementia, and haemodynamic instability [especially requiring vasopressors]) have both poor survival (e.g., less than one year) and poor quality of life, despite successful TAVI. The topic of patient selection is discussed elsewhere in this supplement.
ACCESS-SITE SELECTION, COMPLICATIONS AND BLEEDING
The frequency of (major) vascular complications ranges from 1.9 to 17.3% in larger series of predominantly transfemoral TAVI5,13,14. However, older studies did not report according to VARC definitions12 and should therefore be interpreted cautiously. It has been shown that both vascular complications and major bleeding are associated with increased mortality13,15,16 and prolonged hospital stay17. Centre experience, sheath-to-femoral artery ratio, and moderate/severe femoral calcification have been identified as major predictors of iliofemoral vascular complications13,17, whereas female gender, larger sheaths, percutaneous access/closure, and a history of peripheral artery disease have been associated with major bleeding16.
The impact of sheath size on both vascular complications and major bleeding highlights the importance and necessity of lower profile delivery systems in transfemoral TAVI. In the PARTNER II trial, comparing the first-generation SAPIEN system (sheath profile 24 Fr; Edwards Lifesciences, Irvine, CA, USA) with the lower profile SAPIEN XT system (sheath profile 18 Fr), major vascular complications were significantly reduced from 15.5% to 9.6%18. Furthermore, the latest SAPIEN 3 system uses a 14 Fr expandable sheath (eSheath; Edwards Lifesciences) for the 23 mm and 26 mm valve requiring a minimal vessel diameter of 5.5 mm. In a small feasibility study using this system, no minor/major vascular complications or bleeding were reported19. The SAPIEN 3 trial, which included 96 transfemoral and 54 transapical/transaortic cases, reported an overall major vascular complication rate of 6% with a numeric advantage for the transfemoral group (5.2%) compared to the transapical/transaortic group (7.4%)20. This is the first study reporting a higher incidence of major vascular complications in an access route other than transfemoral, which is most likely due to the low profile delivery system of the SAPIEN 3 prosthesis and the low patient number in the transapical/transaortic cohort. So far, transarterial access has been associated with higher vascular complication rates –20.1% vs. 4.2% in a recent meta-analysis including 3,135 patients21. One may speculate that these promising data from the SAPIEN 3 trial will support even more the transfemoral access route as the primary approach which is already now used in about 75% of all TAVI cases22. Prevention, detection and possible treatment of vascular access-site complications include the proper anatomic evaluation of the access route prior to the implantation, use of a “safety net”, e.g., crossover wire during the implantation, a final angiogram of the access site after closure and, potentially, endovascular techniques, e.g., implantation of covered stents (Figure 1), or surgical reconstruction. The management of vascular complications is discussed in detail elsewhere23.
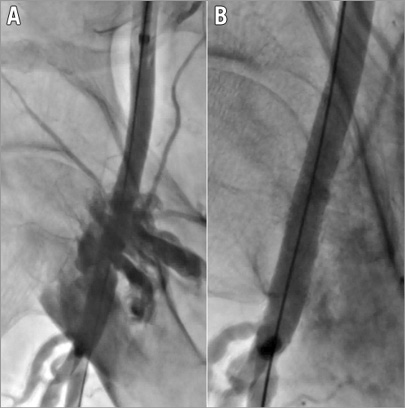
Figure 1. Vascular complication after removal of the sheath.. The patient presented with an acute haemorrhage of the common femoral artery (A) which was successfully managed by the implantation of a GORE® VIABAHN® 8×50 mm covered, self-expanding stent and post-dilatation with an 8 mm balloon (B).
However, the transapical and transaortic approaches are still reserved for patients in whom peripheral access is impossible due to small vessel diameter, severe stenosis, calcification and/or tortuosity of pelvic arteries indicating a high atherosclerotic burden in such patients. The transapical access allows some technical advantages, such as close control of the valve during deployment, but is also associated with an increased length of hospitalisation and risk of 30-day and one-year all-cause mortality21,24. Transaortic access, as compared to a contemporary group of transapical patients, showed a lower combined bleeding and vascular event rate (27% vs. 46%; p=0.05) and a shorter median intensive care unit length of stay (three vs. six days; p=0.01)25.
However, in the SAPIEN 3 trial, 30-day mortality was significantly higher in the transapical/transaortic compared to the transfemoral group (11.1% vs. 2.1% in the as-treated analysis). In this study, the STS score was comparable between transfemoral and transapical/transaortic patient cohorts whereas the logistic EuroSCORE was different with higher values in the latter cohort. Recently, van der Boon et al also compared the short-term and midterm outcomes between transapical and transfemoral TAVI using a multivariable analysis to minimise baseline differences between both groups (higher STS score and logistic EuroSCORE in the transapical group)24. They found an increased risk of all-cause mortality at 30 days and after one year in the transapical group, whereas transfemoral access was associated with a higher incidence of major and minor vascular complications. It is noteworthy that the examined cohort consisted of 882 patients from four centres, of whom 793 (89.9%) underwent transfemoral TAVI and only 89 patients (10.1%) underwent transapical TAVI, so these centres were low-volume centres for the transapical approach.
Despite statistical adjustment, these findings favouring the transfemoral access might be partially explained by differences in underlying baseline comorbidities between the two populations. This underlines the necessity for randomised controlled outcome studies comparing different access routes due to the fact that currently the selection of access for TAVI is driven by practical considerations.
CEREBROVASCULAR EVENTS
Stroke remains a major periprocedural and post-procedural complication leading to increased mortality and a significant deterioration in quality of life. A recent meta-analysis of 53 TAVI studies including more than 10,000 patients showed a reasonable periprocedural stroke rate of 1.5% and a 30-day stroke/transient ischaemic attack rate of 3.3%26. However, many of the included studies were conducted prior to the introduction of standardised definitions for cerebrovascular events12 and, at least in part, events were self-reported without independent monitoring which leads to an underreporting of events27. Therefore, in the randomised U.S. CoreValve trial, the rates of any stroke were 4.9% in the TAVI group and 6.2% in the surgical group at 30 days (p=0.46) and 8.8% and 12.6%, respectively, at one year (p=0.10)3. In the PARTNER trial, major stroke occurred in 3.8% in the transcatheter group and 2.1% in the surgical group at 30 days (p=0.20) and 5.1% and 2.4%, respectively, at one year (p=0.07)2. The overall higher stroke rate in the U.S. CoreValve trial might be explained by a prospective and more subtle diagnosis of cerebrovascular events, e.g., confirmation of the diagnosis by a neurology or neurosurgical specialist, whereas in PARTNER the diagnosis of major and minor stroke was distinguished through a CEC-adjudicated retrospective analysis of neurologic events.
In contrast to clinical presentation, up to 80% of patients treated with TAVI exhibit silent cerebral embolism in diffusion-weighted magnetic resonance imaging irrespective of valve type and access site28,29. The disagreement between silent cerebral embolism and clinically relevant cerebrovascular events, as well as the impact of the former on long-term prognosis, has to be determined in future studies.
Most cerebrovascular events seem to occur within the first 24 hours post procedure, but the risk remains high for the first two months following device implantation. Predilatation of the native valve, manipulation during implantation, e.g., repositioning and post-dilatation of the prosthesis, and post-interventional onset of atrial fibrillation may increase the risk for early and midterm stroke30,31. The SIMPLIFy TAVI trial is underway and aims to investigate the effects of TAVI with and without predilatation (ClinicalTrials.gov, number NCT01539746). Post-dilatation, predominantly performed to reduce paravalvular regurgitation (PVR), has also been associated with an increased risk of particularly early neurologic events (e.g., <7 days)32 but also with conduction abnormalities33 and annular rupture34. With improved valve sizing and the development of new devices reducing the risk of PVR, post-dilatation will hopefully become rare in the near future.
Another subject of interest is the application of cerebrovascular protection devices, e.g., Claret Montage™ Dual Filter System (Claret Medical, Inc., Santa Rosa, CA, USA), TriGuard™ (Keystone Heart, Caesarea Business Park, Israel) or the Embrella Embolic Deflector System (Edwards Lifesciences). Using the former system, 75% of TAVI patients demonstrated bulk material retrieval in filters placed in the right truncus brachiocephalicus and the left common carotid artery. Most of the material was derived from either the native valve or the aortic wall35. Randomised trials are necessary to determine the impact of these devices on neuroimaging and clinical endpoints after TAVI. The CLaret Embolic Protection ANd TAVI - Trial (CLEAN-TAVI; ClinicalTrials.gov, number NCT01833052) is currently underway and results are expected by the end of the year 2014.
The TriGuard™ (Keystone Heart) and the Embrella Embolic Deflector System (Edwards Lifesciences) have also been tested in small studies showing the safety and feasibility of these devices. Currently, randomised studies using these devices (e.g., PROTAVI-C) are underway and preliminary results from the DEFLECT I trial using the Embrella Embolic Deflector System are promising. Data on these devices have been extensively reviewed by Praz et al36.
Another matter of debate regarding the topic of cerebrovascular events and also bleeding is the management of peri- and post-interventional antiplatelet and anticoagulation therapy. Dual antiplatelet therapy with aspirin and clopidogrel should be initiated in all TAVI patients unless they have an indication for oral anticoagulation (e.g., atrial fibrillation). However, there is a lack of evidence about the necessity and dosage of a clopidogrel loading dose, the maintenance dose and duration of therapy. Further evidence is expected from the Aspirin Versus Aspirin+ClopidogRel Following Transcatheter Aortic Valve Implantation trial (ARTE Trial) (ClinicalTrials.gov, number NCT01559298) comparing aspirin alone vs. aspirin+clopidogrel following TAVI.
The “International, Multi-center, Open-label, Randomized Controlled Trial in Patients Undergoing TAVR to Determine the Treatment Effect (Both Safety and Efficacy) of Using Bivalirudin Instead of UFH. (BRAVO 2/3)” (ClinicalTrials.gov, number NCT01651780) is currently recruiting patients to compare bivalirudin vs. unfractionated heparin as a periprocedural anticoagulant. The topic of peri- and post-interventional antiplatelet and anticoagulation regime is also discussed in detail elsewhere37.
PROSTHESIS DYSFUNCTION AND PARAVALVULAR REGURGITATION
The two-year follow-up of PARTNER cohort A patients demonstrated that PVR was more frequent after TAVI (p<0.001), with PVR (none/trace vs. mild/moderate/severe) associated with increased late mortality (hazard ratio 2.11; 95% CI: 1.43 to 3.10; p<0.001)38. Numerous other studies using both self-expandable and balloon-expandable devices were able to show associations between varying degrees of PVR and late mortality after TAVI5,15,39,40. Precise diagnosis of PVR requires a comprehensive assessment of haemodynamics, angiography and in particular echocardiography41,42. The heterogeneity of these data, e.g., increased mortality risk with mild PVR vs. increased mortality in only more-than-mild PVR, may be explained by the difficult assessment of PVR, absence of an echocardiographic/angiographic core lab, and differences in the patient populations. Therefore, improved diagnostic assessment of PVR and its influence on mortality needs to be addressed in future studies.
Underestimation of the annulus diameter, severe and asymmetric calcification of the aortic valvar complex, and valve malalignment have turned out to be the main causes of PVR, and treatment options may include post-dilatation, implantation of a second valve or even the usage of peri-valve vascular plugs41.
To overcome this problem, next-generation devices have been developed to lower the rate of PVR. They are retrievable, repositionable and have, at least in part, a fine positioning control mechanism to avoid valve misalignment. Furthermore, additional cuffs providing an extended seal zone may prevent PVR.
The SAPIEN 3 (Edwards Lifesciences) has just received CE approval and has been studied initially in a small feasibility study in which no patient had more than mild PVR19. Its fine positioning control allows accurate placing during implantation (Figure 2). In the SAPIEN 3 trial, there was no severe PVR and the vast majority of patients had no/trace (72.4%) or mild (24.1%) PVR and only 3.4% had moderate PVR at 30 days20.
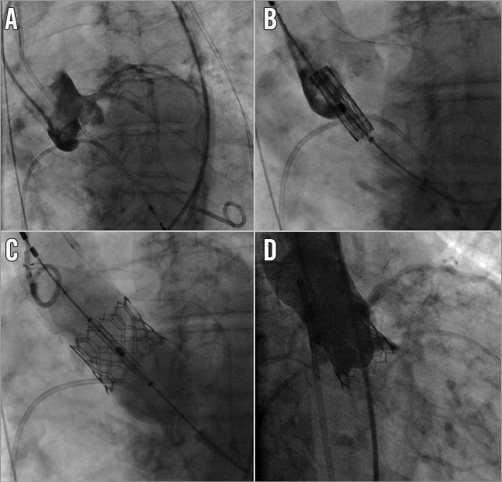
Figure 2. Implantation of the latest balloon-expandable platform SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA). Note the borderline height of the left main ostium (9 mm in CT scan) in the aortic root angiography at baseline (A). Therefore, a guidewire was placed in the left coronary artery as a “safety net” in case coronary occlusion occurred after valve implantation. Following balloon valvuloplasty, the SAPIEN 3 valve (23 mm) was positioned in the aortic annulus (B) and implanted under rapid ventricular pacing (C). Final angiography showing a good result without evidence of paravalvular regurgitation, coronary occlusion or annulus ruptures (D).
The Lotus™ Valve System (Boston Scientific, Natick, MA, USA) (Figure 3) also seems to be promising in this regard, since very low rates of PVR at 30-day follow-up have been described in the REPRISE II trial43. Only one of 60 patients had moderate aortic regurgitation, with more than 80% of patients having no or only trace aortic regurgitation43.
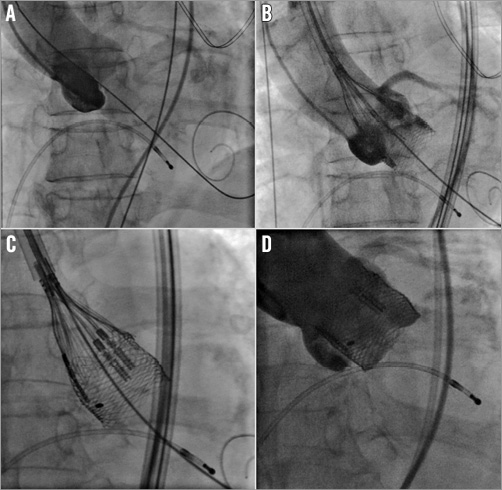
Figure 3. Implantation of the Lotus™ valve (Boston Scientific). Note the CE certified TAVI pre-shaped guidewire Safari™ (manufactured by Lake Region Medical, Wilmington, MA, USA, and distributed by Boston Scientific) in the left ventricle during aortic root angiography at baseline (A). Stepwise release of the self-expandable Lotus valve and confirmation of correct locking (B and C). Until that point the valve can be repositioned and retrieved. Final angiography showing a good result without evidence of paravalvular regurgitation, coronary occlusion or annulus ruptures (D).
A number of other devices, e.g., the Direct Flow Medical® valve (Direct Flow Medical Inc., Santa Rosa, CA, USA), the JenaValve® (JenaValve Technology GmbH, Munich, Germany), the Portico™ valve (St. Jude Medical, St Paul, MN, USA), the Engager™ valve (Medtronic, Minneapolis, MN, USA), the Symetis ACURATE™ valve (Symetis SA, Ecublens, Switzerland), the CoreValve™ Evolut R™ (Medtronic, Minneapolis, MN, USA), the self-expanding CENTERA valve (Edwards Lifesciences), and the HLT® valve (HLT Inc., Maple Grove, MN, USA) have in part received CE approval or are going to be tested in clinical trials. However, whether these new technologies have an impact on intermediate and long-term outcomes will have to be addressed in future studies.
Another issue with regard to prosthesis dysfunction is the long-term durability of currently available transcatheter valves. In the PARTNER trial, improvement in valve areas and transvalvular gradients was comparable between TAVI and surgical replacement, and this was maintained for two years38. However, there are case reports describing degenerated transcatheter valves which have been treated with a second transcatheter valve as a valve-in-valve procedure44. Therefore, data on long-term valve durability and improved anti-calcification technology in the second-generation valves are a prerequisite before indications can be expanded to younger, lower-risk patients.
CONDUCTION ABNORMALITIES
It has been proven that there is a big difference in the need for permanent pacemaker implantation after TAVI between the self-expanding CoreValve and the balloon-expandable SAPIEN valve (median: 28% vs. 6%)45. In the first randomised study comparing the self-expanding and balloon-expandable systems, a significantly higher rate of pacemaker implantation after TAVI was evident among patients receiving a CoreValve system (37.6 vs. 17.3%; p=0.001)46. Device-related factors of the self-expanding CoreValve, such as the depth of device implant in the left ventricular outflow tract and its continuous radial force in this area, appear to heighten the need for a pacemaker. However, patient-specific factors, e.g., age, pre-existing RBBB, LAHB and first-degree AV block, are also multivariate predictors of permanent pacemaker implantation following TAVI45. Although pacemaker implantation occurs frequently after TAVI, it does not seem to have an impact on midterm prognosis47,48.
Furthermore, the incidence of a new LBBB following TAVI is also increased after TAVI with the self-expanding CoreValve (38%-56.8%)49 compared to 10.5% of patients treated with the balloon-expandable SAPIEN in a combined analysis of all PARTNER data50. In the latter, new LBBB was not associated with death, repeat hospitalisation, stroke, or myocardial infarction at one year, which is contradictory to a small retrospective analysis51, but was associated with a higher rate of permanent pacemaker implantation and failure to improve left ventricular ejection fraction.
The topic of second-generation devices and conduction abnormality/pacemaker implantation has to be addressed in future studies. In the SAPIEN 3 trial, pacemaker implantation occurred in 13.3%20, which is somewhat higher than expected. In REPRISE II, the incidence of new pacemaker implantation was as much as 29.3% following implantation of a Lotus valve (Boston Scientific)43. From our point of view, reducing conduction abnormalities and PVR simultaneously will be difficult if not impossible due to valve design and anatomic issues.
Cost-effectiveness of TAVI
A recent meta-analysis of six studies including patients ineligible for surgery aimed to determine whether TAVI compared to medical management is cost-effective. The authors concluded that, despite notable differences in modelling approaches, each study showed that TAVI was likely to be a cost-effective intervention for patients ineligible for SAVR52. Another study, evaluating high-risk patients treated with TAVI compared to SAVR, also concluded that TAVI is likely to be a cost-effective treatment for high-risk patients with AS compared with the reference standard of SAVR. However, uncertainty regarding the long-term outcomes for TAVI patients remains, which could have a substantial impact on estimates of cost-effectiveness53.
Future openings
With the development of second-generation devices, lower profile sheaths and an expanding knowledge of the method, it is likely that clinical indications of TAVI will also expand. This has, at least in part, already started. For example, treatment of degenerated surgically implanted bioprosthetic valves has become an attractive and less invasive treatment option using both self-expanding and balloon-expandable valves in these often old and multimorbid patients54. Knowledge of the different surgical valves and their true internal diameter is crucial for selecting the correct transcatheter valve size55.
Expanding the indication to intermediate-risk patients is the subject of two ongoing clinical trials, PARTNER IIA (SAPIEN XT valve; ClinicalTrials.gov, number NCT01314313) and SURTAVI (CoreValve; ClinicalTrials.gov, number NCT01586910), comparing TAVR vs. SAVR, and future analyses of the more than 4,000 randomised patients from these studies should help to clarify questions regarding the advisability of TAVI in this risk group. However, a study using propensity risk adjustment has already shown that early and late mortality is comparable between intermediate-risk patients treated with TAVI and those treated with SAVR56. Furthermore, patients in the U.S. CoreValve trial had a mean baseline STS score of around 7.3% with about 75% of patients within the range of four to 10, which approximates to intermediate risk for most aortic stenosis patients3. As mentioned above, this study has shown that TAVI is even superior to SAVR after one year of follow-up.
Another issue which needs to be addressed is the need for general anaesthesia and TEE guidance or only conscious sedation and fluoroscopy in transfemoral cases. In the SAPIEN 3 trial, nearly two-thirds of transfemoral procedures were still performed under general anaesthesia (63.5%)20. From our personal point of view and as supported by the literature57, transfemoral TAVI under conscious sedation and fluoroscopic guidance alone is safe, effective and should be the primary way, since it supports the idea of TAVI as being a less invasive method for treatment of old and frail patients.
Furthermore, special entities of aortic stenosis, e.g., “true” or “paradoxical” low-flow low-gradient aortic stenosis, might also benefit from TAVI58. Future studies are warranted to evaluate the impact of TAVI compared to SAVR in these special subgroups.
Summary
TAVI has evolved as an effective treatment option in patients who cannot undergo surgery and as a worthwhile addition to SAVR in high-risk patients. However, despite this impressive evolution, there are many challenges and future requirements for improving outcome. Procedural issues are still controversial, such as the choice of access site, valve sizing, pre- and post-dilatation. Technical and procedural enhancements are therefore required to reduce complications. Moreover, further studies are required to evaluate the second-generation devices in a broader patient population to understand whether the theoretical benefits of improved valve designs translate into fewer complications and a better long-term survival. Adjunctive devices, such as cerebrovascular protection devices, have to be evaluated further in clinical trials. Based on both well conducted and monitored clinical trials and registries evaluating not only efficacy and safety but also economic aspects, the indication for TAVI should be extended to intermediate-risk patients, bioprosthetic valve failure, and other clinical set-ups.
Conflict of interest statement
N. Mangner has received travelling costs from Edwards Lifesciences. A. Linke is a consultant to Medtronic, St. Jude Medical and Claret Medical Inc. He has received speaker’s honoraria from Edwards Lifesciences, Medtronic, Boston Scientific and St. Jude Medical, and is a proctor for Medtronic, Edwards Lifesciences, St. Jude Medical and Boston Scientific. G. Schuler has no conflicts of interest to declare.

