
Abstract
Aims: We sought to determine the long-term outcome of high-risk patients who underwent transcatheter aortic valve replacement (TAVR) with first-generation devices with a focus on the identification of predictors for mortality and valve durability.
Methods and results: Consecutive patients in our prospective single-centre registry undergoing TAVR with first-generation devices (n=214 CoreValve; n=86 SAPIEN) between 06/2007 and 07/2009 were retrospectively analysed (n=300, mean age 81.43±6.55 years, mean STS score 6.5±4.5%). Kaplan-Meier estimates of survival and the Cox proportional hazards model were used to identify independent predictors of all-cause-mortality. At 1, 5, and 7 years, estimated survival rates were 76.0%, 40.2%, and 23.2%, respectively. Age-adjusted baseline predictors of mortality included atrial fibrillation, impaired kidney function, peripheral artery disease, and mitral regurgitation (≥moderate). Baseline risk-adjusted procedure-related predictors for all-cause mortality included acute kidney injury, neurological events, major vascular complications, and major/life-threatening bleeding. At both five and six years, 78.2% of surviving patients were in NYHA Class I or II. PVL was ≤mild in the majority of patients at discharge and throughout follow-up. At seven years, the overall crude cumulative incidence of structural valve deterioration according to the 2017 EAPCI/ESC/EACTS definition was 14.9% (CoreValve 11.8% vs. SAPIEN 22.6%; p=0.01).
Conclusions: Seven years after TAVR, 23.2% of high-risk patients were still alive. Independent predictors of all-cause mortality included both patient- and procedure-related factors. With a cumulative incidence of 14.9% at seven years, there is some suggestion that SVD post TAVR may become increasingly relevant during longer-term follow-up.
Abbreviations
AR: aortic regurgitation
MPG: mean pressure gradient
MR: mitral regurgitation
NYHA: New York Heart Association
PVL: paravalvular leak
SVD: structural valve deterioration
TAVR: transcatheter aortic valve replacement
THV: transcatheter heart valve
TR: tricuspid regurgitation
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve replacement (TAVR) was developed as an alternative treatment modality for patients with severe symptomatic aortic valve stenosis, for whom the operative risk for surgical aortic valve replacement (SAVR) is considered too high or prohibitive. TAVR is now considered to be equivalent or superior to SAVR in high- or intermediate-risk patients1. Follow-up data of up to five years after TAVR are available from different registries and from the Placement of Aortic Transcatheter Valves (PARTNER) trial2-5. Since TAVR is increasingly being applied for patients with intermediate- or even lower-risk profiles, data on long-term transcatheter heart valve durability beyond the five-year horizon will become important but, so far, remain limited.
We sought to determine the long-term outcome of high-risk patients who underwent TAVR focusing on the identification of independent baseline- and procedure-related risk factors for mortality and valve durability.
Patients and methods
STUDY DESIGN AND PATIENT POPULATION
All consecutive patients (N=300) from our single-centre registry who underwent TAVR between June 2007 and September 2009 were analysed retrospectively. Preoperative, intraoperative, and perioperative data were prospectively recorded in a dedicated database. Decisions on the indications for TAVR and procedural planning were based on the consensus of a local multidisciplinary team composed of interventional cardiologists and cardiac surgeons. The designs of the first-generation self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA) and the balloon-expandable SAPIEN (Edwards Lifesciences, Irvine, CA, USA) transcatheter heart valves (THVs) have been described previously6. The study complied with the Declaration of Helsinki and was approved by the local ethics committee of the Technical University of Munich. All patients provided informed consent.
DATA AND DEFINITIONS
The Society of Thoracic Surgeons’ predicted risk score (STS score) and the logistic European System for Cardiac Operative Risk Evaluation score (logEuroSCORE) were used preoperatively to calculate the risk of operative mortality and morbidity. Functional status was graded according to the New York Heart Association (NYHA) classification. Postoperative outcomes were recorded according to criteria of the updated Valve Academic Research Consortium (VARC-2)7. Structural valve deterioration (SVD) and bioprosthetic valve failure (BVF) were defined according to the EAPCI/ESC/EACTS definitions8.
FOLLOW-UP
Clinical and echocardiographic follow-up data were collected at discharge, six months and yearly thereafter. Data on vital status, NYHA functional class, and adverse events were obtained at outpatient clinic patient visits, through telephone calls with the patient or the referring cardiologists/family doctor, and from collected external medical reports. The date of follow-up census was September 2016, which allowed a minimum potential follow-up duration of seven years. The median follow-up as calculated by the reverse Kaplan-Meier estimator was 7.14 years. Median observed follow-up (until death or lost to follow-up) was 4.1 years (ranging from 0 to nine years), resulting in a cumulative follow-up time of 1,147.7 years.
STATISTICAL ANALYSIS
Categorical variables are presented as frequencies and percentages, and continuous data are expressed as mean±SD. Survival was assessed using the Kaplan-Meier estimator, with curves plotted along with the 95% confidence interval (CI). A log-rank test was used for survival comparisons between groups. The reverse Kaplan-Meier method was used to calculate the median follow-up time. A life table of the general German population (2010-2012) was downloaded from www.destatis.de and used to calculate the expected cumulative survival of the study population. The Cox proportional hazards regression method was used for univariate and multivariate assessment of candidate predictors of all-cause mortality. The STS score was added to the model for baseline risk adjustment of the multivariate analysis used to identify the independent procedural predictors of all-cause mortality. Potential covariates in the multivariate analysis were considered because of their described or supposed clinical relevance regardless of their statistical significance in the univariate analysis. The cumulative incidence function for competing risk was used to estimate the crude incidence of SVD. The hazard ratio is presented as mean plus 95% CI. A p-value<0.05 was considered to indicate statistical significance. The SPSS statistical software package, Version 22 (IBM Corp., Armonk, NY, USA) and R statistics version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
Results
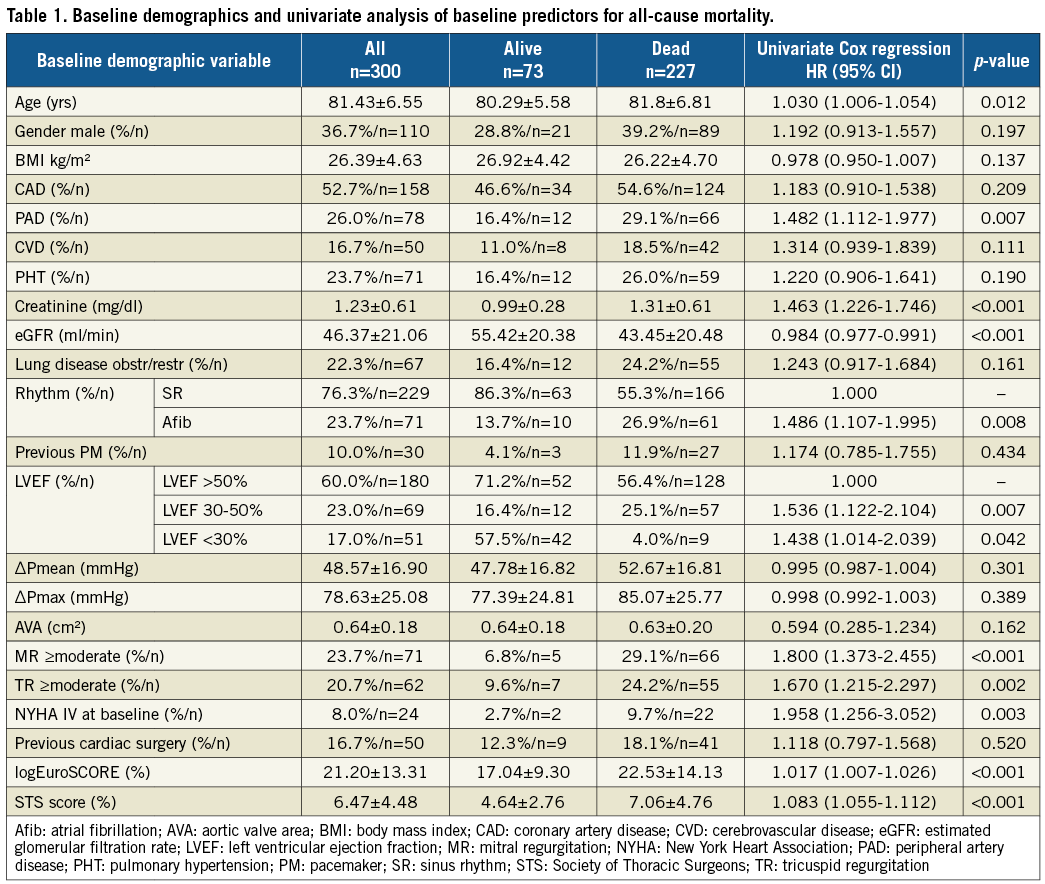
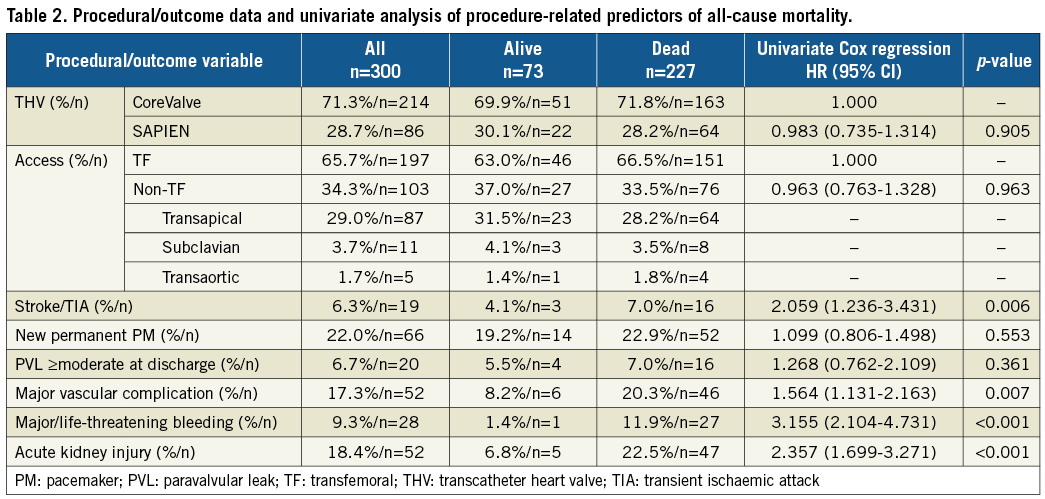
A total of 300 patients (mean age 81.43±6.55 years, mean STS score 6.5±4.5%, mean logEuroSCORE 21.2±13.3%) were included in the study. A CoreValve or SAPIEN THV was used in 214 patients (71.3%) and 86 patients (28.7%), respectively. Baseline demographic, procedural and periprocedural data are listed on the left side of Table 1 and Table 2, respectively.
SURVIVAL
At census, 227 mortality events were documented. There were 73 survivors at a median follow-up of 7.14 years. The median survival was 4.12 years (95% CI: 3.57-4.67 years). The estimated cumulative survival rates at 1, 5, and 7 years were 76.0%, 40.2%, and 23.2%, respectively. The risk for death was highest within the first three months after the index procedure and decreased thereafter, such that the expected age- and sex-adjusted survival curve was almost parallel to the observed curve (Figure 1A). Survival stratified according to STS score intervals is depicted in Figure 1B.

Figure 1. Kaplan-Meier survival estimates. A) Kaplan-Meier estimate of overall survival. B) Kaplan-Meier estimates of survival stratified according to STS score intervals (<4%/4%-8%/>8%). C) Kaplan-Meier survival estimates of patients with or without moderate-to-severe MR at baseline. D) Kaplan-Meier survival estimates of patients according to the presence or absence of significant PVL at discharge.
PREDICTORS OF ALL-CAUSE MORTALITY
Univariate predictors of all-cause mortality are shown in Table 1 and Table 2, and the multivariate baseline and procedural predictors are presented in Figure 2A and Figure 2B. Univariate Cox regression analysis revealed a significant effect on mortality for age, peripheral artery disease, elevated creatinine level, decreased estimated glomerular filtration rate (eGFR), atrial fibrillation, impaired left ventricular ejection fraction (LVEF), moderate-to-severe mitral and tricuspid regurgitation (MR/TR), and NYHA Class IV at baseline. Both the STS score and logEuroSCORE were significantly associated with mortality (both p<0.001). Multivariate age-adjusted baseline predictors of overall all-cause mortality included peripheral artery disease (p=0.037), impaired kidney function (p=0.005), chronic atrial fibrillation (p=0.026), and the presence of preoperative MR ≥moderate (p=0.003). The Kaplan-Meier survival curve stratified for the presence or absence of MR ≥moderate at baseline shows a progressive separation of both curves (p<0.001) (Figure 1C). Moderate-to-severe PVL was not associated with worse long-term survival (Figure 1D). A strong trend, just barely missing statistical significance, was found for the presence of chronic lung disease (p=0.063). Likewise, a trend was found for the presence of cerebrovascular disease (p=0.101) and TR ≥moderate (p=0.128). Results of multivariate analysis for baseline predictors are depicted in Figure 2A.

Figure 2. Multivariate analysis. Age-adjusted baseline (A) and procedure-related (B) predictors of all-cause mortality for all patients and after exclusion of death events occurring up to one year.
All procedure-related factors found significant for association with mortality by univariate analysis (Table 2) were also confirmed to have an independent effect on mortality by the final multivariate prediction model (Figure 2B). The multivariate baseline risk-adjusted (by inclusion of STS score into the model) procedure-related predictors of overall all-cause mortality included the following periprocedural complications (all within 30 days after the index procedure): neurological event (p=0.004), major vascular complication (p=0.001), major/life-threatening bleeding (p<0.001), and acute kidney injury (p=0.001) (Figure 2B). The relationship between the need for pacemaker implantation and the risk of mortality was not significant (p=0.574). Moreover, none of the following candidates was found to be independently predictive of mortality: type of access site (p=0.226), type of implanted valve (p=0.724), or the presence of paravalvular leakage (PVL) ≥moderate at discharge (p=0.244) (Figure 2B).
FUNCTIONAL OUTCOME
The majority of patients showed significant improvement and were in NYHA functional Class I or II early post TAVR and throughout follow-up. At five and six years, 68 (78.2%) of 87 and 49 (78.2%) of 61 surviving patients, respectively, were in NYHA Class I or II (Supplementary Figure 1A). The proportion of patients with freedom from NYHA Class III, IV, or death at 1, 5, and 7 years was 74.7%, 33.1%, and 11.4%, respectively (Supplementary Figure 1B).
HAEMODYNAMIC PERFORMANCE AND VALVE DURABILITY
PVL in the majority of patients at discharge and throughout follow-up was none/trace or mild. Only a small proportion of patients (between 4.4% and 11.7%) exhibited moderate PVL throughout the follow-up period, and only 0.7% to 1.0% of patients showed severe PVL. Postoperative MPG remained stable at lower levels throughout the follow-up period (Supplementary Figure 2A). The mean pressure gradient (MPG) at discharge was 11.8±4 mmHg. MPGs over time are depicted in Supplementary Figure 2B. A total of 37 patients showed an MPG ≥20 mmHg at least once during follow-up. In 91.9% (34 of 37) of these patients, the MPG was ≥20 and <30 mmHg; only three patients showed an MPG ≥30 mmHg. At seven years, the overall crude cumulative incidence of SVD according to the 2017 EAPCI/ESC/EACTS definition was 14.9% (CoreValve 11.8% vs. SAPIEN 22.6%; p=0.01) (Figure 3). Late BVF (severe haemodynamic SVD or repeat intervention following confirmed bioprosthetic valve dysfunction >30 days after initial TAVR procedure) occurred in 11 patients (n=8 SAPIEN/n=3 CoreValve) of whom four underwent reintervention (all valve-in-valve).

Figure 3. Estimates for freedom from SVD, freedom from SVD or death and crude cumulative incidence function according to the 2017 EAPCI/ESC/EACTS definition. A), C) & E) All patients. B), D) & F) Stratified for valve type.
Discussion
Although positive short-term and midterm results after TAVR have been reported, few long-term data are available. To the best of our knowledge, our study represents the largest long-term follow-up study, with a median follow-up of >7 years and a sizeable number of patients remaining alive and at risk. The five-year survival rate of 40.3% seen for our cohort is comparable to five-year survival rates ranging from 26% to 46% in various other study cohorts2-5,9. Since patients included in this study were elderly and either inoperable or at high risk for surgery because of multiple comorbidities, the high mortality we observed during follow-up is not surprising.
An age-adjusted multivariate model revealed that the following preoperative parameters were independently associated with all-cause mortality: peripheral artery disease, impaired kidney function, atrial fibrillation, and moderate-to-severe mitral regurgitation, confirming the findings of other studies10-13.
With a reported prevalence of approximately 15%, significant MR is a common concomitant condition that is usually left untreated in patients with severe aortic stenosis who undergo TAVR14. In our cohort, MR ≥moderate at baseline was present in 23.7% of patients and was the strongest independent predictor of all-cause mortality, even after excluding death events occurring up to one year postoperatively. A recent meta-analysis which included 8,000 patients showed that persistent MR ≥moderate was associated with increased early and late mortality after TAVR15. We think that a staged double-valve procedure should be considered for such cases, especially since a variety of interventional treatment tools is available.
In addition to patient-related factors, procedure-related factors also affect mortality after TAVR. Although in our study the type of THV device and access route did not affect outcome, various TAVR-related complications including stroke/TIA, major vascular complication, major/life-threatening bleeding and acute kidney injury were independently associated with all-cause mortality, confirming the findings of other studies16,17.
Interestingly, we found that moderate-to-severe PVL at discharge was not associated with increased mortality (Figure 1D). Post-TAVR PVL of varying degrees remains a frequent complication. A systematic review found a pooled estimate of 11.7% for the overall incidence of moderate or severe PVL18. The PARTNER IB trial found that moderate-to-severe PVL was associated with an increased five-year mortality risk in patients who underwent TAVR5. A potential explanation might be the use of all-cause mortality instead of cardiovascular mortality as an endpoint. In the recent five-year follow-up report of the PARTNER 1B trial, moderate-to-severe PVL affected cardiovascular mortality only and not all-cause mortality19. Since moderate-to-severe PVL only occurred in a small fraction of patients included in this study, statistical power may have been limited.
With the broadening of indications for TAVR to include lower-risk and younger patients, the long-term durability of THVs is of increasing importance. There is a body of evidence demonstrating the durability of THV devices up to five years. The five-year results of the randomised controlled PARTNER 1 trial were favourable regarding valve durability; SVD that required surgical replacement was not observed5. For a multicentre study that included 339 patients, Rodes-Cabau et al analysed data only from patients with complete serial echocardiographic follow-up examinations. Changes in residual AR and valve failures were not seen during follow-up3. A recent study conducted by Dvir et al analysed 704 patients who had undergone TAVR between April 2002 and May 2011 and followed 378 of the patients for up to 10 years. They observed 35 cases of SVD according to VARC-2 criteria in 100 patients who survived at least five years, with a significant number of deteriorating valves occurring between five and seven years post TAVR. Freedom from SVD was seen in 82% of patients at six years and 50% at eight years, results that are comparable to our results (Dvir D. First look at long-term durability of transcatheter heart valves: assessment of valve function up to 10 years after implantation. Presented at: EuroPCR 2016, Paris, France). The overall crude cumulative incidence of SVD according to the 2017 EAPCI/ESC/EACTS definition in our study was 14.9% at seven years. It should be noted that SVD estimates and incidences reported in this manuscript were unadjusted for factors that might have an important impact on results (i.e., patient selection, annulus/valve size, underexpansion/overexpansion, balloon expansion or self-expansion, implantation depth, etc.). The reasons for the numerical and statistical differences between devices remain elusive and require further investigation. SVD includes permanent morphological changes of the valve which may finally result in stenosis and/or intraprosthetic regurgitation. While THVs seem to degenerate in a manner similar to surgical bioprostheses, there are also fundamental differences between transcatheter and surgical aortic valve replacement that may impact on the natural history of SVD. THV leaflet injury may occur at the time of initial valve crimping, balloon expansion or dilation, or as a result of suboptimal leaflet coaptation, underexpansion/overexpansion, leaflet folding, or leaflet-frame contact due to asymmetrical frame expansion potentially predisposing to earlier tissue degeneration. Another more recently recognised issue is valve thrombosis20,21. Of note, an in silico fatigue simulation study compared the durability of transcatheter and surgical bioprostheses under identical loading conditions and with identical leaflet material properties. Significantly higher leaflet stress and strains in THVs compared to surgical valves were observed. The results of this study suggest that, even when properly deployed, the durability of THVs will be significantly reduced compared to surgical bioprostheses to about 7.8 years. The durability of THVs deployed in non-optimal (elliptical or underexpanded) configurations is expected to be further reduced22. Extended follow-up evaluations of elderly high-risk patients will be challenging because of the high mortality rates at late follow-up. Comprehensive clinical and echocardiographic evaluations in intermediate-risk patients are crucial before the indications for TAVR can be safely extended to include lower-risk and younger patients.
Limitations
Our study is a non-randomised, observational single-centre study and the results are self-reported with no external validation from independent data, which certainly limits their generalisability. The number of patients was relatively small; the multivariate predictors of mortality identified as significant in our study should be confirmed in a larger sample. Moreover, because our study enrolled the first 300 consecutive patients who underwent TAVR between 2007 and 2009 at our centre, results may have been affected by a “learning curve”. Several important variables have changed, including new-generation devices, smaller delivery systems, greater experience in the selection of appropriate patients, and greater experience in surgical procedures. Echocardiography was not evaluated by an external core laboratory. Finally, we reported the date of death, but not the cause of death. Therefore, we only reported all-cause mortality, but not cardiac- or valve-related mortality. The predictors of late mortality were examined in a high-risk group of patients, among whom non-cardiac death might have been frequent or cardiac death might have been unrelated to valvular complications; therefore, many “true” predictors of mortality might have been biased by the overall high rates of mortality due to other causes.
Conclusions
After seven years, 23.2% of 300 high-risk patients remained alive after undergoing TAVR with first-generation devices. Baseline- and procedure-related factors were identified as being independently associated with all-cause mortality. With an overall cumulative incidence of 14.9% at seven years, there is some suggestion that SVD post TAVR may become increasingly relevant during longer-term follow-up. With the broadening of indications for TAVR to include lower-risk and younger patients, close attention is required to determine the long-term durability of THVs.
| Impact on daily practice Over the long term, a high proportion of survivors maintained good clinical status. Independent predictors of all-cause mortality included both patient-related (atrial fibrillation, impaired kidney function, peripheral artery disease, and mitral regurgitation) and procedure-related factors (acute kidney injury, neurological events, major vascular complications, and major/life-threatening bleeding). There is some suggestion that SVD post TAVR may become increasingly relevant during longer-term follow-up. Thus, SVD post TAVR warrants further investigation. |
Conflict of interest statement
H. Ruge is a proctor for Boston Scientific. N. Piazza is a proctor and consultant for Medtronic, and a JenaValve advisory board member. S. Bleiziffer is a proctor and consultant for Medtronic, and a proctor for Boston Scientific, JenaValve Technology Inc., HighLife Inc. and XPand Medical SAS. R. Lange is member of the Medtronic advisory board. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. NYHA functional class over time, and freedom from the composite endpoint NYHA III/IV or death.
Supplementary Figure 2. Degree of PVL over time, and mean transprosthetic pressure gradients over time.
To read the full content of this article, please download the PDF.

