
Abstract
Aims: Our aim was to assess one-year outcomes of patients enrolled in the pilot European Sentinel Registry of Transcatheter Aortic Valve Implantation (TAVI).
Methods and results: One-year outcomes of 4,571 patients (81.4±7.2 years, 2,291 [50.1%] male) receiving TAVI with the SAPIEN XT (57.3%) or CoreValve prosthesis at 137 European centres were analysed using Kaplan-Meier and Cox proportional hazards regression techniques. At one year, 3,341 patients were alive, 821 had died, and 409 were lost to follow-up. Of 2,125 patients who underwent functional assessment, 1,916 (90%) were in New York Heart Association (NYHA) Class I/II at one year, with functional improvement from baseline noted in 1,682 patients (88%). One-year survival based on 4,564 patients was estimated at 79.1%. Independent baseline predictors of mortality were increasing age and logistic EuroSCORE, the presence of NYHA III/IV, chronic obstructive pulmonary disease, and atrial fibrillation. Female gender was associated with a 4% survival benefit at one year. Vascular access routes other than transfemoral were associated with poorer survival. Procedural failure and major periprocedural complications had an adverse impact on survival.
Conclusions: Contemporary European experience attests to the effectiveness of routine TAVI in unselected elderly patients.
Introduction
The number of elderly patients with degenerative aortic valve stenosis has increased with ageing of the European population. Advances in science and technology have offered an alternative to conventional surgical valve replacement with the evolution of transcatheter aortic valve implantation (TAVI). There is a need to monitor indications, techniques and outcomes to identify those patients best treated with such devices. However, at present there is no doubt that this technology has greatly improved the symptomatic and prognostic outcomes for patients with an otherwise dismal prognosis1-4.
The pilot Sentinel Registry of Transcatheter Aortic Valve Implantation of the EURObservational Research Programme (EORP) represents a collaborative effort of 137 centres in 10 European countries. Over a 16-month period, 4,571 patients were recruited. In-hospital outcomes of this cohort have recently been published5. Whilst a number of national and industry-sponsored, valve-specific registries have been reported, the EORP pilot Sentinel Registry is unique in its unbiased reflection of national and international variation in patient selection, procedural approach, and choice of valve prosthesis.
In this paper, the one-year follow-up results from the registry with respect to patient selection, procedural technique, and predictors of one-year mortality are presented.
Methods
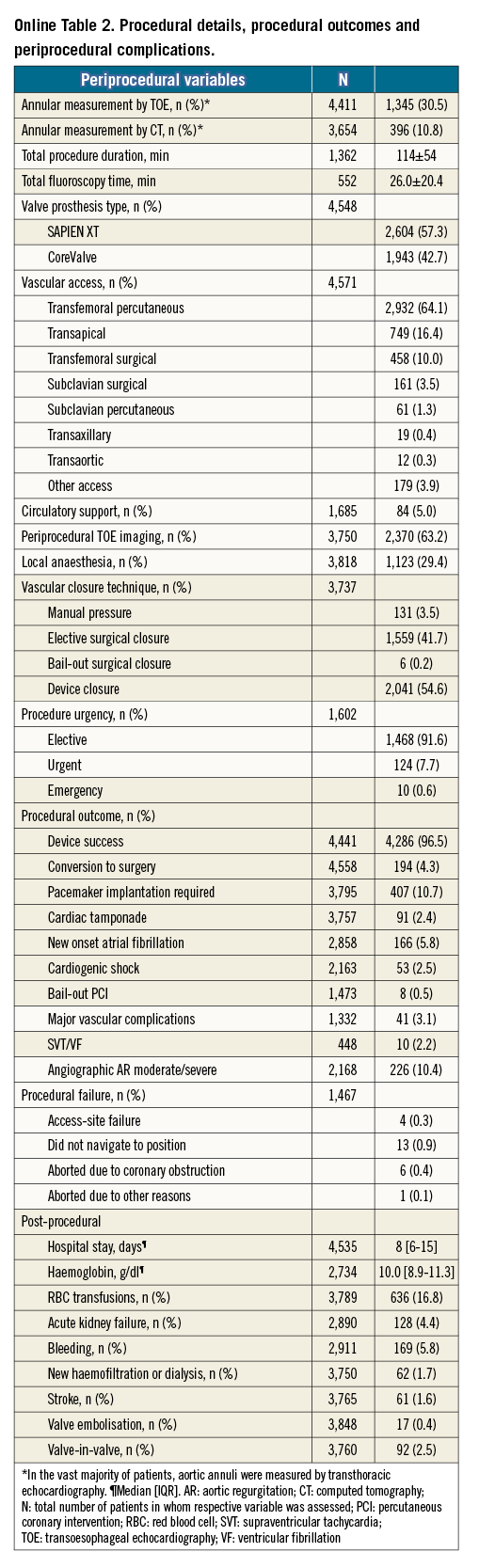
Between January 2011 and June 2012, 4,571 patients were enrolled at 137 centres in 10 European countries. Full details of this process and data collection have been described before5. In brief, patients were consecutively recruited and informed consent was obtained for both the TAVI procedure and the clinical data collection and processing. The valve prostheses used in this study were the balloon-expandable SAPIEN XT (Edwards Lifesciences Inc., Irvine, CA, USA) and the self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA) devices exclusively. Information was obtained either via a web-based electronic case record form (eCRF) or by direct transfer of national registry data. All data collection was monitored centrally at the European Heart House (Sophia Antipolis, France), where the EORP team generated queries to clean the database and validate entries.
At participating centres all consecutive patients receiving TAVI were prospectively entered into the registry. In accordance with national legislation, patients provided written informed consent, including consent for anonymous processing of data. Patients directly entered into the TCVT database signed an individual specific consent approved by the ethics committees of the participating centres.
All recorded variables were defined in accordance with, or as close as possible to, the updated Valve Academic Research Consortium-2 (VARC-2) criteria6, and attempts were made to align the national databases with the EORP data set. Where ambiguity was a concern, outcomes were limited to recording unequivocal events only (e.g., death).
STATISTICAL ANALYSIS
Continuous variables are reported as mean±standard deviation or as median plus interquartile range (IQR), where appropriate. Between-group comparisons were made using the Kruskal-Wallis test. Categorical variables are presented as absolute numbers and percentages. Between-group comparisons were made using a chi-square test or Fisher’s exact test if any expected cell count was less than five. Survival up to 12 months was assessed using the Kaplan-Meier method, with differences between survival curves assessed by the log-rank test. All analyses were performed using SAS statistical software, version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Cox proportional hazards regression analysis was used to explore the association between baseline covariates and mortality up to one year. Baseline covariates were compared in univariate analyses at 12 months between deceased and surviving patients; all covariates statistically significant on univariate analysis were then entered into a “full” multivariate proportional hazards model. Multivariate Cox regression was performed with 100 multiple imputations for missing values using the R software (http://www.Rproject.org/) and the package Hmisc (http://CRAN.Rproject.org/package-Hmisc). Two-tailed p-values <0.05 were considered statistically significant.
Results
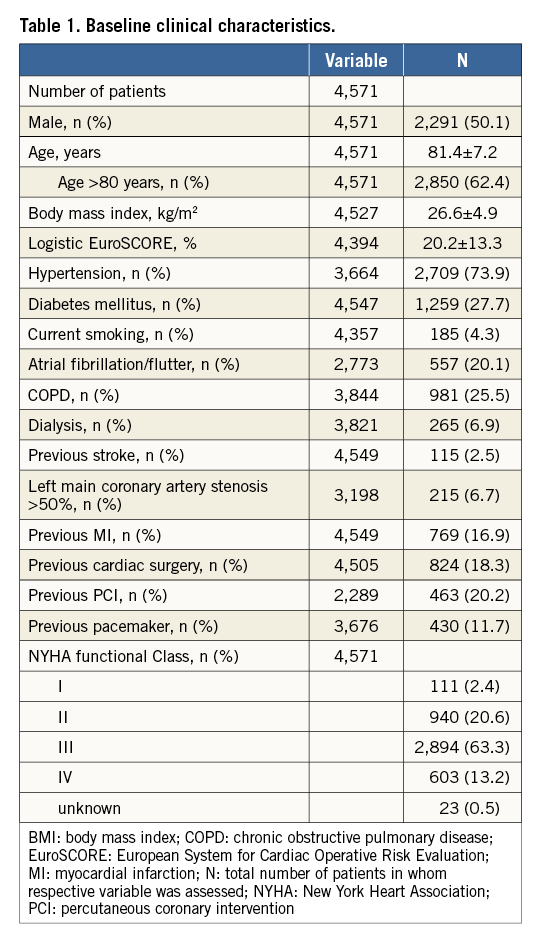
Baseline clinical characteristics of the 4,571 patients are given in Table 1. Baseline and post-interventional echocardiographic variables are presented in Online Table 1.
PROCEDURAL OUTCOMES
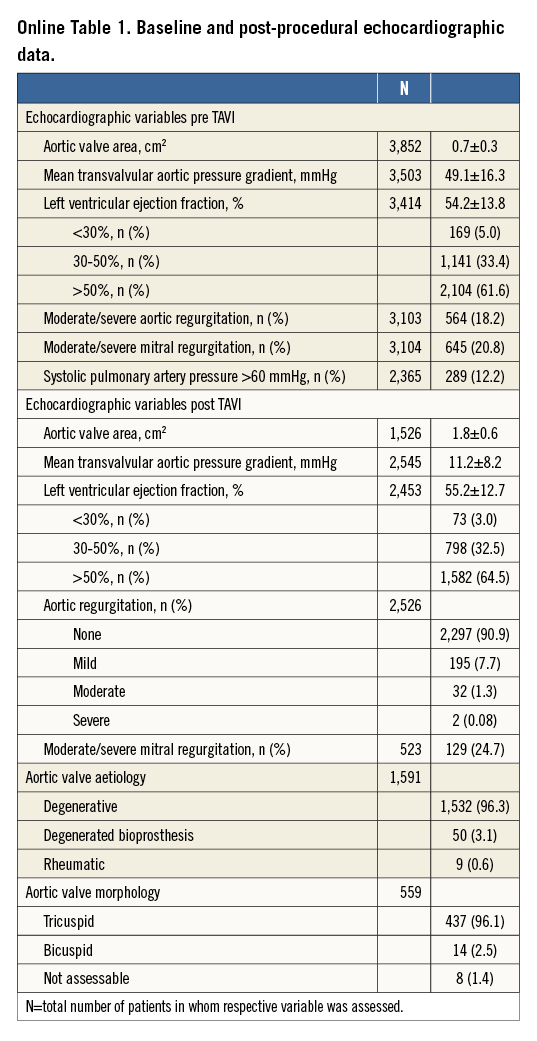
Device success was 96.5%. Procedural details and periprocedural complications are listed in Online Table 2.
CLINICAL OUTCOMES AT ONE YEAR
Of the 4,571 patients, vital status at one year could be assessed in 4,162 patients (91.1%), with 3,341 patients (73.1%) alive, 821 (18.0%) deceased, and 409 patients (8.9%) lost to follow-up. Of the surviving patients, New York Heart Association (NYHA) functional class at baseline and at one year was available in 2,125 patients (Figure 1). The vast majority of these patients (n=1,916 [90.2%]) were in NYHA Class I or II at one year. Changes in NYHA functional class from baseline to one year are shown for the 2,125 patients in Online Figure 1.
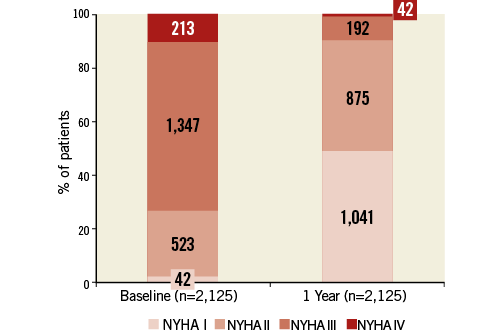
Figure 1. Functional status according to New York Heart Association (NYHA) class at baseline and one year in the 2,125 patients in whom functional status was assessed at both time points. Numbers in columns denote numbers of patients.
Of the 821 patients who had died by one year, in-hospital deaths occurred in 338 (41% [Figure 2]). A further 188 patients (23%) had died out of hospital by one month after TAVI, and 295 patients (36%) died between the one-month and one-year time points. It must be noted that the proportion of unknown causes of death increased considerably, reaching 48% (142 of 295) in patients who died between one month and one year after TAVI.
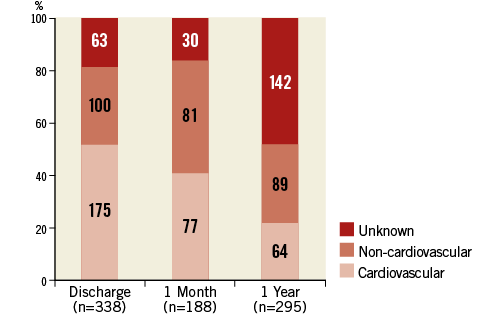
Figure 2. Classification of death at discharge, one month, and one year after TAVI in 821 patients. Numbers in columns denote numbers of patients.
SURVIVAL AT ONE YEAR
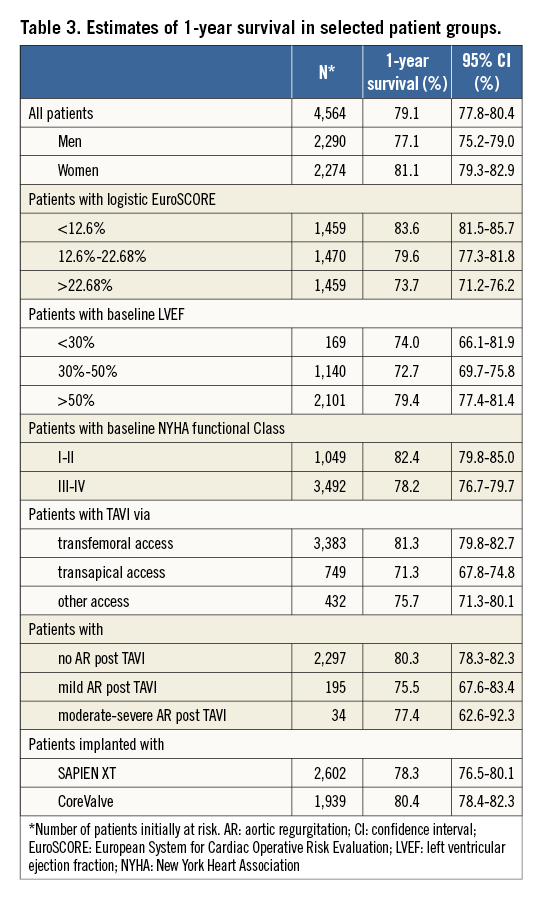
Cumulative survival of 4,564 patients initially at risk (follow-up dates were missing in seven patients) is shown in Figure 3A. The Kaplan-Meier estimate of cumulative survival at one year was 79.1%.
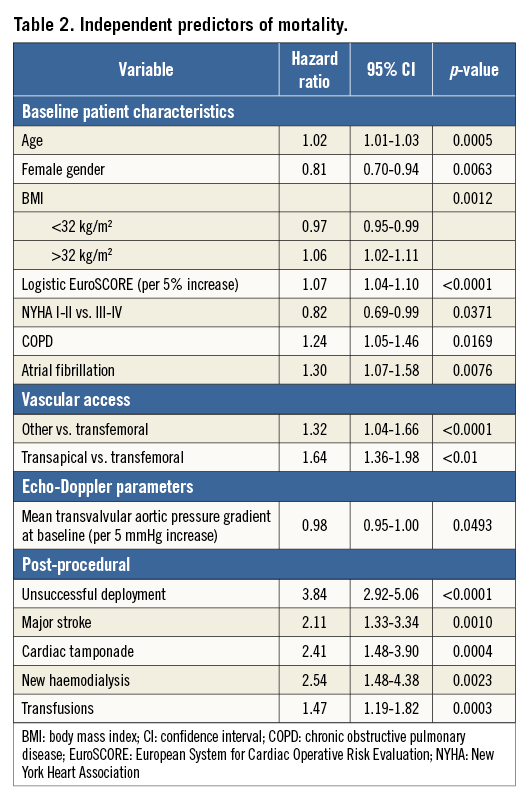
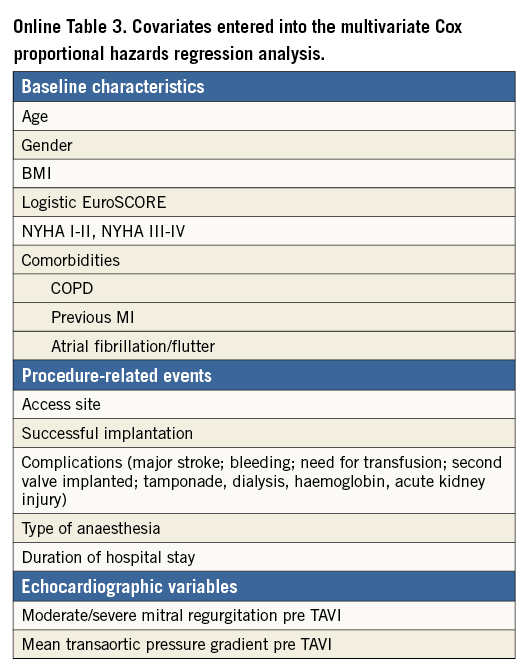
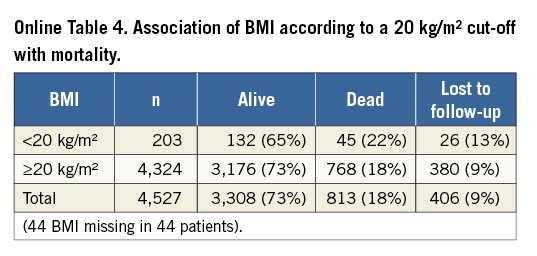
Independent predictors of mortality as assessed by multivariate Cox proportional hazards regression analysis are shown in Table 2. Covariates statistically significant on univariate analysis and subsequently entered into the multivariate analysis are listed in Online Table 3. Of the baseline patient characteristics, expected predictors were age (2% increase in the probability of death per one-year increase in age) and logistic EuroSCORE (7% increase in the probability of death per 5% increase in EuroSCORE). Female gender was associated with a 19% lower probability of death at one year. A non-linear relation of body mass index (BMI) with survival was noted, such that, in patients with BMI <32 kg/m2, a 1-kg/m2 increase was associated with a 3% decrease in the probability of death, whereas, in patients with BMI >32 kg/m2, a 1-kg/m2 increase was associated with a 6% increase in the probability of death. The association of BMI with mortality according to a 20 kg/m2 cut-off is shown in Online Table 4. Not surprisingly, patients with baseline NYHA functional Class I or II (as opposed to III or IV) had a lower probability of death within one year (by 18%), whereas increases in mortality (by 24% and 30%, respectively) were observed in those with chronic obstructive pulmonary disease and atrial fibrillation.
Vascular access routes other than transfemoral were associated with an increased probability of death (by 32% if any “other” route was used and by 64% if the transapical route was used).
Borderline statistical significance was observed for the pre-TAVI mean transvalvular aortic pressure gradient, for which an increase by 5 mmHg was associated with a 2% decrease in the probability of death. Failure to deploy the prosthetic valve increased the probability of death by a factor of nearly four; an increased risk of death was also associated with periprocedural adverse events such as major stroke, cardiac tamponade, the need for haemodialysis, and transfusions.
Of note, neither valve prosthesis type (hazard ratio [CoreValve vs. SAPIEN XT] 0.88 [95% CI: 0.76-1.01], p=0.0693) nor the implantation of a permanent pacemaker (hazard ratio [yes vs. no] 1.16 [95% CI: 0.93-1.45], p=0.1979) was found to impact on survival on univariate Cox regression analysis. Therefore, these covariates were not included in the multivariate model.
The relationship between mortality and any variable is also reflected in the cumulative survival curves. Thus, Figure 3B shows a small, yet statistically significant survival benefit for women (estimated one-year survival [Table 3] 81.1% vs. 77.1% in men; p [log-rank]=0.0080), and Figure 3C shows decreasing one-year survival estimates (from 83.6% to 73.7%; p<0.0001) in terciles of patients with increasing logistic EuroSCORE (from <12.6% to >22.68%, respectively). One-year survival was, at 79.4%, significantly higher (p=0.0002) in patients with a left ventricular ejection fraction (LVEF) >50% than in patients with an LVEF between 30% and 50% or an LVEF <30% in whom survival curves overlapped and one-year estimates were in the order of 73% (Figure 3D).
Patients in NYHA functional Class I or II at baseline had a significantly higher one-year survival (82.4%) than patients in NYHA Class III or IV (78.2%; p=0.0028) (Figure 3E), and transfemoral vascular access was associated with significantly better one-year survival (81.3%; p<0.0001) than transapical access (71.3%) or any other vascular access route (75.7%) (Figure 3F).
With respect to residual aortic regurgitation (AR) severity post TAVI, no statistically significant difference in survival curves was observed between patients with no residual AR vs. those with mild or moderate-severe AR, despite the finding that one-month survival in the few patients with moderate-severe AR appeared to be markedly lower than in the other two patient groups (Figure 3G).
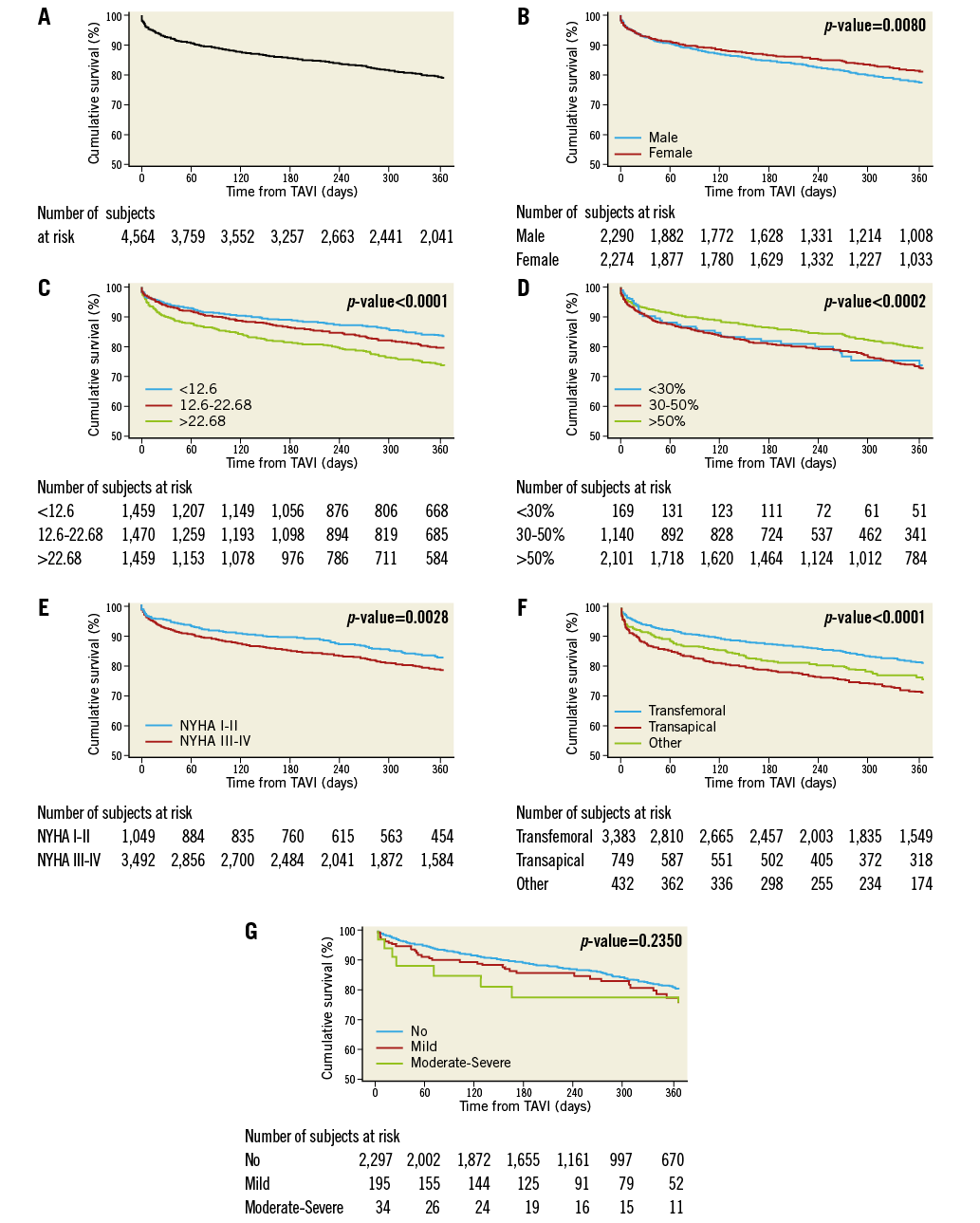
Figure 3. Cumulative survival for the first 12 months after TAVI. A) Total patient cohort (n=4,564 patients). B) According to gender in 4,564 patients. C) According to terciles of logistic EuroSCORE in 4,388 patients. First tercile (n=1,459) <12.6% (red curve); second tercile (n=1,470) 12.6%-22.68% (green curve); third tercile (n=1,459) >22.68% (blue curve). D) According to left ventricular ejection fraction (LVEF) in 3,410 patients. E) According to New York Heart Association (NYHA) functional class at baseline in 4,541 patients. F) According to vascular access route in 4,564 patients. G) According to residual aortic regurgitation post TAVI in 2,526 patients.
Discussion
THE REGISTRY
This large, contemporary, non-industry-sponsored multinational registry of more than four thousand patients provides a unique opportunity to examine the “real-world” one-year outcomes of unselected patients who were deemed at high surgical risk or inoperable undergoing routine TAVI in Europe. Utilising exclusively the SAPIEN XT or CoreValve prostheses, vascular access was predominantly transfemoral (74%), and device success was achieved in 96.5% of cases. The registry was extremely well balanced with respect to gender (50.1% male vs. 49.9% female), the patients had a mean age of 81.4 years, with 62.4% of patients older than 80 years, and a mean logistic EuroSCORE of 20.2%; before TAVI, 76.5% of patients were in NYHA functional Class III or IV.
MAIN FINDINGS
Based on a total of 4,564 patients, one-year survival was estimated at 79%. With 409 patients lost to follow-up, actual mortality was 18% (821 patients). Thus, the survival rate observed in this registry was similar to the rates reported in previous major national registries4,7,8. The vast majority of patients surviving up to one year experienced a significant improvement in functional capacity, with 90% of 2,125 patients in whom functional status was assessed at one year presenting in NYHA Class I or II. This finding confirms the persistent long-term benefits of TAVI.
A number of patient and procedural characteristics associated with mortality were identified. Not surprisingly, increasing age as well as increasing logistic EuroSCORE, a left ventricular ejection fraction ≤50%, and poor functional status at baseline (NYHA Class III or IV) increased mortality.
Interestingly, lower mortality was observed with an increase in BMI up to 32 kg/m2, probably reflecting increased physical robustness; however, in patients with a BMI beyond 32 kg/m2 – which includes patients regarded as severely or even morbidly obese – the probability of death after TAVI increased with increasing BMI. This finding appears to reflect that reported previously for patients undergoing surgical aortic valve replacement9-11, yet is not concordant with results from the FRANCE-2 TAVI registry, in which a steady increase in survival with increasing BMI was observed12.
Regardless of possible differences in baseline characteristics according to gender, lower mortality was observed in female patients, reflected in survival curves that diverge steadily over time and result at one year in a 4% survival benefit for women. This finding confirms previous reports13-15. However, female gender has also been associated with higher rates of life-threatening bleeding16 and major vascular complications17 after TAVI.
Borderline statistical significance was observed for an increasing mean transvalvular pressure gradient associated with decreasing probability of death, possibly explained by relief of aortic stenosis resulting in an increased left ventricular ejection fraction.
A clear benefit was noted for patients in whom the transfemoral vascular access route was used (81.3% survival at one year), as opposed to the transapical (71.3%) or other access routes (75.7%). These results confirm previous reports assessing the relationship between access route and mortality2,5,18,19.
In accordance with previous studies reporting no differences in terms of outcomes between different types of valve prosthesis4,20,21, valve type was not found to affect survival in the patients of this registry.
The severity of residual AR post TAVI did not impact on mortality in this registry, even though distinct survival curves were observed for patients with no residual AR vs. those with mild residual AR and patients with moderate-severe residual AR. However, statistical significance was not reached, possibly because the numbers of patients in the latter two groups were fairly low (195 and 34, respectively). The echocardiographic grading of residual AR (1.4% moderate/severe) was obviously dissociated from the angiographic grading (10.4% moderate/severe). However, the angiographic grading depends on the position of the catheter, the volume of contrast agent, and the fact that it was performed in the catheter laboratory immediately after TAVI. The echocardiographic grading, performed one or two days after the intervention, is not influenced by these factors. Also, the lack of statistical significance of residual AR post TAVI may be due to the absence of standardisation in the evaluation of AR severity in a large multicentre registry such as ours.
Finally, failure to deploy the valve prosthesis and severe periprocedural complications such as major stroke, cardiac tamponade, new haemodialysis, and the need for transfusion of red blood cells all had an adverse impact on survival.
CLINICAL RELEVANCE
It is becoming increasingly evident that procedural practice significantly impacts on patient prognosis. Whilst we can use clinical characteristics to inform patient selection by the Heart Team, the technique of valve implantation does confer worse outcomes if suboptimal results are achieved. Failure to deploy the valve and major complications are most powerful predictors of mortality. Operators must strive to minimise AR after valve deployment, and the introduction of new valves (Direct Flow Medical®; Direct Flow Medical, Santa Rosa, CA, USA, Lotus™; Boston Scientific, Marlborough, MA, USA, JenaValve; JenaValve Technology, Irvine, CA, USA) or new iterations of the valves used in this study (SAPIEN 3 and CoreValve Evolut) are expected to minimise AR. Previous reports suggested that any level of AR was a marker of worse outcome5,22. However, in this registry, mild AR did not influence survival and there were too few patients with more severe AR to draw statistically meaningful conclusions. Recent studies using mainly the CoreValve have shown a reduction of AR in serial echocardiograms which may explain the different result.
LVEF has been demonstrated to impact on outcome, independent of prior myocardial infarction, historical revascularisation, or the presence or extent of coronary artery disease4,23,24. The observation in this registry that patients with a baseline LVEF >50% have better survival than patients with an LVEF ≤50% supports these findings.
Our observations add to an already substantial body of work aiming to identify patients most likely to respond well to TAVI and to guide further advances in procedural technique by identifying aspects that have a deleterious impact on survival. As more knowledge and experience is gained, clinical outcomes will probably improve, and TAVI may become a competitive alternative in patients with moderate surgical risk – now currently under investigation in trials such as PARTNER 2A, SURTAVI and UK TAVI25. Formulation and validation of TAVI-specific risk scores will aid in the process of patient selection, taking into account patient characteristics that are not traditionally included in surgical risk scores.
Limitations
The TCVT registry is a voluntary pilot registry. Procedural complications, adverse events and echocardiographic parameters are self-reported. With the exception of national audit protocols for some of the registries, no other specific monitoring was performed. We acknowledge the restrictions evident in the direct transfer of national data. Missing values can be generated by national registry data transfer, as the list of collected variables may differ or definitions vary. We attempted to standardise endpoints, using only unequivocal clinical endpoints. Nonetheless, the analysis of this registry has necessitated the use of statistical techniques to account for missing values, while maintaining the validity of our observations and conclusions. This registry only included patients treated with the SAPIEN XT or CoreValve prostheses, two devices that are in the process of being replaced with more sophisticated new designs and are now joined by a variety of other CE-marked devices due to be assessed in the next (long-term) phase of this registry.
Conclusions
The use of TAVI to treat severe symptomatic aortic stenosis is an effective and safe option, improving symptomatic and prognostic outcome in patients who are unable to undergo surgical intervention or in patients considered at high surgical risk according to the Heart Team.
Impact on daily practice
This follow-up study offers a broad comparison basis from the recent past to understand the rapid improvement of transcatheter aortic valve technology. The higher periprocedural mortality in surgical and especially transapical approaches shows a further increase at one year, justifying the steep increase in the percutaneous transfemoral approach allowed by the miniaturisation of the valve delivery systems. The limited percentage of 18% patients dead at one year with a clear cardiovascular cause of mortality confirms the efficacy of TAVI, but also suggests a more careful selection of cases excluding patients whose general health is too poor to benefit from any intervention.
Acknowledgements
Members of the Executive Steering Committee, Steering Committee and Investigators were provided in the primary paper by Di Mario et al5. This study includes data collected on behalf of the relevant audit’s professional society under the auspices of the National Institute for Cardiovascular Outcomes Research (NICOR) for the United Kingdom data.
We thank the EURObservational Research programme (EORP) team, national coordinators, and investigators for their contribution in performing the survey.
Data monitoring and technical support team: data collection conducted by the EORP Department of the European Society of Cardiology by Gérard Gracia, statistical analyses performed by Cécile Laroche with the support of Renato Urso, and overall activities coordinated by Aldo Maggioni, EORP Scientific Coordinator, Thierry Ferreira, Head of EORP Department, and the EORP Oversight Committee (Roberto Ferrari, Panos Vardas, Michel Komajda, Fausto Pinto, Angeles Alonso, David Wood, Nikolaos Maniadakis, Patrizio Lancellotti, Carina Blomström-Lundqvist, Stephan Windecker, Stefan Anker, Uwe Zeymer, Aldo Maggioni, Luigi Tavazzi).
From the EURObservational Research Programme of the European Society of Cardiology, 2035 Route des Colles – Les Templiers, CS 80179 BIOT - 06903 Sophia Antipolis Cedex – France. Tel: +33 492 94 76 00 - Fax: +33 492 94 76 29. E-mail: [email protected]
Funding
At the time of the registry, the following companies were supporting the EURObservational Research programme: Abbott Vascular Int., Amgen, Bayer Pharma AG, Boehringer Ingelheim, Boston Scientific International, The Bristol Myers Squibb and Pfizer alliance, The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company, Menarini International Operations, Merck & Co. Int., Novartis Pharma, Sanofi-Aventis, Servier. At the time of the registry, none of these companies was involved in manufacturing or selling the two TAVI devices with CE approval.
Conflict of interest statement
H. Eltchaninoff served as a proctor for Edwards Lifesciences and received lecture fees from the company. N. Moat served as a consultant and a proctor for Medtronic. P. Wenaweser declares proctoring and lecture fees from Medtronic, Edwards Lifesciences, Boston Scientific, and a research grant to the institution from Medtronic. S. Price declares an educational contract with Medtronic and is part of the medical advisory board of Abbott and MitraClip. B. Iung has received consultant’s fees from Abbott, Boehringer Ingelheim, Valtech and speaker’s fees from Edwards Lifesciences. B. Prendergast received lecture fees from Edwards Lifesciences. S. Windecker has received research contracts to the institution from Abbott, Biotronik, Boston Scientific, Biosensors, Edwards Lifesciences, Medicines Company, Medtronic and St. Jude, and lecture fees from AstraZeneca, Eli Lilly, Bayer, Abbott, Biotronik, Boston Scientific, Edwards Lifesciences, and Medtronic. A. Witkowski received proctorship fees from Medtronic. H. Danenberg serves as a clinical proctor for Medtronic CoreValve. The other authors have no conflicts of interest to declare.
Supplementary data
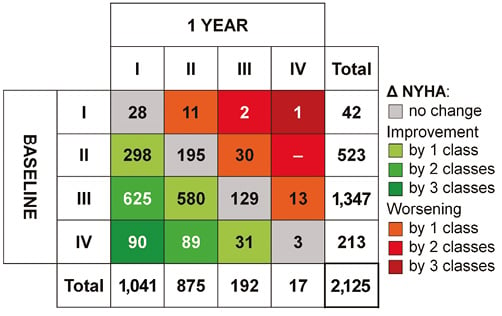
Online Figure 1. Schematic showing TAVI-related changes in NYHA functional class from baseline to one year in 2,125 patients. Shades of green denote improvement, shades of red denote worsening, e.g., 625 patients with NYHA III at baseline improved to NYHA I within one year, whereas 30 patients with NYHA II at baseline worsened to NYHA III.

