Abstract
Over the last decade, transcatheter aortic valve implantation (TAVI) has emerged to become the treatment of choice for inoperable patients and the preferred alternative for high-risk patients with severe, symptomatic aortic stenosis (AS). Questions about the long-term durability of TAVI valves were raised early in the history of the procedure. Although there has not yet been a significant signal of early structural valve deterioration (SVD), these concerns remain important today, especially if TAVI is to be considered for use in lower-risk and younger patients with longer life expectancy. Durability expectations for TAVI to some degree parallel those of surgical bioprostheses, but the different tissue, mounting design and crimping of TAVI devices might adversely influence long-term results. The experience with surgical bioprostheses has shown that deterioration of these valves is a slow and gradual process. Thus, despite promising midterm results of many surgical bioprostheses at five to seven years, design faults with higher failure rates have become manifest eight to 10 years after implantation. Similarly, although the initial five-year outcomes of TAVI are promising, these results cannot yet be extrapolated to predict long-term durability with any firm degree of assuredness, especially in younger patient populations. Thus, a high degree of caution is necessary when considering TAVI in intermediate-risk and younger patients until more evidence of durability equivalent to that of surgical bioprostheses is forthcoming.
Introduction
Advances in tissue preservation techniques have led to improved long-term durability of third- and fourth-generation surgical aortic valve tissue bioprostheses. This significant step forward (in combination with the ability to forgo long-term anticoagulation when using such devices) has led to their more frequent use, with a corresponding decrease in the rate of implantation of mechanical prostheses in the Western hemisphere. The 2014 Annual Report of the German Society for Thoracic and Cardiovascular Surgery revealed that tissue bioprostheses (xenografts) were used in 87% of all isolated aortic valve replacements (surgical and transcatheter)1. Similarly, Chiang and colleagues showed that bioprosthetic valve usage in patients aged 50 to 69 in New York State increased from 15% in 1997 to 74% in 20122.
Concurrent with this surge in bioprosthesis utilisation has been the emergence of transcatheter aortic valve implantation (TAVI) over the last decade. TAVI is now the treatment of choice for inoperable patients and the preferred alternative for high-risk patients with severe, symptomatic aortic stenosis (AS). While long-term durability (>5 years) may be of secondary importance in this mainly elderly population, the current trend towards performing TAVI in lower-risk and younger patients requires more definitive answers regarding the long-term durability of TAVI devices. In this manuscript, we present current knowledge about long-term clinical outcomes of TAVI as well as the durability of both surgical and TAVI bioprostheses.
Types of tissue valve
Tissue valves for aortic valve replacement can be divided into the categories of autografts (Ross procedure), homografts and xenografts. While indications for autografts (children, females of child-bearing years) and homografts (infective endocarditis) are currently very limited, xenografts are the most commonly employed type of bioprosthesis. The different valve types vary not only in their composition but also in terms of haemodynamics, durability, and thrombogenicity. Xenografts are derived from either bovine or porcine (or infrequently equine) sources and can be either stented or stentless. Porcine xenografts are most often aortic valves from porcine hearts reinforced with scaffolding, and bovine valves are composed of bovine pericardium. Stentless porcine valves consist of a porcine aortic root and mimic the human anatomy. This structure results in improved haemodynamics and an increased effective orifice area as compared to stented valves. Whether these factors lead to improved clinical outcomes, including freedom from structural valve deterioration (SVD), has never been clearly demonstrated.
Why tissue valves fail
Without surgical damage or manufacturing flaws in a surgical bioprosthesis, deterioration is a slow and gradual process due to the fact that the tissue is non-viable. Structural valve failure is mainly caused by calcification that is histologically evident within three years of implantation; failure is therefore accelerated by any diseases that influence calcium metabolism, especially chronic kidney disease (Figure 1, Figure 2). Calcification most often occurs in the commissural and basal areas of the cusps3. These depositions result in cusp stiffening or tears, leading to either valve stenosis or regurgitation. The mode of deterioration differs according to valve type, with bovine pericardial valves more frequently stiffening to cause stenosis and porcine native valves tearing to cause regurgitation. Developments in newer generations of bioprostheses have attempted to prevent or at least delay this calcification and tissue deterioration by improvements in tissue fixation and anti-mineralisation treatment. A final mechanism of valve deterioration is extensive tissue overgrowth. Although more common with mechanical valves, the resulting pannus can thicken the valve cusps, causing either stenosis or regurgitation. The extent of pannus formation might be related to the amount of prosthesis in contact with host tissue.
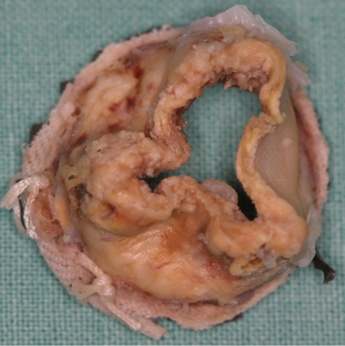
Figure 1. Degenerated surgical bioprosthesis.
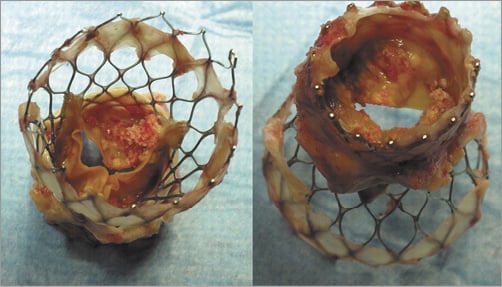
Figure 2. Degenerated CoreValve with extensive calcification on the outflow and inflow aspects of the porcine leaflets (reproduced with permission of Ong et al11).
Experience in surgical valves
Reported midterm failure rates of porcine and bovine bioprostheses are very low (less than 1% before five years and 10% at 10 years for patients >65 years)4. However, several studies with different valves have demonstrated that the risk of SVD is age-related and highest in patients <35 years at the time of implantation. This inverse relationship between durability and age may be related to greater competence of the immune system and faster metabolic rate in younger patients. Additionally, higher postoperative atrioventricular gradients in younger patients increase mechanical stress, augmenting the risk of SVD.
Experience with the Ionescu-Shiley pericardial tissue valve, in particular, taught surgeons that SVD can occur fairly early after implantation despite excellent initial results and haemodynamics5. This first-generation pericardial bioprosthesis was affected by SVD beginning only five to six years after implantation, when suture fixation of the leaflets resulted in cusp tear and aortic regurgitation, and freedom from reoperation of only 38% at 13 years. Changes in design and tissue treatment resulted in improved durability in the next generation. After initial five-year data with the St. Jude Toronto SPV bioprosthesis (St. Jude Medical, Inc., St. Paul, MN, USA) appeared promising, these valves developed SVD at eight to 10 years. Due to increased mechanical stress on the cusps and late dilatation of the sinotubular junction, freedom from SVD and moderate or severe aortic regurgitation were only 69% and 48% after 12 years, respectively6. Both examples suggest that good midterm outcomes cannot be extrapolated to long-term durability. Thus, long-term follow-up is necessary to establish the unique expected durability of each bioprosthesis.
Clinical outcomes after TAVI
Several randomised trials have demonstrated excellent clinical outcomes after TAVI in inoperable and high-risk patients. The Placement of Aortic Transcatheter Valves (PARTNER I) trial investigated outcomes of both patient cohorts after TAVI with the first-generation SAPIEN (Edwards Lifesciences, Irvine, CA, USA) TAVI system. In the inoperable cohort, mortality at one year was 30.7% with a significant decrease in cardiac symptoms: both of these results were superior to standard treatment (medical therapy, mostly combined with balloon aortic valvuloplasty)7. After five-year follow up, the rate of all-cause mortality was 71.8% in the inoperable TAVI group versus 93.6% in the standard treatment group (p<0.0001)8. At five years in the high-risk cohort of the PARTNER I trial, which compared TAVI against surgical aortic valve replacement, the risk of death was 67.8% in the TAVI group and 62.4% in the surgical group (p=0.76). Of note, moderate or severe paravalvular leak (PVL) existed in 14% of the TAVI patients compared to only 1% after conventional surgery. An Italian nation-based clinical data repository recently reported five-year outcomes after CoreValve (Medtronic Inc., Minneapolis, MN, USA) implantation. In these 353 consecutively treated patients, five-year mortality was 55%. This patient cohort was characterised by a lower STS PROM score compared to the PARTNER trial, but the Italian patients still had a high rate of subsequent moderate or severe PVL (23.6%)9. In both trials, moderate and severe PVL was associated with a higher mortality rate. However, the incidence of severe PVL after TAVI has been decreasing in recent experience due to improvements in imaging and device design (allowing optimal valve deployment and minimisation of the risk of PVL using special sealing cuffs, skirts or inflatable cuffs). Of particular relevance is the fact that rates of reoperation for SVD at five years were not reported in either the TAVI or the surgical arms of the PARTNER I trials. It is noteworthy, however, that the number of patients with SVD who did not undergo repeat intervention is also unknown.
Durability of TAVI valves
The tissue used for TAVI valves is similar to that used for surgical valves with equivalent anti-calcification treatment. However, there are a number of reasons why the durability of surgical and TAVI valves may differ. First, although the tissue used for surgical and TAVI bioprostheses has proven to be durable when mounted on a rigid ring, different mechanical stresses result from mounting within the various transcatheter valve designs with possible impact on durability. Second, bench studies have shown that crimping the leaflets to allow transcatheter valve delivery results in microscopic tissue damage observable at the surface layers. Although a correlation with SVD has not yet been demonstrated, these non-visible defects may influence the long-term durability of transcatheter valves. Third, the trend to reduce sheath sizes for device delivery requires even smaller crimped valve diameters, requiring increasingly thinner leaflet material and possible shorter durability. Fourth, some transcatheter valves use porcine pericardium which has no track record of durability in surgical valves. Lastly, the inability to remove native valve calcification, as is standard in SAVR, may negatively influence transcatheter valve stent geometry, causing distortion or incomplete valve expansion and resulting in undue mechanical stress on the implant leaflets. Likewise, both underexpansion and overexpansion of the stent result in different mechanical stresses on the leaflet than those for which the stents were designed. All of these factors might further shorten the durability of TAVI valves.
In the longest reported experience encompassing >1,000 transcatheter valve implantations in Vancouver (with a few valves implanted over nine years ago), only five failed valves have been identified to date. However, due to the relatively small number of valves implanted this long ago, no reliable long-term data concerning TAVI devices currently exist beyond five years. In the PARTNER I trial, no SVD requiring redo surgical aortic valve replacement was detected five years after SAPIEN implantation8. Another group reported the absence of SVD four years after SAPIEN implantation, but, after five years, 9.7% of living patients in the study had moderate prosthetic valve failure (albeit without need for reoperation or reintervention)10. With the CoreValve device, the five-year rate of midterm prosthesis failure was 1.4%9. A valve-in-valve procedure was performed in two of the five patients with failing devices from this cohort. In summary, the majority of reports have identified only mild to moderate prosthesis dysfunction in a minority of patients up to five years after TAVI with no need for intervention. However, as development of many different valve designs continues, the long-term data for one valve may differ from others. Thus, the reported long-term data of the early TAVI valves might not apply for the current generation of TAVI devices.
Why don’t we have reliable data about TAVI durability?
In addition to the rapid turnover from one device generation to the next, several other factors prevent the acquisition of reliable TAVI durability data. 1) TAVI is a relatively new treatment and implantations could not be performed on a sufficiently large scale until the first devices received CE mark approval in 2007 and FDA approval in 2011. 2) Because the initial patients treated with TAVI were either inoperable or high-risk elderly patients, their survival was limited despite (and independent of) a successful TAVI procedure, leading to a small number of patients at risk in the long term. 3) Many of the patients who present with SVD are too sick to undergo redo surgery or valve-in-valve procedures, which may result in under-reporting of the true incidence of SVD. 4) Our experience with surgical prostheses suggests that SVD is more common in younger patients, whereas mainly elderly patients were included in both the initial and the current TAVI trials. Given all of these issues, it will be several more years before reliable data concerning the long-term durability of TAVI devices are available. Even then, however, these outcomes will not be readily extrapolated to younger patients.
Conclusions
So far, no study demonstrating high rates of SVD in transcatheter valves has been published. However, reliable data are only available at five years of follow-up. Experience with several surgical bioprostheses has taught us that increasing rates of SVD may occur beyond this period. The reported durability of TAVI devices appears adequate for an elderly, high-risk cohort. Long-term studies, much greater than five years in duration, are necessary to prove non-inferior durability compared to surgical valves for younger, lower-risk patients. Because TAVI durability is only established in elderly and high-risk patients, much caution is necessary when considering TAVI in intermediate-risk and younger patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.

