
Abstract
Aims: Our aim was to evaluate the long-term device performance and clinical outcomes of patients with symptomatic, severe aortic valve stenosis (AS) who underwent transcatheter aortic valve implantation (TAVI) with the CoreValve bioprosthesis.
Methods and results: The CoreValve CE Pivotal Study was a prospective, multicentre, single-arm TAVI trial using the CoreValve system. Valve performance, patient quality of life (QoL), New York Heart Association (NYHA) class, and mortality at four years were analysed in 126 patients (mean age 82.4 years, 42.9% male, mean logistic EuroSCORE 23.4%) with severe AS. Mean aortic valve gradient decreased from 46.9±16.1 mmHg at baseline to 9.8±4.1 mmHg at discharge and to 7.8±2.7 mmHg at four years. Mean effective orifice area increased from 0.7±0.2 cm2 to 1.8±0.4 cm2 after TAVI and was 1.6±0.5 cm2 at four years. There were no reports of structural valve deterioration or valve migration. There was sustained improvement in QoL and NYHA class in surviving patients. All-cause and cardiac survival was 45.3% and 62.6%, respectively, at four years.
Conclusions: The CoreValve bioprosthesis demonstrates long-term durability, stable haemodynamic function, and no evidence of structural deterioration. Most surviving patients continued to have improved NYHA class and QoL at four years. ClinicalTrials.gov Identifier: NCT01051518
Abbreviations
AS: aortic stenosis
CEC: clinical events committee
EOA: effective orifice area
EQ-5D: European Quality of Life-5 Dimension
EuroSCORE: European System for Cardiac Operative Risk Evaluation
MACCE: major adverse cardiovascular and cerebrovascular events
NYHA: New York Heart Association
QoL: quality of life
SAVR: surgical aortic valve replacement
TAVI: transcatheter aortic valve implantation
VAS: visual analogue scale
Introduction
Ongoing technological advances in percutaneous techniques and in the evolution of collapsible bioprosthetic aortic valves have allowed transcatheter aortic valve implantation (TAVI) to emerge as a viable therapeutic option for high-risk or otherwise inoperable patients with severe, symptomatic aortic stenosis (AS). To date, short-term and midterm clinical results with current TAVI technology have been encouraging, with patients demonstrating sustained clinical and functional cardiovascular improvement1-12. However, data regarding long-term outcomes, including serial echocardiographic valve assessment, following TAVI remain limited13,14, and long-term data on valve durability are needed if TAVI is to expand to additional patients, including those at lower risk of surgical mortality.
CoreValve® (Medtronic, Minneapolis, MN, USA) is a self-expanding, bioprosthetic, porcine pericardial tissue valve for TAVI1. Buellesfeld and colleagues previously reported the two-year experience with the device based on data from the CoreValve Pivotal CE Study6. Here we report the final, four-year clinical outcomes and performance of the CoreValve bioprosthesis in the same patient cohort.
Methods
STUDY DESIGN
The CoreValve CE Pivotal Study was a prospective, multicentre, single-arm trial to evaluate the safety and performance of the CoreValve bioprosthesis in patients undergoing TAVI for treatment of severe AS. The primary endpoint of major adverse cardiovascular and cerebrovascular events (MACCE) at 30 days has already been reported6 and we report this outcome up to the final follow-up at four years. In addition, we report pre-specified secondary endpoints of New York Heart Association (NYHA) functional class status, patient quality of life (QoL), echocardiographic measures of valve function, and aortic regurgitation. Patient inclusion criteria were the presence of severe aortic stenosis (≤0.6 cm2/m2), aortic annulus diameter ranging from 20 to 27 mm as determined by echocardiography, ascending aorta diameter ≤45 mm at the sinotubular junction, age ≥75 years, or surgical risk with logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) ≥15%, or one to two high-risk comorbidities such as cirrhosis of the liver, pulmonary insufficiency (forced expiratory volume in one sec <1 L), previous cardiac surgery, pulmonary hypertension (systolic pulmonary pressure >60 mmHg), porcelain aorta, right ventricular failure, or history of mediastinal radiation therapy.
The CoreValve CE Pivotal Study utilised two independent cardiovascular surgeons with recognised expertise in aortic valve surgery to perform a post hoc risk stratification using commonly accepted surgical criteria to identify patients considered high-risk or moderate-risk for surgical aortic valve replacement (SAVR). The surgeons were blinded to procedural details and outcomes but were provided with baseline case report forms, logistic EuroSCORE, and available source documentation for each patient. Reviewers scored the patients according to the following scale: moderate-risk―probability of all-cause 30-day or in-hospital mortality from SAVR <10%; high-risk operable―probability of all-cause 30-day or in-hospital mortality from SAVR >10%; high-risk inoperable―if high risk was established, the reviewer also determined if the patient met the inclusion criteria for participation in the inoperable study arm, in which the probability of all-cause 30-day or in-hospital mortality exceeded 50%.
The study complied with the Declaration of Helsinki. All patients provided written informed consent before the procedure. The study was approved by the local ethics committees at each institution.
DEVICE AND PROCEDURE
The CoreValve prosthesis design and procedural characteristics have been reviewed previously1,3. In summary, the current 18 Fr generation of the CoreValve prosthesis consists of a trileaflet bioprosthetic porcine pericardial tissue valve mounted and sutured in a self-expanding nitinol stent frame. The device is implanted in a retrograde manner.
DEFINITIONS
Major adverse cardiovascular and cerebrovascular events were defined as the composite of all-cause death, myocardial infarction, emergent cardiac reintervention (including emergent revascularisation procedures and emergent aortic valve repair surgery), and stroke. Myocardial infarction was defined as elevation of creatine kinase twice the upper limit of normal in the presence of elevated creatine kinase-myocardial band above the upper limit of normal, with electrocardiographic evidence of ischaemia. Stroke was defined as a new prolonged (>24 hrs) or permanent neurological deficit and radiographic imaging demonstrating an acute ischaemic cerebrovascular event or leading to death, with no apparent cause other than that of vascular origin. Structural valve deterioration was defined as any change in function of the study valve resulting from an intrinsic abnormality of the valve that causes stenosis or regurgitation.
Quality of life (QoL) was evaluated using the European Quality of Life-5 Dimension (EQ-5D) questionnaire15. The questionnaire contained the following self-assessments: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Patient responses to the five EQ-5D questions were rated on a 3-point scale: 1) no problems, 2) some or moderate problems, and 3) significant problems. The EQ-5D questionnaire provides a simple, descriptive profile and generates a summary index score for which full health is assigned a value between 1 (perfect health) and 0 (dead). Additionally, there was an overall health state score. The EQ-5D health state score was measured on a 10-point visual scale (10=best imaginable health state to 0=worst imaginable health state) that was completed by the patient.
DATA COLLECTION AND ECHOCARDIOGRAPHIC FOLLOW-UP
Conduct of the trial was monitored by MedPass International (Paris, France) and Medtronic Bakken Research Center (Maastricht, The Netherlands). All serious adverse events up to 30-day follow-up and all deaths to one year were adjudicated by an independent clinical events committee (CEC). After one year, serious adverse events and deaths were recorded based on site reports and patient medical records.
Clinical and echocardiographic evaluations were performed at baseline and after the procedure at discharge, one, six, and 12 months, and annually thereafter at the study sites. All study echocardiograms were analysed at an independent core laboratory (Hôpital Henri Mondor Laboratoire d’Echocardiographie, Créteil, France). Image acquisition quality was ensured by use of a detailed acquisition protocol and site qualification. For study site qualification, echocardiographers had to undergo a certification process that required submission of an echocardiographic study to the core lab demonstrating 100% adherence to the study protocol. Study site echocardiograms were transferred digitally (by use of a compact disc) to the core lab for analysis. Transvalvular gradients were calculated using the modified Bernoulli equation, and the prosthetic valve effective orifice area (EOA) was calculated with the continuity equation (velocity-time integral method). Aortic regurgitation was classified as none, mild, moderate, moderate/severe, and severe16.
STATISTICAL METHODS
Since this study was a prospective, multicentre, single-arm study, all patients enrolled were intended to receive the same treatment. Descriptive statistics were used to report demographic clinical characteristics and procedural, echocardiographic, and QoL data. For categorical data, the number and percentage of patients in the category are presented. For continuous data, the mean±standard deviation is presented. Kaplan-Meier analyses of event-free rates were performed to analyse safety endpoints.
Results
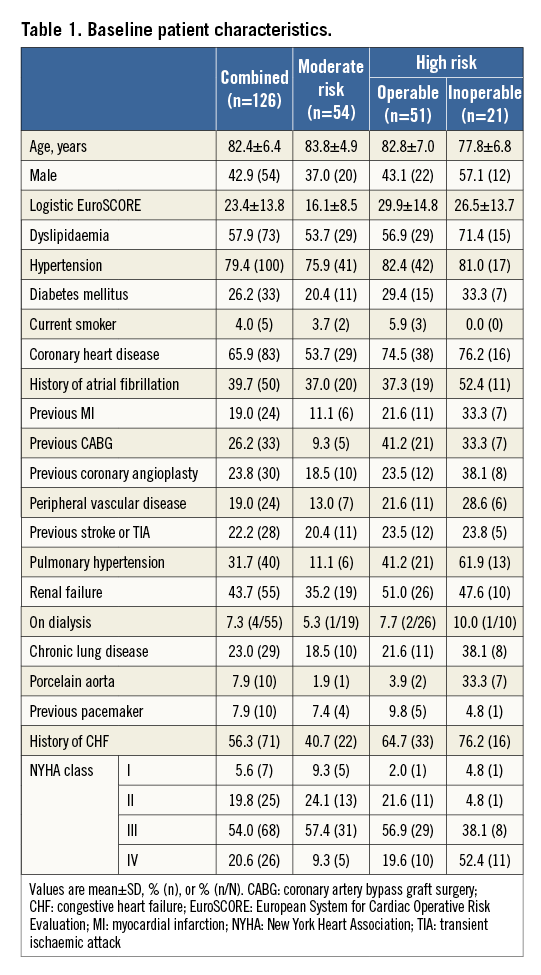
PATIENT CHARACTERISTICS
The CoreValve CE Pivotal Study prospectively enrolled 126 patients at nine sites in Europe and Canada between May 2006 and November 2008. Baseline patient characteristics are presented in Table 16.
CLINICAL OUTCOMES
At four years, a total of 67 patients had died: 18 died within 30 days after the TAVI procedure, and 49 died during the remainder of the four years. Six patients (4.8%) were lost to follow-up. Figure 1 illustrates the freedom from all-cause mortality up to four years post-procedure. Of the 67 patients who died during the follow-up period, 40 died from cardiac causes. Of the cardiac deaths, 12 occurred within 30 days of the procedure, and 28 died during the remainder of the follow-up period. Figure 2 illustrates the freedom from cardiac mortality up to four years post-procedure. Among the subgroups, the high-risk groups had numerically higher all-cause and cardiac mortality than the moderate-risk group up to four-year follow-up (Table 2). A total of 18 patients had a stroke up to four-year follow-up: of these, 12 (66.7%) occurred within 30 days of the procedure (Table 2). Overall, 75 patients had a MACCE during the follow-up period, with more patients in the high-risk groups compared with the moderate-risk group (51 vs. 24; Table 2). Figure 3 illustrates the freedom from MACCE up to four years post-procedure.
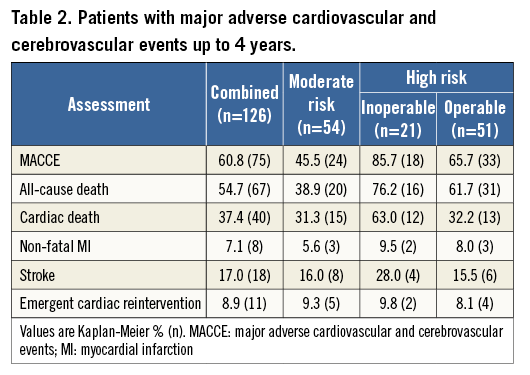
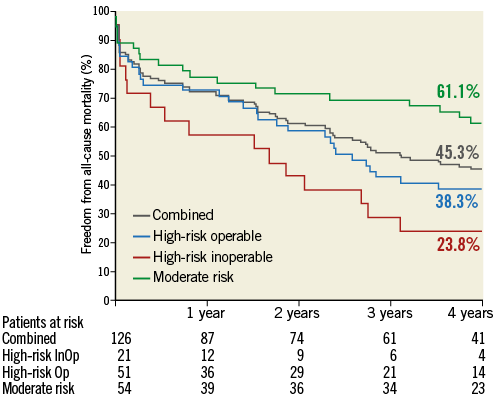
Figure 1. Freedom from all-cause mortality. Kaplan-Meier estimates of freedom from all-cause mortality up to four-year follow-up. InOp: inoperable; Op: operable
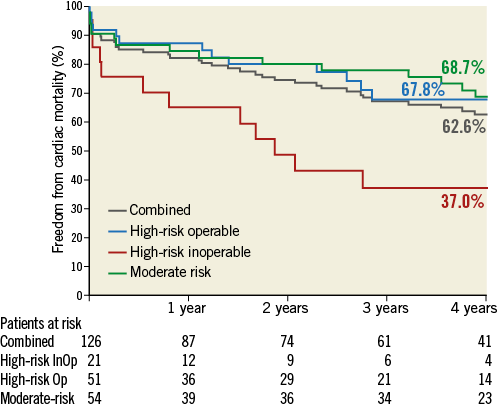
Figure 2. Freedom from cardiac mortality. Kaplan-Meier estimates of freedom from cardiac mortality up to four-year follow-up. InOp: inoperable; Op: operable
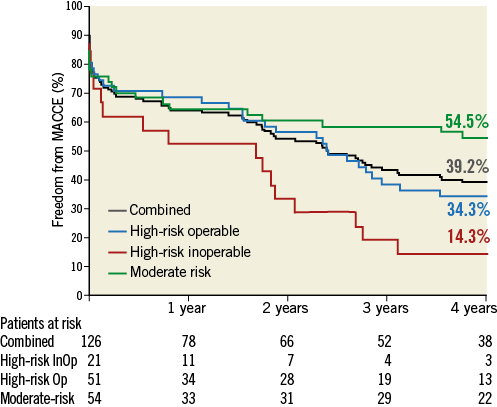
Figure 3. Freedom from MACCE. Kaplan-Meier estimates of freedom from major adverse cardiovascular and cerebrovascular events (MACCE) up to four-year follow-up. InOp: inoperable; Op: operable
FUNCTIONAL STATUS
For the overall implanted population, the percentage of surviving patients in NYHA Class III or IV was 75.0% at baseline, 15.8% at discharge, 12.0% at one year, and 10.6% at four years. Figure 4 shows the change in NYHA classification compared with baseline status at discharge, one year, and four years for implanted patients with paired data in the overall study cohort as well as for the subgroups. At discharge, 81.2% of the total implanted patient population with paired data improved by at least one NYHA level, while 13.9% remained unchanged, and 4.9% had worsened. At four years, 74.5% reported improvement, 17.0% remained unchanged, and 8.5% had worsened.
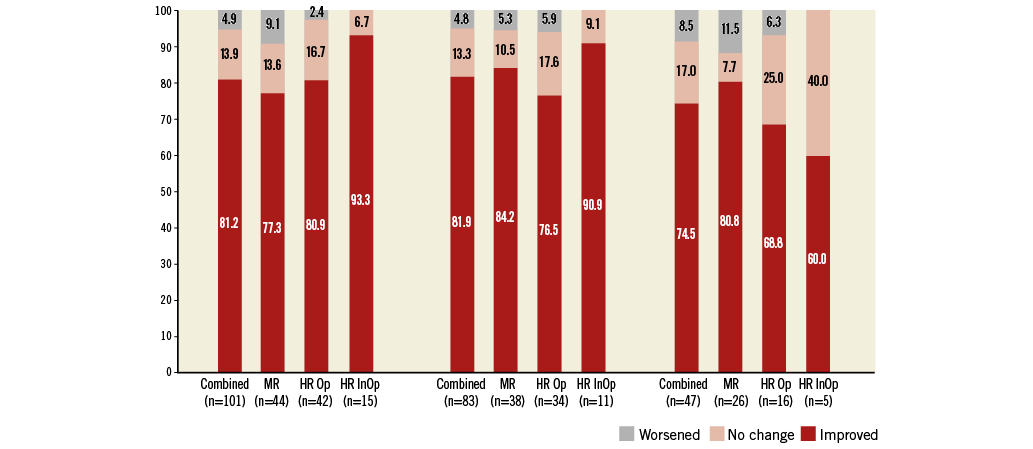
Figure 4. NYHA functional status. Change in functional status among survivors expressed by New York Heart Association (NYHA) classification at discharge and at one-year and four-year follow-up compared with baseline. HR InOp: high-risk inoperable; HR Op: high-risk but operable; MR: moderate-risk
QUALITY OF LIFE
The EQ-5D questionnaire descriptive data for all implanted patients at baseline and at one and four years are shown in Figure 5. For those patients in the combined risk group with available data, the mean summary index (scale: 0-1; 1=full health) was 0.6 at baseline and 0.7 at one and four years post-procedure. The mean response to health state (10-point visual scale: 10=best imaginable health state, 0=worst imaginable health state) was 5.3 at baseline, 6.7 at one year, and 7.4 at four years post-procedure.
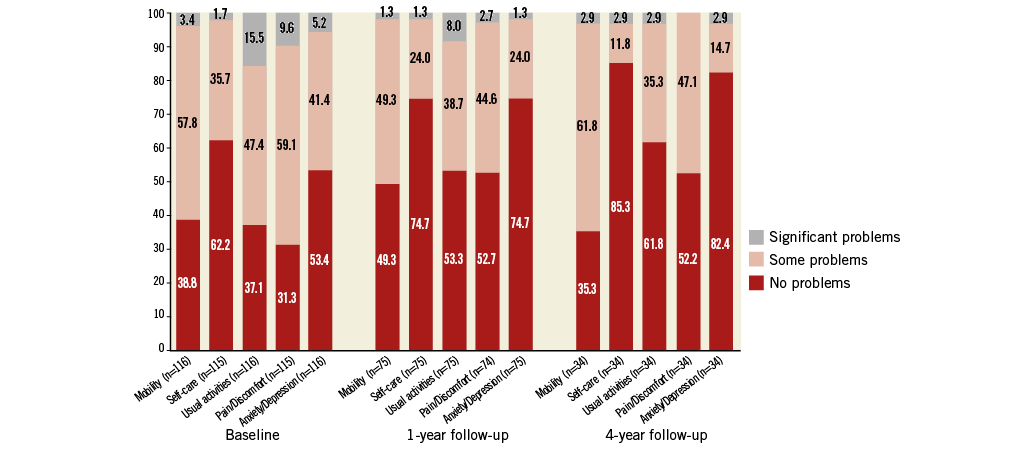
Figure 5. EQ-5D patient quality of life assessment. EQ-5D quality of life questionnaire descriptive data for all implanted patients at baseline, and at one-year and four-year follow-up.
VALVE PERFORMANCE AND DURABILITY
For all implanted patients, the mean EOA was 0.7±0.2 cm2 at baseline, 1.8±0.4 cm2 at discharge, 1.7±0.3 cm2 at one year, and 1.6±0.5 cm2 at four years (Figure 6). The mean aortic valve gradient was 46.9±16.1 mmHg at baseline, 9.8±4.1 mmHg at discharge, 10.3±3.8 mmHg at one year, and 7.8±2.7 mmHg at four years (Figure 6). At baseline, moderate aortic regurgitation (AR) was documented in 12.4% of patients (Figure 7). Following TAVI, the percentage of patients with moderate AR was 6.0% at discharge, 4.2% at one year and 4.8% at four years. Due to the small number of patients at four-year follow-up with echocardiographic measurements, it was not feasible to calculate paired serial changes throughout the study. There was no moderate-to-severe or severe AR (3+/4+) throughout the follow-up period. Emergent cardiac reintervention was required in 11 of the 126 patients up to six months with no additional events up to four-year follow-up. Of the emergent reinterventions, three were adjudicated by the CEC to be related to a malposition of the initially implanted valve and all occurred on the same day as the initial procedure. There were no reports of structural valve deterioration or valve migration throughout the study. One case of endocarditis involving the CoreValve occurred 523 days post-procedure.
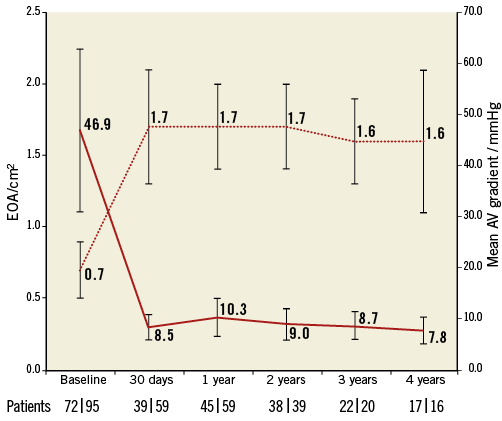
Figure 6. Mean aortic valve (AV) pressure gradient and effective orifice area (EOA). Mean AV pressure gradient and EOA assessed by echocardiography up to four years. Presented are the mean AV gradient (n) | EOA (n) evaluated at each time point. Error bars represent standard deviations.
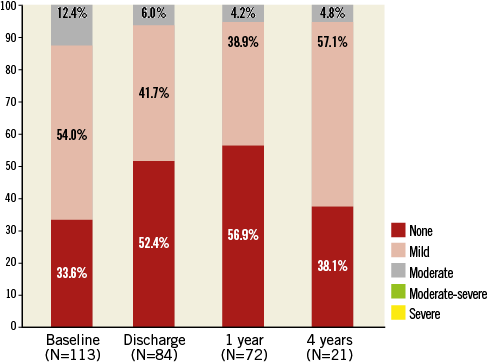
Figure 7. Aortic valve regurgitation. Aortic valve regurgitation assessed by echocardiography up to four years.
Discussion
This study reports the results of four years’ experience with the CoreValve bioprosthesis. As such, it is one of the longest follow-up studies of a first-generation TAVI device reported to date. Overall, the results continue to demonstrate the efficacy and durability of the self-expanding CoreValve bioprosthesis for the treatment of patients with severe AS.
Major adverse cardiovascular and cerebrovascular events occurred in 75 study patients. At four-year follow-up, 67 patients had died, of whom 40 died from cardiac causes. Kaplan-Meier estimates of all-cause and cardiac mortality rates were 54.7% and 37.4%, respectively, at four years. This level of mortality, while high, is not unexpected given the advanced age and extremely high risk of the patients treated with the device during early experience with TAVI therapy. Indeed, among the study’s risk groups, the high-risk inoperable group’s all-cause mortality and cardiac mortality were twice those of the moderate-risk group, demonstrating the importance patient risk characteristics have on outcomes. Similar mortality results have also been reported with the Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) transcatheter valve. In a study by Gurvitch and co-workers describing 70 patients who received the SAPIEN device, death occurred in 43% at a median of 3.7 years of follow-up7. In another study of 339 patients who received the device, Rodés-Cabau and colleagues reported mortality of 55.5% after a mean follow-up of 42±15 months13. Additionally, Toggweiler and co-workers reported five-year mortality of 65% in 88 patients who received the SAPIEN device14.
Of the MACCE monitored during the study, stroke was the second most common adverse event, occurring in 18 patients. However, the majority of strokes occurred early in the follow-up period, with 12 occurring within 30 days of the procedure.
Improvements in survival and health-related QoL are the main goals of any cardiovascular surgery. To this end, the CoreValve CE Pivotal Study used the EQ-5D instrument as well as a related visual analogue scale (VAS) to evaluate patients’ QoL at yearly intervals throughout the study. The EQ-5D is a societal-based, composite global health-related QoL summary index, and it has been extensively used in trials of new cardiovascular treatments17. In the present study, the majority of surviving patients (≥80%) had improved or had no change in assessed QoL domains with the exception of mobility over the four-year study period. Similarly, the average VAS, which provides a direct global health-related QoL assessment from the patient’s perspective17, showed improvement among implanted patients with paired data up to four years. In conjunction with these findings was an improvement in patients’ functional status, with 74.5% of surviving patients improving by at least one NYHA class at four years.
Unique to the CoreValve Pivotal CE Study was its use of an echo core laboratory for serial long-term transcatheter valve assessment. The importance of utilising an echo core laboratory to reduce variability and enhance the precision of study results is well documented18-21. The long-term echo data from the CoreValve Pivotal CE Study continue to demonstrate the durability of the CoreValve bioprosthesis. Four years after implantation, there was no evidence of structural valve deterioration, valve migration, or significant changes in the haemodynamic status of the prosthesis. These results are encouraging, since proof of valve durability is vital to the expansion of TAVI to younger patients, who will require a functional valve over a longer time period, and those at lower risk of surgical mortality.
Study limitations
This study had several limitations. First, as a non-randomised trial, it was not possible to compare the study’s data with data obtained from patients with severe AS treated by surgery or medical therapy. Second, small subgroup sizes, which diminished further due to patient deaths throughout the course of the study, may have affected interpretation of the data. Third, the study was designed and implemented prior to the introduction of Valve Academic Research Consortium (VARC) standardised endpoint definitions for TAVI trial22,23. Fourth, in some patients we were not able to collect echocardiographic measures at the later follow-up time points; however, based on clinical assessment we were able to summarise their overall clinical status. Fifth, the echocardiographic core laboratory documented aortic regurgitation, but not whether it was central or paravalvular. Thus, the results of this trial must be interpreted carefully when used for comparison in later trials that implement VARC definitions.
Conclusions
This study demonstrated favourable long-term outcomes after TAVI using the self-expanding CoreValve aortic bioprosthesis for the treatment of patients with severe AS. After four years, the valve’s haemodynamic status remained stable, and no valve migration or structural dysfunction occurred. Clinically, there was sustained improvement in QoL and NYHA class among surviving patients with evaluable data who underwent TAVI with the CoreValve device.
| Impact on daily practice The CoreValve CE Pivotal Study is one of the first studies to have assessed longer-term performance of transcatheter valves implanted in high-risk candidates for surgical aortic valve repair. Valve durability is vital to the expansion of TAVI to younger patients and those at lower risk of surgical mortality. The present study demonstrated favourable long-term outcomes using the self-expanding CoreValve aortic bioprosthesis for the treatment of patients with severe AS. Additional multidisciplinary research will help identify the use of TAVI in lower-risk patient groups, and research into applied technology will result in further miniaturisation of the procedural devices as well as in further improvement in the safety of the procedure. |
Guest Editor
This paper was guest edited by Josep Rodés-Cabau, MD; Cardiology Department, Quebec Heart & Lung Institute, Laval University, Quebec City, Quebec, Canada.
Acknowledgements
The authors thank Janice Hoettels, PA, MBA, and Jane Moore, MS, ELS for their assistance in the preparation of this manuscript. Angie Zhang, MS, performed all statistical analyses and verified the accuracy of the data presented. Julie Houle, MBA, and Kelly Hendrickson, MS, were responsible for overall study management.
Funding
The CoreValve CE Study was funded by Medtronic.
Conflict of interest statement
J. Kovac serves as a consultant and a proctor for Medtronic. U. Gerckens has received consultant and lecture fees and study-related travel expenses from Medtronic and Edwards Lifesciences, and serves as a proctor for Medtronic and Boston Scientific. R. Bonan serves as a consultant to and proctor for Medtronic. M. Labinaz serves as a proctor for Medtronic. P. den Heijer serves as a proctor and consultant for Medtronic. E. Grube serves as a proctor and a member of an advisory board for Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor has received research grants from Edwards Lifesciences, St. Jude Medical and Medtronic.

