![]()
TAVI has become the “standard of care” for the treatment of high-risk and surgically inoperable patients with symptomatic aortic stenosis (AS). Studies are currently recruiting which will assess whether TAVI can also be safely used in the “intermediate risk” group of patients. As a now well-established therapy, recent years have seen impressive reductions in complications and consequent improvements in outcomes and survival. The efforts of both physicians/surgeons and the device industry are now directed at refinement of the technique: addressing complications to improve outcomes and striving to make the procedure easier and more predictable. The move towards conscious sedation, smaller delivery systems, improvements in percutaneous access closure techniques and rendering the valve prosthesis fully repositionable and retrievable are examples of recent advances which have gone some way towards these goals. But will TAVI be good enough to compete with surgical aortic valve replacement in the intermediate-risk patient?
There are still several shortfalls in the management of AS patients, and three studies that examine some of these issues are addressed in this editorial. There remain very few effective alternatives to TAVI in inoperable patients (medical therapy and balloon valvuloplasty [BAV] do not alter prognosis), there are recent suggestions that valve leaflets may be compromised in the crimping process necessary to make newer devices low-profile, and rare complications such as coronary occlusion still occur during valve prosthesis deployment.
In current practice, little is done to alter the rate of progression of AS and no one has yet attempted to alter the morphology of a native aortic valve or “prepare” a stenotic valve for future device implantation. Jonas et al present a new concept in a preclinical feasibility study of the Leaflex™ Catheter System (Pi-Cardia, Beit Oved, Israel), described as a “novel percutaneous device for fracturing valve calcification using mechanical impact in order to regain leaflet mobility”1. The Leaflex is a disposable 13.2 Fr transfemoral catheter that includes two expandable nitinol elements, both connected by a unique set of shafts to an external “impact generator”, which transforms pneumatic energy into mechanical movements of the catheter shaft.
This study demonstrates that the Leaflex system causes fractures of certain patterns of calcification, which leads to improved leaflet compliance and increased aortic valve area in a bench-top model. It is well known that aortic valve calcification is the predominant feature of severe AS2 and its presence has significant prognostic implications. Freeman et al2 reported a worse outcome at five years among subjects with moderate to severe aortic valve calcification and, more recently, Aksoy et al3 showed that patients with a higher Agatston calcium score had a higher mortality if treated conservatively. However, little is known about the mechanical effects of calcium, valve leaflet compliance and whether this can be therapeutically modified. If the calcium can be fractured and compliance improved, will the valve stenosis (at least in the short term) lessen?
Clearly the Leaflex™ system is still a conceptual model and its application has to be demonstrated in vivo. However, the concept of altering the properties of the native stenosing valve is an exciting one and opens up the possibility of slowing down the AS process, thus deferring the time at which intervention is needed. It may also provide an alternative to the rather suboptimal BAV. Its theoretical advantages over BAV are: 1) its effect is not influenced by aortic recoil as it affects only the calcium hinge points and as such there may be less restenosis, and 2) valve integrity seems to be maintained, suggesting potentially less embolisation to the brain.
Will this new technique really prove more sophisticated than BAV? Staubach et al4 demonstrated that there were no differences among patients with and without severe calcification in terms of in-hospital death, cerebrovascular events, myocardial infarction, thromboembolic events, aortic dissection, or severe vascular complications after TAVI. Moreover, it is well established that BAV does cause calcification fractures and thus the claimed theoretical superiority of the Leaflex system will have to be tested in further studies.
Technological advances have seen a progressive decline in TAVI delivery system calibre, which involves tighter crimping of valve prostheses. The crimping process has always generated concerns about damage to the tissue leaflets, which might influence long-term durability and thrombogenicity. Recent studies have demonstrated a higher than expected incidence of thrombus on TAVI valve leaflets, which may be in part related to damage of these leaflets during crimping. This is currently only speculative, but, as the drive to smaller calibre delivery systems continues, preserving leaflet integrity is a fundamental priority. Kiefer et al5 have previously demonstrated increased fragmentation of valve tissue when prostheses are crimped for a longer duration.
Kheradvar et al6 presented a novel fully repositionable, retrievable valve (FOLDAVALVE; FOLDA LLC, Rancho Santa Margerita, CA, USA) with an ingenious leaflet system which remains outside the valve frame during crimping and is pulled into place as the valve self-expands on deployment6. The ability to reposition and retrieve TAVI prostheses has been the focus of several companies’ TAVI programmes and valves are already in use with these features (Table 1).

Figure 1 illustrates how the leaflets are spared during crimping but also raises the question of whether exposing the valve leaflets to the shear force during valve delivery and during crossing a stenotic calcified native valve is less harmful than current crimping techniques.
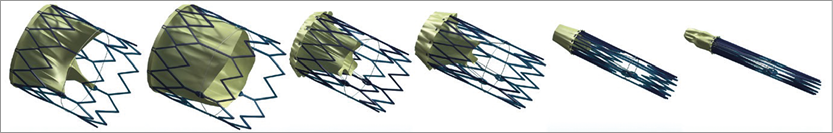
Figure 1. FOLDAVALVE leaflets are spared during crimping.
Significant CAD is present in 40% -75% of patients undergoing TAVI7. Coronary occlusion at the time of TAVI prosthesis deployment is a rare but feared complication with a high mortality. In the study by Abramowitz et al, reported in this issue, a pre-emptive technique is presented to manage potential CAD obstruction during TAVI after identifying 25 high-risk patients out of 623 cases performed8. This technique is not new to most TAVI operators but literature supporting its utility is lacking. Moreover, the ability to predict coronary ostial occlusion confidently is sometimes difficult.
The incidence of TAVI-induced coronary ostial occlusion varies between 0% and 1.2%6. Table 2 summarises previously published studies.
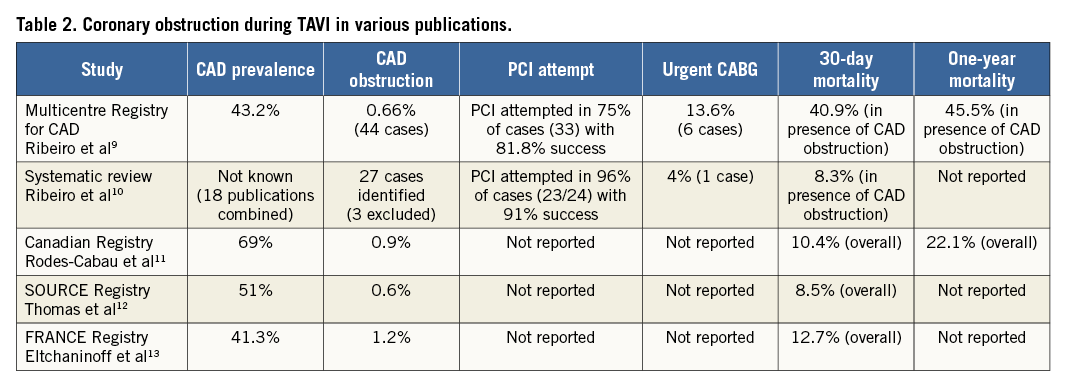
Coronary ostial obstruction during TAVI is typically manifest by persistent severe haemodynamic disturbance and the majority of cases are due to left coronary artery involvement (83.3%). However, this study highlighted the fact that significant left main stem (LMS) compromise can occur without obvious immediate haemodynamic effects. However, can such a potentially catastrophic event be predicted and prevented?
The study highlights predictive features of increased risk of ostial coronary obstruction:
– Anatomical features:
Left main ostium height above the aortic valve annulus of less than 9 mm
A difference of less than 2 mm between the sinus of Valsalva and prosthesis diameter
Severe aortic valve calcification with the presence of left cusp bulky calcium nodule(s)
– Significant LMS disease, defined as ≥50% angiographic stenosis and intravascular ultrasound (IVUS) minimal luminal area of <6 mm² or a previous LMS ostial stent
– Previous bioprosthetic valve - for “valve-in-valve” procedures, particularly with certain prosthesis types.
Abramowitz et al “protected” the LMS with a guide catheter, one or two guidewires and an uninflated angioplasty balloon/stent placed in the LAD prior to TAVI prosthesis deployment. This proved an effective strategy to “rescue” coronary flow when LMS compromise occurred. The most impressive aspect of the study was the degree to which coronary ostial occlusion could be predicted using their predefined criteria: there was one out of 598 coronary occlusions in patients who did not meet these criteria, compared to five out of 25 in patients who did –perhaps a lesson for all TAVI operators.
These studies are indicative of the direction that TAVI research and development is taking. The technique is firmly established but, as the indications for TAVI encroach on the intermediate-risk population, the limitations of TAVI must be acknowledged, addressed and studied. It is only in this way that technological and procedural advances can improve outcomes further in this increasingly large patient population.
Conflict of interest statement
P. MacCarthy is a proctor for Edwards Lifesciences. O. Aldalati has no conflicts of interest to declare.

