Abstract
Transcatheter aortic valve implantation (TAVI) is now utilised as a less invasive alternative to surgical aortic valve replacement (SAVR) across the whole spectrum of surgical risk. Long-term durability of the bioprosthetic valves has become a key goal of TAVI as this procedure is now considered for younger and lower-risk populations. The purpose of this article is to present a state-of-the-art overview on the definition, aetiology, risk factors, mechanisms, diagnosis, clinical impact, and management of bioprosthetic valve dysfunction (BVD) and failure (BVF) following TAVI with a comparative perspective versus SAVR. Structural valve deterioration (SVD) is the main factor limiting the durability of the bioprosthetic valves used for TAVI or SAVR, but non-structural BVD, such as prosthesis-patient mismatch and paravalvular regurgitation, as well as valve thrombosis or endocarditis may also lead to BVF. The incidence of BVF related to SVD or other causes is low (<5%) at midterm (5- to 8-year) follow-up and compares favourably with that of SAVR. The long-term follow-up data of randomised trials conducted with the first generations of transcatheter heart valves also suggest similar valve durability in TAVI versus SAVR at 10 years, but these trials suffer from major survivorship bias, and the long-term durability of TAVI will need to be confirmed by the analysis of the low-risk TAVI versus SAVR trials at 10 years.
Transcatheter aortic valve implantation (TAVI) is now utilised as a less invasive alternative to surgical aortic valve replacement (SAVR) across the whole spectrum of surgical risk from extreme/high to low surgical risk. Ideally, the proven durability of the bioprosthetic valve should match or exceed the expected life expectancy of the patient. Structural valve deterioration (SVD) is the main factor limiting the durability of the bioprosthetic valves used for TAVI or SAVR. The long-term durability of bioprosthetic valves has become a key goal of TAVI as this procedure is now considered for younger and lower-risk populations with longer life expectancy12. The utilisation of TAVI will likely further extend to even lower-risk populations including patients with asymptomatic severe aortic stenosis or at-risk moderate aortic stenosis. The purpose of this article is thus to present a state-of-the-art overview on the definition, aetiology, risk factors, mechanisms, diagnosis, clinical impact, and management of bioprosthetic valve dysfunction (BVD) and failure (BVF) following TAVI with a comparative perspective versus SAVR (Central illustration).
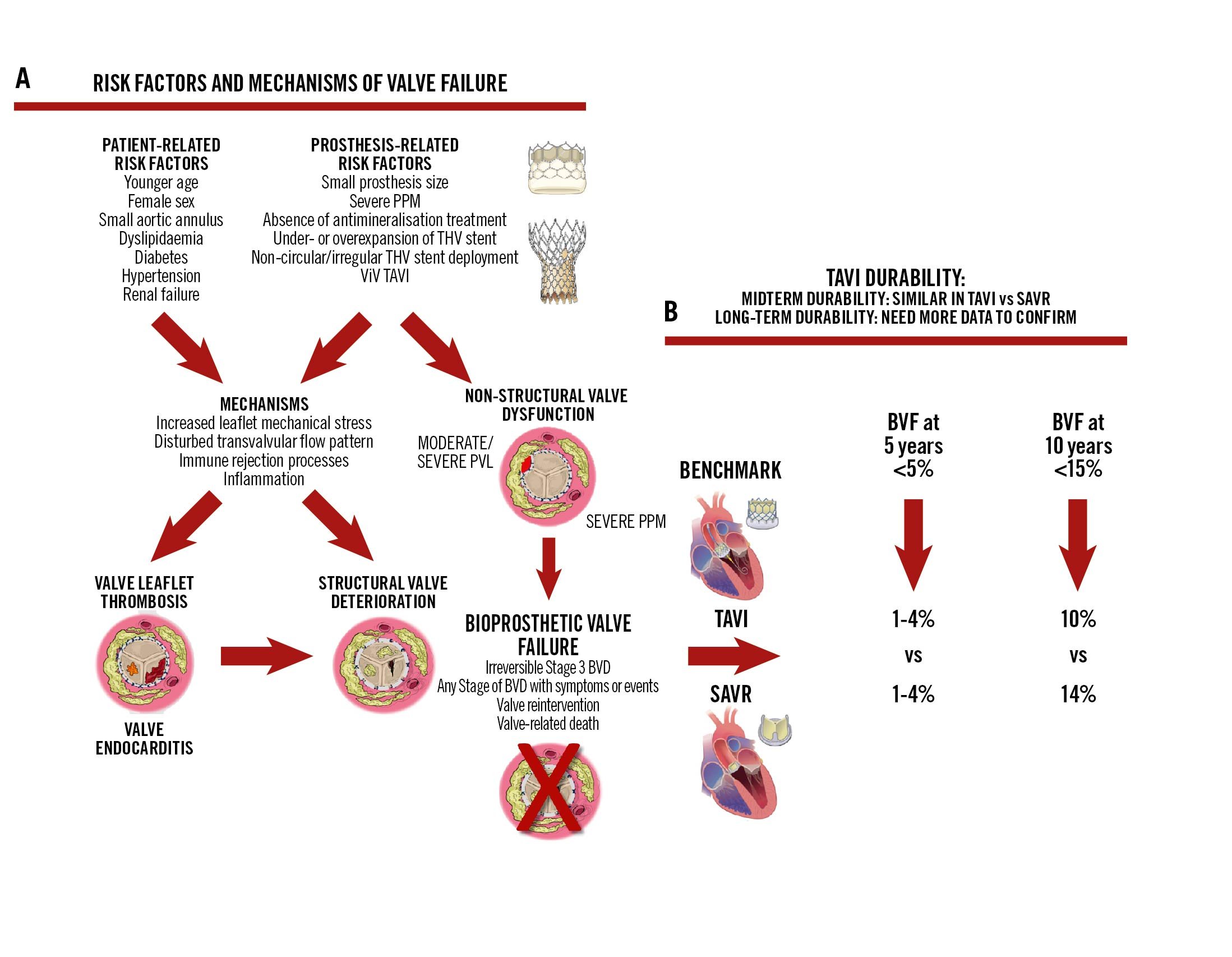
Central illustration. Durability of TAVI. The risk factors and mechanisms of valve failure are represented in (A), while (B) presents the data concerning mid- and long-term TAVI durability. BVD: bioprosthetic valve dysfunction; BVF: bioprosthetic valve failure; PPM: prosthesis-patient mismatch; PVL: paravalvular leak; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; ViV: valve-in-valve
Definition, diagnosis, and staging of bioprosthetic valve dysfunction and failure
BVD may be related to non-structural dysfunction, which is defined as any abnormality not intrinsic to the valve, resulting in BVD (Figure 1)345. Non-structural BVD includes prosthesis-patient mismatch (PPM) and paravalvular regurgitation. This type of BVD occurs at the time of the TAVI or SAVR procedure and is generally stable during follow-up. Structural BVD, or SVD, is defined as intrinsic permanent changes to the prosthetic valve leaflets, stent or strut resulting in valve stenosis and/or regurgitation (Figure 1). This type of BVD develops progressively during follow-up. Valve thrombosis and endocarditis are classified neither as structural nor as non-structural BVD. Indeed, in some cases, thrombosis or endocarditis may be reversible with pharmacotherapy (anticoagulation or antibiotherapy), whereas in other cases, these BVD may cause permanent damage to the valve leaflets (Figure 1). All 4 of these types of BVD (non-structural BVD, SVD, thrombosis, endocarditis) may ultimately lead to BVF and may thus impair valve durability.
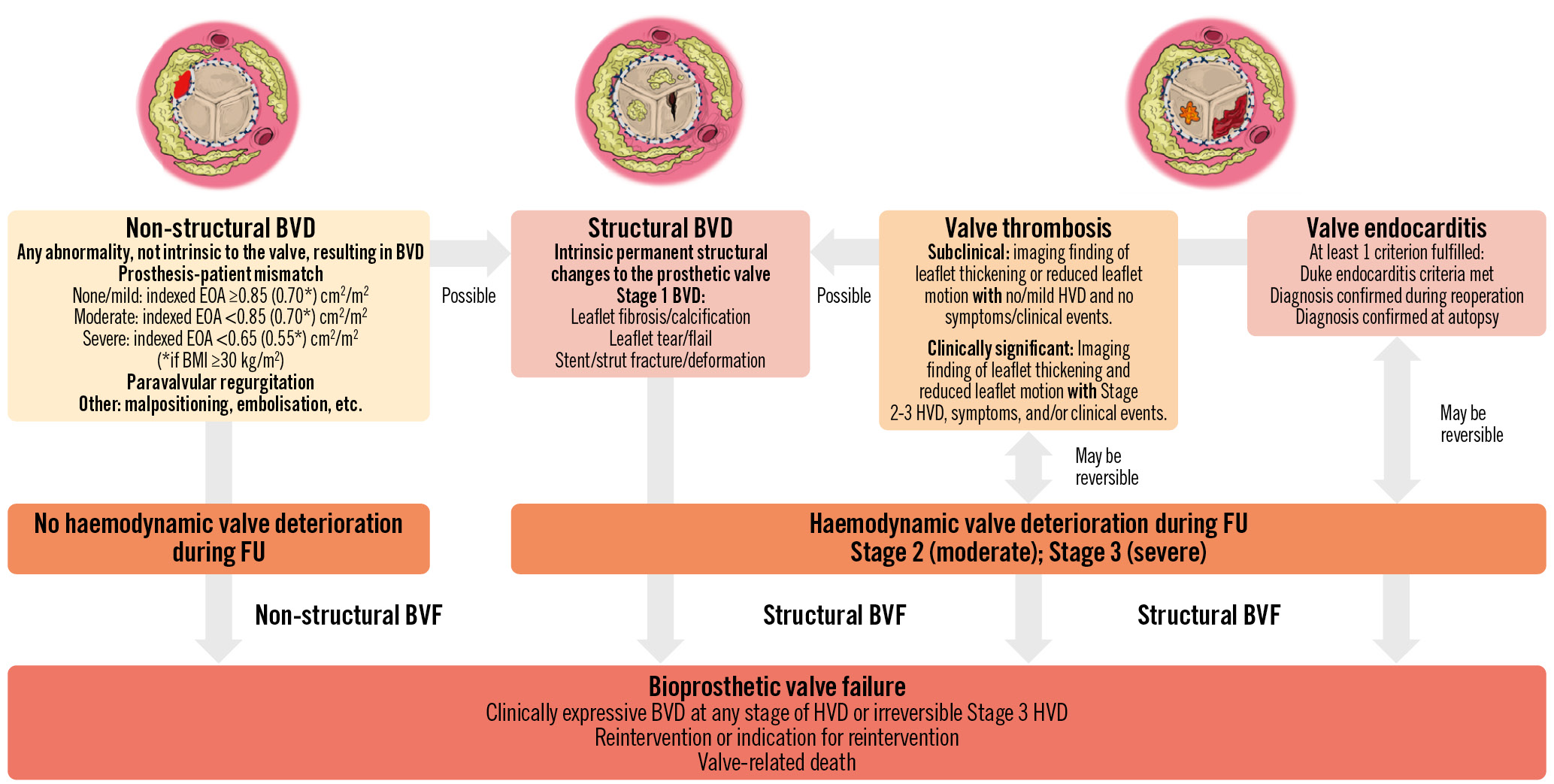
Figure 1. Category, aetiology, and definition of bioprosthetic valve dysfunction and failure. BMI: body mass index; BVD: bioprosthetic valve dysfunction; BVF: bioprosthetic valve failure; EOA: effective orifice area; FU: follow-up; HVD: haemodynamic valve deterioration
DEFINITIONS AND DIAGNOSIS OF BIOPROSTHETIC VALVE DYSFUNCTION
Historically, SVD has been defined by the Society of Thoracic Surgeons as the occurrence of aortic valve reintervention or valve-related death, and the vast majority of the SAVR durability data are based on this endpoint. However, this definition markedly underestimates the true incidence of SVD, because several patients with significant SVD may not be referred for reintervention. Indeed, in many cases, SVD is not detected or is underestimated, because the patients are considered too old or too high risk to undergo reintervention5. Furthermore, in this elderly population with multiple comorbidities, the adjudication of the cause of death may be difficult and may miss or underestimate the contribution of SVD to the fatal event. For these reasons, the European Association of Percutaneous Cardiovascular Interventions (EAPCI)/European Association for Cardio-Thoracic Surgery (EACTS) and the Valve Academic Research Consortium 3 (VARC-3) have proposed new standardised definitions based on the identification and staging of structural and haemodynamic valve deterioration (HVD) at Doppler echocardiographic follow-up (Figure 1)345.
The VARC-34 and the Heart Valve Collaboratory5 have proposed a 4-step algorithm to assess the presence, type, severity (stage) and clinical consequences of BVD (Figure 2). Step 1 is to identify “red flags” of BVD on clinical examination (new onset of symptoms) or transthoracic echocardiography (TTE): i.e., high transvalvular gradient, small valve effective orifice area (EOA) or low Doppler velocity index (DVI), or new onset of intraprosthetic aortic regurgitation (Table 1). Step 2 is to confirm the presence and aetiology of BVD by assessing valve leaflet morphology and mobility. Non-structural BVD, such as PPM, is characterised by normal valve leaflet morphology and mobility, whereas the other types of BVD imply the presence of valve leaflet abnormalities. Hence, this assessment of leaflet morphology and mobility is key for determining the type and aetiology of BVD. However, this step may be difficult to achieve by TTE only and often requires the use of multimodality imaging including transoesophageal echocardiography (TOE), cardiac computed tomography (CT), and positron emission tomography-CT (PET-CT) (Figure 2, Table 2)5. Contrast-enhanced CT is helpful to assess the presence of hypoattenuated leaflet thickening (HALT) and reduced leaflet mobility (RLM), which are markers of valve leaflet thrombosis, whereas non-contrast CT may be used to detect and quantitate valve leaflet calcification, a marker of SVD (Table 2, Figure 3)678. The morphological and haemodynamic valve abnormalities associated with infective endocarditis are often less obvious and more insidious in TAVI patients than in SAVR patients. The TTE findings are indeed often limited to mild thickening of leaflets with some degree of obstruction and an ensuing moderate increase in transvalvular gradient. A clinical or TTE suspicion of endocarditis should prompt a more comprehensive assessment, including blood culture, TOE, contrast CT and PET-CT imaging (Table 2). Several types of BVD may coexist or occur sequentially, which may render the differential diagnosis more difficult. For example, patients may present with both severe PPM and structural BVD.
Table 1. Red flags for suspicion of bioprosthetic valve dysfunction.
| Reduced or excessive leaflet mobility |
| Leaflet thickening |
| Colour Doppler of transvalvular flow shows systolic restriction (paucity) |
| Mean transvalvular gradient ≥20 mmHg (≥30 mmHg)* |
| Increase in mean gradient ≥10 mmHg (≥20 mmHg)* during follow-up |
| Valve effective orifice area <1.1 cm2 (<0.8 cm2)* |
| Doppler velocity index <0.35 (<0.25)* |
| Acceleration time/LV ejection time ratio >0.32 (>0.37)* |
| New onset or worsening of intraprosthetic aortic regurgitation ≥mild |
| New onset or worsening of symptoms or heart failure |
| *Cutoff associated with a higher level of suspicion of bioprosthetic valve dysfunction. LV: left ventricular |
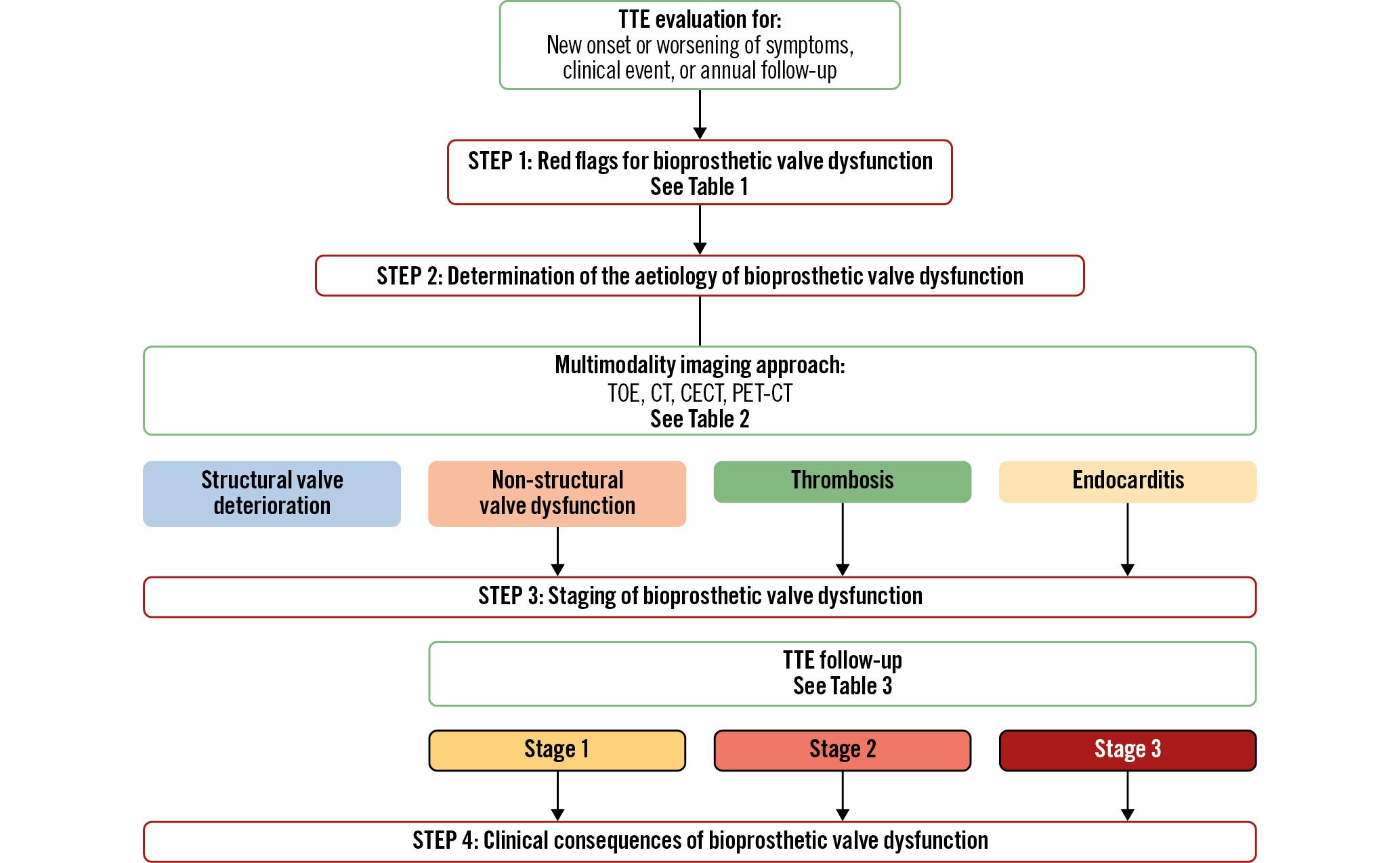
Figure 2. Four-step algorithm for the determination of the presence, aetiology, stage, and clinical consequences of bioprosthetic valve dysfunction. CT: computed tomography; CECT: contrast-enhanced CT; FU: follow-up; PET: positron emission tomography; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography
Table 2. Multimodality imaging of morphological abnormalities of valve leaflets or stent for determination of the type of bioprosthetic valve dysfunction.
| Imaging modality | Prosthesis-patient mismatch | Valve thrombosis | Pannus | Valve endocarditis | Structural valve deterioration |
|---|---|---|---|---|---|
| TTE/TOE | Normal valve leaflet morphology and mobility | Diffuse or focal hypoechogenic leaflet thickening (>2 mm) of at least 1 leaflet | Dense fixed hyperechogenic tissue involving periannular region or sewing ring | Presence of vegetation(s)Valve leaflet thickening | Diffuse or focal hyperechogenic leaflet thickening (>2 mm) of at least 1 leaflet |
| Normal or reduced leaflet mobility | Normal leaflet morphology | Possible torn/avulsed/perforated leaflets or reduced leaflet mobility | Reduced mobility and/or torn/avulsed/perforated leaflets | ||
| Paucity (restriction) of colour Doppler transvalvular flow | Leaflet mobility may be normal or abnormal | Paravalvular complications: abscess, pseudoaneurysm, fistula, dehiscence | Paucity (restriction) of colour Doppler transvalvular flow | ||
| Multidetector CT | |||||
| Non-contrast CT | No leaflet calcification | No leaflet calcification | No leaflet calcification | No leaflet calcification | Leaflet calcification |
| Contrast-enhanced CT | Normal leaflet morphology | Hypoattenuated leaflet thickening (HALT)Hypoattenuation affecting leaflet motion (HAM) (possible) | Hypodense semicircular or circular structure along and beneath the valve ring/stent | Paravalvular complications: vegetations, abscess, pseudoaneurysm, fistula, dehiscence | Calcific or non-calcific hyperdense leaflet thickening affecting leaflet motion |
| 4D contrast-enhanced CT | Normal leaflet mobility | Reduced leaflet motion (RLM) (possible) | Presence of vegetation(s)Valve leaflet thickening | Reduced leaflet motion (possible) | |
| Nuclear imaging | |||||
| 18F-NaF PET/CT | No 18F-NaF uptake at the level of the bioprosthetic valve leaflets* | Increased 18F-NaF uptake at the level of the bioprosthetic valve leaflets (possible)* | Unknown | Increased 18F-NaF uptake at the level of the bioprosthetic valve leaflets (possible) | Increased 18F-NaF uptake at the level of the bioprosthetic valve leaflets (possible)* |
| 18F-FDG PET/CT | No increased 18F-FDG uptake at the level of the valve or paravalvular region* | Unknown | Unknown | Increased 18F-FDG uptake at the level of the bioprosthetic valve and/or paravalvular region | No increased 18F-FDG uptake at the level of the bioprosthetic valve or paravalvular region* |
| Adapted with permission5. *For research use. 18F-FDG: 18F-fluorodeoxyglucose; 18F-NaF: 18F-sodium chloride; 4D: four-dimensional; CT: computed tomography; PET: positron emission tomography; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography | |||||
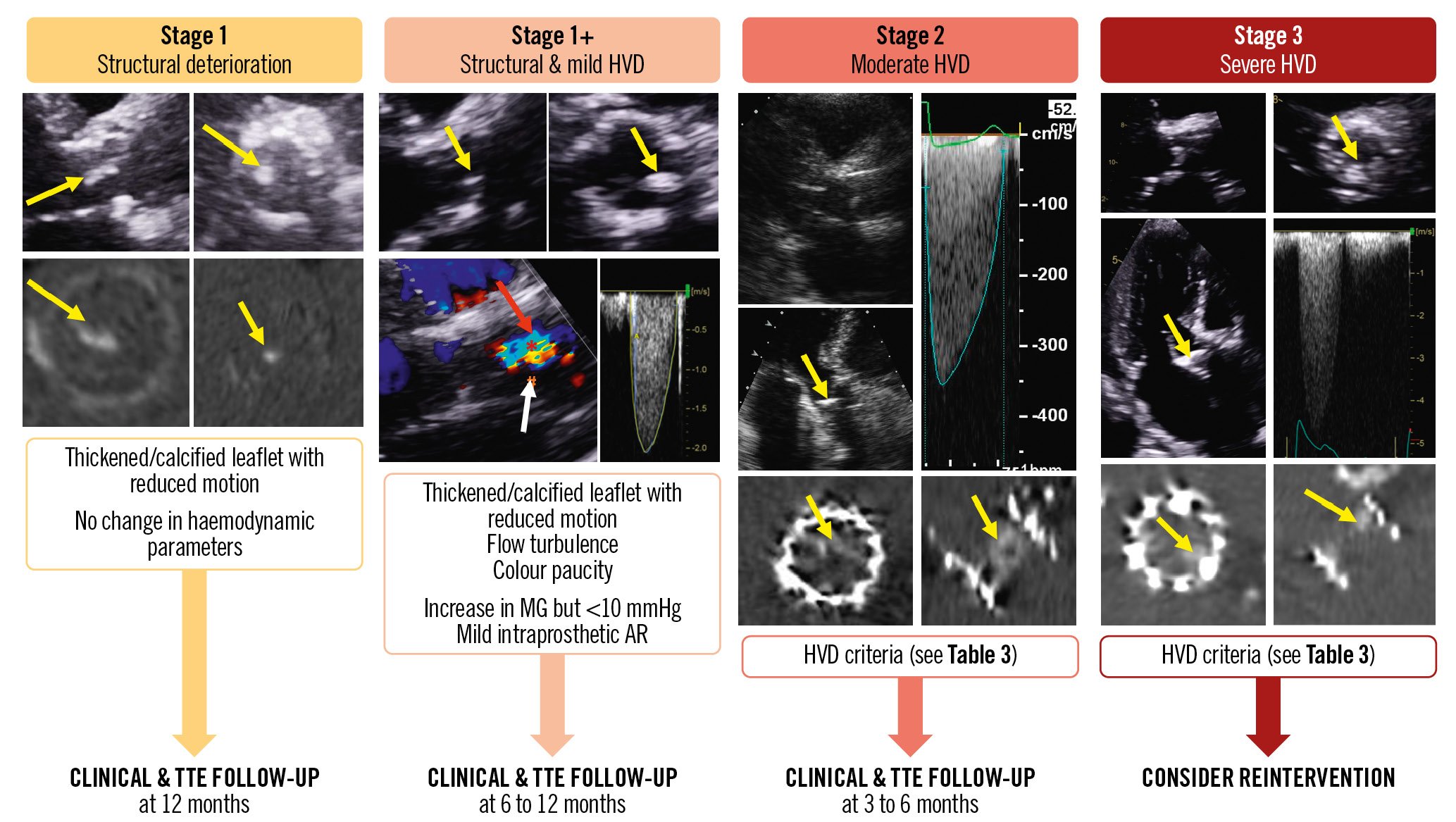
Figure 3. Representative examples of the different stages of bioprosthetic valve dysfunction and proposed management. Yellow arrows indicate valve thickening and reduced motion; red arrow: colour Doppler flow restriction and turbulence; white arrow: colour Doppler flow paucity. AR: aortic regurgitation; HVD: haemodynamic valve deterioration; MG: mean gradient; TTE: transthoracic echocardiography
STAGING OF BIOPROSTHETIC VALVE DYSFUNCTION
Step 3 of the algorithm presented in Figure 2 consists of staging the severity of the structural and haemodynamic valve deterioration (Table 3, Figure 3). This staging classification is based on the presence and magnitude of changes in the leaflet morphology and valve haemodynamic performance at TTE follow-up. It can be applied to BVD related to SVD, valve thrombosis, or valve endocarditis but not to non-structural BVD, such as PPM, where the BVD is already present at the outset of the TAVI or SAVR procedure and generally does not change during follow-up.
Stage 1: SVD is defined as the occurrence of abnormalities of valve leaflet morphology (leaflet thickening, fibrocalcific remodelling, tear or prolapse) and mobility during follow-up (Table 3, Figure 3)45. CT valve leaflet calcium density (calcium score/aortic annulus area) can be used as an early and sensitive marker for Stage 1 valve deterioration, and a density >58 Agatston Units (AU)/cm2 has been shown to be a powerful predictor of outcomes8. Stage 2: moderate HVD is defined as the presence of Stage 1 plus a moderate increase in the mean gradient from early postprocedural TTE to follow-up TTE with a concomitant decrease in EOA and DVI and/or new onset or worsening of intraprosthetic aortic regurgitation. Stage 3: severe HVD is defined with the same parameters as for Stage 2 but with more severe criteria.
The main difference between the EAPCI/EACTS and the VARC-3 standardised definitions34 is that the former considers that structural BVD is present when there is either (i) a high gradient at any echocardiography after aortic valve replacement (AVR) or (ii) an increase in gradient during follow-up, whereas the latter requires that both criteria are met (Table 3, Figure 3). The major disadvantage of the EAPCI/EACTS definition is that it markedly overestimates the actual incidence of structural BVD because, according to the first criterion, it may also include several cases of PPM, which is a non-structural BVD. Hence, if this definition is applied to compare valve durability in treatment groups that have different rates of PPM (e.g., TAVI vs SAVR), this may lead to the false conclusion that the incidence of structural BVD or BVF differs between the groups (i.e., higher in the group with more PPM) whereas in fact this difference is only explained by the difference in PPM. On the other hand, the advantage of the EAPCI/EACTS definition is that it can also be useful for detecting risk factors for structural BVD and BVF, i.e., severe PPM and high residual gradient.
Table 3. Staging of bioprosthetic valve dysfunction and failure.
| Parameters | STAGE 0 No structural/ haemodynamic valve deterioration | STAGE 1 Structural valve deterioration | STAGE 2 Moderate haemodynamic valve deterioration | STAGE 3 Severe haemodynamic valve deterioration |
|---|---|---|---|---|
| Assessment of structural valve deterioration by TTE, TOE, and/or CT | ||||
| Thickening ≥2 mm | Absent | Present | Present | Present |
| Calcifications on TTE, TOE or CT(CT calcium density >58 AU/cm2) | Absent | Often present | Often present | Generally present |
| Reduced mobility | Absent | Absent or mild | Mild to moderate | Moderate to severe |
| Tear/prolapse/avulsion | Absent | Absent | Possible | Possible |
| Assessment of haemodynamic valve deterioration by TTE | ||||
| HVD criteria | ||||
| 1 – Mean gradient increase during FU | <10 mmHg | <10 mmHg | 10 to 19 mmHg | ≥20 mmHg |
| 2 – Final mean gradient at last FU | <20 mmHg | <20 mmHg | 20 to 29 mmHg | ≥30 mmHg |
| 3 – AVA decrease during FU | <0.3 cm2 | <0.3 cm2 | 0.3 to 0.6 cm2 | ≥0.6 cm2 |
| 4 – DVI decrease during FU | <10% | <10% | 10 to 20% | ≥20% |
| 5 – Intraprosthetic AR increase | Absent | Absent | ≥1 grade | ≥2 grades |
| 6 – Final intraprosthetic AR | ≤Mild | ≤Mild | Moderate | Severe |
| Diagnosis of BVD according to: | ||||
| VARC-3 criteria | HVD criteria 1 and 2 combined with criteria 3 or 4 and/or criteria 5 and 6 | |||
| EAPCI/EACTS criteria | HVD criteria 1 or 2 and/or criteria 5 or 6 | |||
| Clinical consequences | ||||
| Symptoms | Absent | Absent | Often absent | Often present |
| LV remodelling/dysfunction | Absent | Absent | Variable | Usually present |
| Pulmonary hypertension | Absent | Absent | Variable | Usually present |
| Bioprosthetic valve failure | ||||
| Criterion 1a | Any BVD with clinically expressive criteria (new onset or worsening symptoms, LV dilation/hypertrophy/dysfunction, or pulmonary hypertension) | |||
| Criterion 1a | Irreversible Stage 3 BVD with confirmatory imaging of leaflet/stent abnormalities and/or confirmatory invasive assessment of BVD | |||
| Criterion 2 | Aortic valve reintervention or indication for reintervention | |||
| Criterion 3 | Valve-related death | |||
| AR: aortic regurgitation; AU: Agatston Unit; AVA: aortic valve area; BVD: bioprosthetic valve dysfunction; CT: computed tomography; DVI: Doppler velocity index; EACTS: European Association of Cardio-Thoracic Surgery; EAPCI: European Association of Percutaneous Cardiovascular Interventions; FU: follow-up; HVD: haemodynamic valve deterioration; LV: left ventricular; THV: transcatheter heart valve; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography; VARC-3: Valve Academic Research Consortium 3 | ||||
DEFINITIONS OF BIOPROSTHETIC VALVE FAILURE
All types of BVD, even if only mild or moderate, may lead to BVF and thus impair valve durability (Figure 1)345. According to VARC-3, BVF is defined and classified as follows (Table 3): criterion 1: (a) mild (Stage 1) SVD or moderate (Stage 2) or severe (Stage 3) HVD associated with symptoms or signs of heart failure; (b) irreversible severe (Stage 3) HVD, even if not associated with symptoms or heart failure; criterion 2: valve reintervention or indication for reintervention; criterion 3: valve-related death. Also, it is important to report not only structural BVF but also all-cause BVF, given that non-structural BVD as well as valve thrombosis and endocarditis may lead to BVF and thus impair valve durability. All-cause BVF is therefore the most important and accurate endpoint to assess valve durability, and an incidence of BVF <5% at 5 years and <15% at 10 years appears to be a reasonable benchmark for biological SAVR or TAVI9 (Central illustration).
Aetiology, mechanisms, and risk factors of structural valve deterioration
SVD generally consists of bioprosthetic valve leaflet thickening and stiffening due to fibrocalcific remodelling of leaflet tissues and/or leaflet tear, perforation, prolapse or flail. Approximately 30% of surgical bioprosthetic valves that fail because of SVD exhibit no leaflet calcification10. BVD was categorised as stenosis in 40%, regurgitation in 30% and mixed dysfunction in 30% of failed surgical bioprostheses11. This distribution appears to be different in failed transcatheter heart valves (THVs), where >80% present with predominant stenosis12.
RISK FACTORS
The risk factors of SVD include patient-related and prosthesis-related factors. The patient-related risk factors of SVD after SAVR or TAVI are younger age, female sex, small annulus size, atrial fibrillation (AF), diabetes, metabolic syndrome, hypercholesterolaemia, hypertension, end-stage renal disease, hyperparathyroidism, pregnancy, and elevated circulating levels of the following: apolipoprotein B and apolipoprotein B/apolipoprotein A ratio, remnant cholesterol, non-high-density lipoprotein cholesterol, lipoprotein-associated phospholipase A2, proprotein convertase subtilisin/hexin type 9 (PCSK9), and calcium-phosphorus product (Figure 4, Central illustration)1213141516. The main risk factors that have been associated with SVD following TAVI specifically are younger age, female sex, larger body surface area, diabetes, hypertension, and AF1217. The association with AF may be related to the fact that this risk factor may predispose a patient to valve leaflet thrombosis, which in turn may increase the risk of accelerated SVD. AF is a well-established risk factor for thrombotic and thromboembolic complications. However, in TAVI series, the published data on the association between AF and valve leaflet thrombosis have been conflicting, with several studies reporting a protective rather than a detrimental effect of AF1819 This is most likely related to the fact that patients with known AF are generally anticoagulated and thus protected against valve thrombosis. On the other hand, a substantial proportion of patients with AF are not detected and are thus not anticoagulated, and approximately 50% of the patients with known AF are not adequately treated; these patients may be at increased risk for valve leaflet thrombosis.
Prosthesis-related factors that may accelerate SVD include small bioprosthesis size, severe PPM, high residual transprosthetic pressure gradients, absence of preimplant antimineralisation treatment of the bioprosthetic valve tissues, and some specific bioprosthetic valve designs/models such as surgical bioprosthetic valves with bovine pericardial leaflets mounted outside of the stent (e.g., Mitroflow [Sorin], Trifecta [Abbott]) (Figure 4)6202122. PPM is defined as an EOA of a normally functioning prosthesis that is too small for the patient’s body size. According to the VARC-3 definition4, PPM is not clinically significant (i.e., mild or no PPM) if the indexed EOA (EOAi) is>0.85 cm2/m2 (or>0.70 cm2/m2 if the patient is obese: body mass index ≥30 kg/m2), moderate if it is>0.65 cm2/m2 but ≤0.85 cm2/m2 (or>0.55 cm2/m2 but ≤0.70 cm2/m2 if obese), and severe if it is ≤0.65 cm2/m2 (or ≤0.55 cm2/m2 if obese)4. The main haemodynamic consequence of PPM is the persistence of high residual transprosthetic gradients, which is defined in VARC-3 as a mean gradient >20 mmHg.
TAVI-specific risk factors, such as (i) leaflet injury at the time of the procedure by crimping, loading or balloon post-dilatation, (ii) under- or overexpansion of the THV stent, and (iii) non-circular or irregular stent deployment, may also contribute to the risk of valve thrombosis and of accelerated SVD (Figure 4, Central illustration)2324252627282930313233.
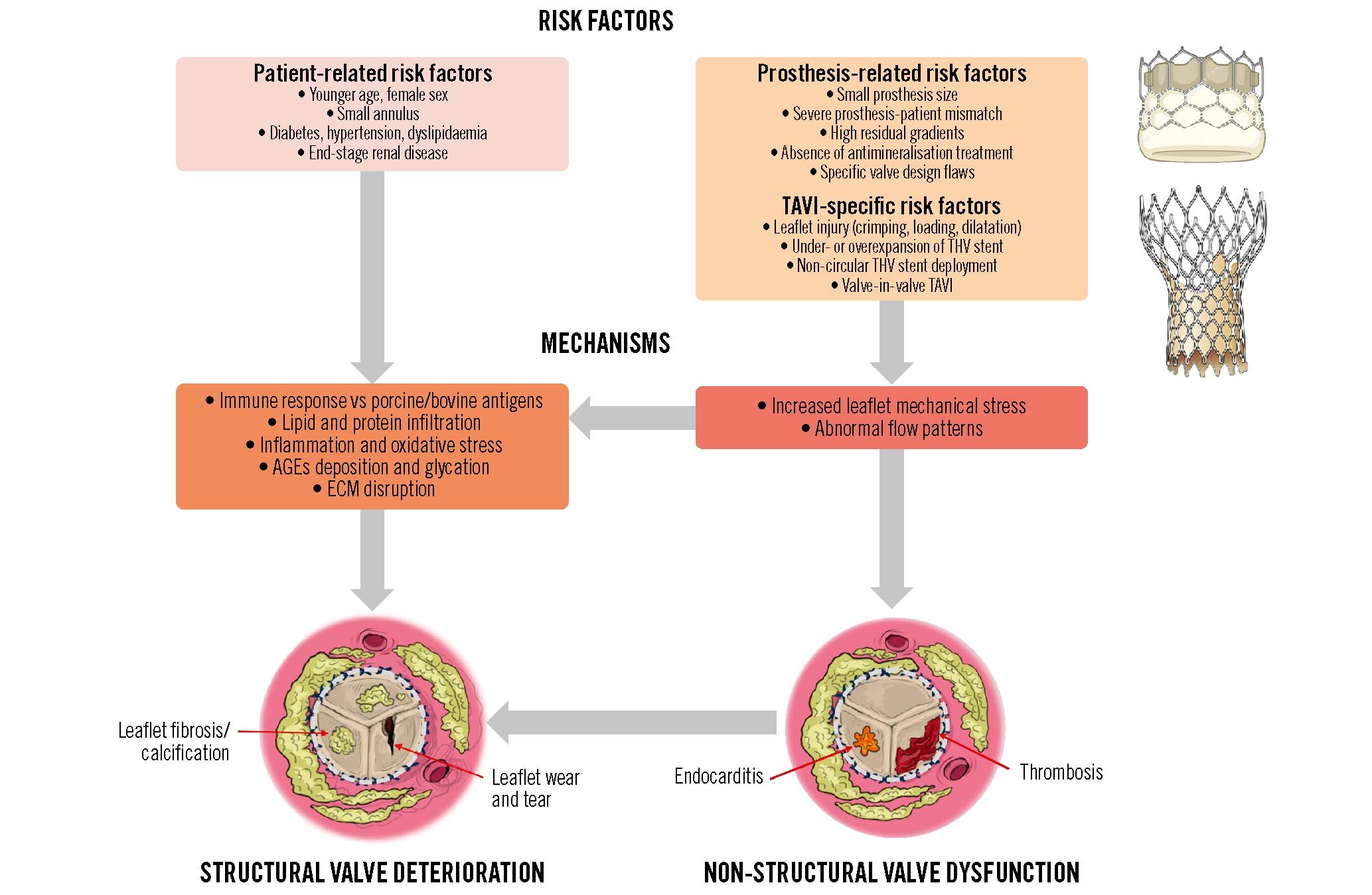
Figure 4. Risk factors and mechanisms of structural valve deterioration and failure. AGEs: advanced glycation end products; ECM: extracellular matrix; TAVI; transcatheter aortic valve implantation; THV: transcatheter heart valve
MECHANISMS
SVD is believed to result from repetitive leaflet mechanical stress and wear and tear processes leading to fibrocalcific degeneration of the leaflet tissues. Inflammation triggered by low-grade immune rejection, oxidised lipids, or thrombosis may also cause fibrocalcific remodelling and disruption of leaflet tissues (Figure 4)34. Bioprosthetic valve tissues are crosslinked with glutaraldehyde to reduce their antigenicity and ensure chemical stabilisation. Despite this fixation process, bioprosthetic valves may not be completely “immunologically inert”, and residual animal antigens could trigger humoral and cellular immune responses leading to tissue degeneration35. A more robust immune system might also explain the more rapid SVD reported in younger patients. Moreover, the preimplant treatment of bioprosthetic valve tissues with glutaraldehyde may induce a calcium influx due to membrane damage, which, combined with the residual phospholipids of the membranes, provides an environment prone to calcium crystal nucleation36. In addition, several patient-related factors, such as lipid-mediated inflammation, the deposition of advanced glycation end products (AGEs) and serum albumin, glycation processes, and the dysregulation of phosphocalcific metabolism, contribute to the growth of calcium crystal and may cause disruption of the extracellular matrix373839.
Among prosthesis-related factors, increased leaflet mechanical stress is a key determinant of SVD and predisposes to the fibrocalcific remodelling and disruption of leaflet tissues. Patients with small bioprostheses, stent deformation, severe PPM or high residual gradients are at higher risk of faster SVD, likely because of increased leaflet mechanical stress and disturbance of the transprosthetic flow pattern (Figure 4)640. Patients undergoing a valve-in-valve TAVI procedure within failed surgical bioprostheses generally have severe PPM and a high residual gradient and may thus be predisposed to both valve thrombosis and SVD. Mechanically induced structural damage to the THV leaflets due to crimping, loading, or balloon post-dilatation during the TAVI procedure may also account for bioprosthetic valve degeneration by enhancing plasma molecules and blood cellular component infiltration and enzymatic degradation by opening pathways within the chemically treated tissue matrix10.
INTERACTION BETWEEN NON-STRUCTURAL BVD, THROMBOSIS, ENDOCARDITIS, AND SVD
By disturbing the valve leaflet kinetics and the transvalvular flow pattern, non-structural BVD such as severe PPM, and possibly paravalvular regurgitation, may predispose patients to valve thrombosis and SVD (Figure 1, Figure 4, Central illustration)41. Subclinical leaflet thrombosis as documented by HALT on contrast-enhanced CT may occur in ~20% (15 to 32%) of patients following TAVI (Table 2)1842. Valve thrombosis occurs predominantly during the first 2 years post-AVR, with the initial 3- to 6-month period being at highest risk43, but may also occur later during follow-up118. Although subclinical thrombosis can resolve spontaneously in 50% of cases and clinical thrombosis is reversible with anticoagulation therapy in the majority of cases, this complication could trigger leaflet tissue inflammation and subsequently lead to leaflet fibrosis and then calcification (Figure 4, Figure 5)4445. Similarly, infective endocarditis, even if treated successfully with antibiotherapy, could result in accelerated SVD. These findings support the concept that the occurrence of non-SVD complications such as thrombosis and endocarditis could accelerate SVD and BVF (Figure 4).
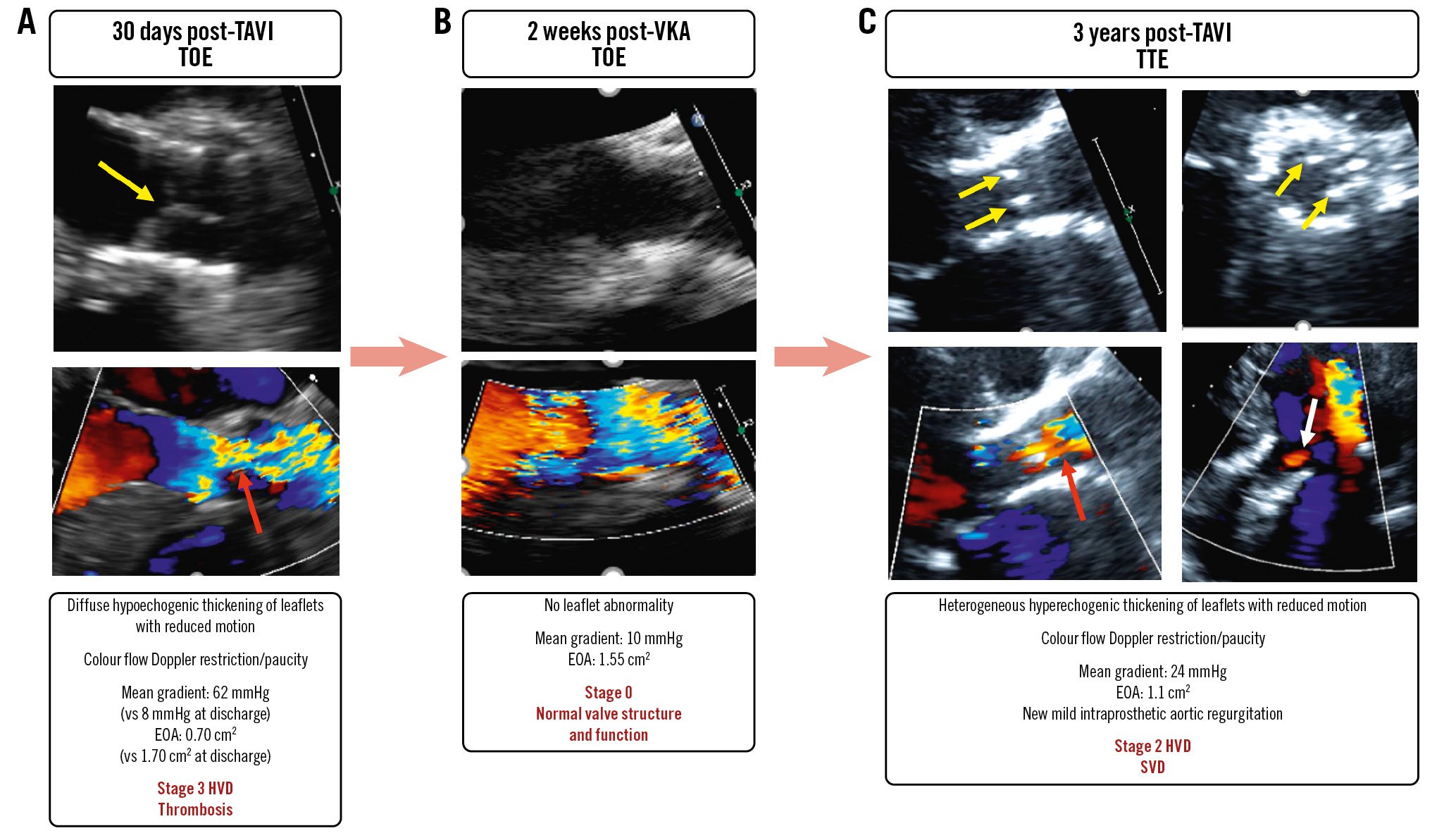
Figure 5. A case of early valve thrombosis followed by accelerated structural valve deterioration. A female patient aged 73 years underwent TAVI with a SAPIEN XT 23 mm. At 30 days, she presented with clinical valve thrombosis resulting in severe (Stage 3 HVD) bioprosthetic valve stenosis (A). There was a quasi-complete resolution of the valve thrombosis and stenosis following 2 weeks of VKA treatment (B). However, 3 years later there was evidence of structural valve deterioration resulting in moderate (Stage 2 HVD) bioprosthetic valve stenosis (C). Yellow arrows indicate valve thickening and reduced motion; red arrows: colour Doppler flow restriction/paucity; white arrow: intraprosthetic aortic regurgitation (AR). EOA: effective orifice area; HVD: haemodynamic valve deterioration; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography; VKA: vitamin K antagonist
Durability of TAVI versus SAVR
Until recently, the durability of surgical bioprostheses has been essentially based on the incidence of reintervention, which grossly underestimates the actual incidence of SVD46. The reported rates of reintervention are low: <7% at 10 years and <15% at 20 years, with some substantial differences depending on the patient’s age and the design/generation/model of the bioprosthetic valve implanted474849. The new standardised definitions of SVD that have recently been proposed (Figure 1, Table 3) allow for a more accurate estimation of the true incidence of SVD following SAVR or TAVI.
VALVE DURABILITY FOLLOWING TAVI
Table 4 summarises the valve durability data following TAVI; these data essentially come from single- or multicentre registries and from randomised controlled trials (RCTs) and continued access registries. The incidence of Stage 2 or 3 (i.e., moderate or severe) SVD is between 3.6% and 15.8% at 5- to 6-year follow-up and between 4.6% and 14.9% at 7 to 8 years when using the EAPCI/EACTS definition, between 1.8% and 9.5% at 5 years and 13.9% at 10 years when using the VARC-3 definition (Table 4). The incidence of BVF related to SVD is between 1.6% and 3.3% at 5 to 6 years, between 1.8% and 3.7% at 7 to 8 years and 9.7% at 10 years. And finally, the incidence of all-cause BVF (i.e., related to SVD, PPM, paravalvular regurgitation, thrombosis, or endocarditis), which is the most important endpoint with regard to valve durability, is between 1.1% and 3.7% at 5 to 6 years, between 2.5% and 4.5% at 7 to 8 years, and between 2.6% and 9.7% at 10 years, depending on the studies (Table 4).
Table 4. TAVI durability data.
| Study | Study design (number of patients) | Surgical risk (mean age) | Definition of BVD/BVF | Type of AVR | Stage 2-3 SVD-related BVD (Stage 3 SVD) | SVD-related BVF (all-cause BVF) | Duration of follow-up |
|---|---|---|---|---|---|---|---|
| Barbanti et al892015 | Multicentre registry (n=353) | High risk (81.5 y) | VARC-1 | TAVI-SEV | 4.2% | 1.4% | 5 years |
| FRANCE 2 RegistryDidier et al522018 | Multicentre registry (n=4,210) | High risk (83 y) | EAPCI/EACTS | TAVITAVI-BEVTAVI-SEV | 13.3% (2.5%) (2.2%) (1.8%) | Not reported | 5 years |
| CoreValve HRGleason et al902018 | RCT (n=391) | High risk (83 y) | EAPCI/EACTS | TAVI-SEVSAVR | 9.5%26.6% | 1.7% (3.0%) 0.8% (1.1%) | 5 years |
| Orvin et al912019 | Multicentre registry (n=450) | High risk (82 y) | EAPCI/EACTS | TAVI-BEV | 12.3% | 3.3% | 5.6 years |
| Deutsch et al922018 | Single-centre registry (n=300) | High risk (81 y) | EAPCI/EACTS | TAVI-BEV | 14.9% | 3.7% | 7 years |
| Eltchaninoff et al1012018 | Single-centre registry (n=378) | High risk (83 y) | EAPCI/EACTS | TAVI-BEV/SEV | 12.8% | 3.4% (3.4%) | 8 years |
| Holy et al932018 | Single-centre registry (n=152) | High risk (81 y) | EAPCI/EACTS | TAVI-SEV | 0% | 0% (4.5%) | 8 years |
| Barbanti et al1022018 | Single-centre registry (n=288) | High risk (81 y) | EAPCI/EACTS | TAVI-BEV | 8.26% | 4.51% | 8 years |
| Panico et al942019 | Single-centre registry (n=278) | High risk (82 y) | EAPCI/EACTS | TAVI-SEV | 3.6% | 2.5% | 6.8 years |
| UK TAVIBlackman et al952019 | Multicentre registry (n=241) | High risk (79 y) | EAPCI/EACTS | TAVI-BEV/SEV | 9.1% | Not reported | 6 years |
| Durand et al962019 | Multicentre registry (n=1,403) | High risk (83 y) | EAPCI | TAVI-BEV | 10.9% | 1.9% (1.9%) | 7 years |
| PARTNER II-S3iPibarot et al172020 | RCT & registry (n=1,665) | Intermediate risk (82 y) | VARC-3 | TAVI-SAPIEN XTTAVI-SAPIEN 3SAVR | 9.5% 3.9% 3.5% | 3.7% (4.7%) 1.1% (2.6%) 0.8% (1.3%) | 5 years |
| Ferreira-Neto et al1032020 | Single-centre registry (n=212) | High risk (80 y) | VARC-3 | TAVI-BEV | 30.2% | 9.3% | 8 years |
| Murray et al972020 | Single-centre registry (n=452) | High risk (80 y) | EAPCI/EACTS | TAVI-BEV/SEV | 10.2% | 3.8% (3.8%) | 7 years |
| Testa et al1042020 | Single-centre registry (n=990) | High risk (82 y) | EAPCI/EACTS | TAVI-SEV | 4.6% | 2.5% (2.5%) | 8 years |
| CHOICEAbdel-Wahab et al512020 | RCT (n=241) | High risk (82 y) | EAPCI/EACTS | TAVI-SAPIEN XTTAVI-CoreValve | 6.6%0.0% | 4.1%3.4% | 5 years |
| NOTIONJorgensen et al982021 | RCT(n=145) | Low risk (79 y) | EAPCI/EACTS | TAVI-SEVSAVR | 13.9%28.3% | 8.7%10.5% | 8 years |
| Sathananthan et al1052021 | Single-centre registry(n=235) | High risk (82 y) | EAPCI/EACTS | TAVI-BEV | 6.5% | 2.6% | 10 years |
| Stehli et al992023 | Multicentre registry(n=693) | High/int risk (83 y) | EAPCI/EACTS | TAVI-BEV/SEV | 5.3% | 1.8% | 8 years |
| UK TAVIAli et al532023 | Multicentre registry(n=221) | High/int risk (79 y) | EAPCI/EACTS | TAVI-BEVTAVI-SEV | 22.4%9.8% | 4.5% 1.4% | 7 years |
| O’Hair et al122023 | RCT & registry(n=4,762) | High/int risk (83 y) | VARC-3 | TAVI-SEVSAVR | 1.8%2.7% | Not reported | 5 years |
| NOTIONThyregod et al542024 | RCT(n=280) | Low risk (79 y) | VARC-3 | TAVI-SEVSAVR | 12.5%13.9% | (9.7%)(13.8%) | 10 years |
| PARTNER 3Mack et al12023 | RCT(n=280) | Low risk (79 y) | VARC-3 | TAVI-SAPIEN 3SAVR | 4.2%3.8% | 1.6% (3.3%) 2.4% (3.8%) | 5 years |
| AVR: aortic valve replacement; BEV: balloon-expandable valve; BVD: bioprosthetic valve dysfunction; BVF: bioprosthetic valve failure; CoreValve HR: Medtronic CoreValve U.S. Pivotal Trial; EACTS: European Association for Cardio-Thoracic Surgery; EAPCI: European Association of Percutaneous Cardiovascular Interventions; int: intermediate; RCT: randomised controlled trial; SAVR: surgical aortic valve replacement; SEV: self-expanding valve; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation; VARC: Valve Academic Research Consortium; y: years | |||||||
VALVE DURABILITY IN BALLOON-EXPANDABLE VERSUS SELF-EXPANDING TAVI
Some in vitro studies performed in a pulse duplicator suggest that leaflet mechanical stress is higher in balloon-expandable valves (BEVs) versus supra-annular self-expanding valves (SEVs)50. In addition, BEVs generally exhibit somewhat more frequent severe PPM and ensuing high residual gradients, which have been associated with a higher risk of SVD following SAVR. It is still uncertain whether these differences in the haemodynamic and biomechanical behaviour of BEVs versus SEVs will translate into significant differences in the long-term durability of these 2 THV platforms. There are very few clinical studies comparing THV durability in the different TAVI devices and, in particular, in BEVs versus SEVs, and the results of these studies are conflicting (Table 4). In the CHOICE RCT, which compared the first/second generations of SAPIEN (Edwards Lifesciences) versus CoreValve (Medtronic) THVs, the rate of Stage 2 or 3 SVD at 5 years was 6.6% in BEVs versus 0% in SEVs, whereas the rate of all-cause BVF was similar and low in both groups (4.1% vs 3.4%)51. In the FRANCE TAVI registry, the rate of Stage 3 SVD was similar in BEVs versus SEVs (2.2% vs 1.8%)52. In a subanalysis of the UK TAVI registry (n=221) with the first and second generations of THVs, Stage 3 SVD according to the EAPCI/EACTS definition was more frequent in BEVs versus SEVs (11.9% vs 3.5%; p=0.02), but the rate of valve reintervention was not statistically different at 7-year follow-up (4.5% vs 1.4%; p=0.17)53. Large RCTs with long follow-up are thus needed to determine if the valve durability is comparable in BEVs versus SEVs. Two major trials comparing new generations of BEVs versus SEVs are ongoing and will address this objective: the SMART Trial (ClinicalTrials.gov: NCT04722250) and the BEST trial (ClinicalTrials.gov: NCT05454150). Both trials are expected to extend follow-up until 10 years to compare THV durability in BEVs versus SEVs.
VALVE DURABILITY IN TAVI VERSUS SAVR
The analyses of RCTs or the propensity-score matched analyses from registries comparing TAVI versus SAVR revealed reassuring results on the midterm durability of TAVI, which compares favourably with that of SAVR (Table 4). The incidence of Stage 2 or 3 SVD defined by VARC-3 was as follows: (i) PARTNER 2A RCT and SAPIEN 3 intermediate-risk registry (S3IR): 9.5% in TAVI with SAPIEN XT and 3.9% in TAVI with SAPIEN 3 versus 3.5% in SAVR at 5 years17; (ii) pooled analysis of the Medtronic CoreValve U.S. Pivotal Trial (CoreValve HR) and SURTAVI studies: 1.82% in TAVI with CoreValve or Evolut (Medtronic) versus 2.67% in SAVR at 5 years12; (iii) PARTNER 3 RCT: 4.2% in TAVI with SAPIEN 3 versus 3.8% in SAVR at 5 years1; and (iv) NOTION RCT: 12.5% in TAVI with CoreValve versus 13.9% in SAVR at 10 years54 (Figure 6, Table 4). The incidence of all-cause BVF was as follows: (i) PARTNER 2A-S3IR: 4.7% in TAVI with SAPIEN XT, 2.6% in TAVI with SAPIEN 3 versus 1.3% in SAVR at 5 years; (ii) PARTNER 3: 3.3% in TAVI with SAPIEN 3 versus 3.8% in SAVR at 5 years (Figure 6); and (iii) NOTION RCT: 9.7% in TAVI with CoreValve versus 13.8% in SAVR at 10 years54.
Hence, except for the first generations of BEVs, the midterm (up to 7-8 years) durability of TAVI valves is at least as good as that of SAVR valves. There are still very limited data on the long-term (10 years and beyond) durability of THVs. The NOTION RCT is the only trial that has reached this 10-year milestone. Although the results of this trial are very reassuring, no definitive conclusion can be made on the long-term durability of TAVI on the basis of these results, because this study suffered from several limitations, the most important one being a major survivorship bias with only 25% of the patients still being alive at 10 years54. Furthermore, the TAVI arm of the NOTION trial only included first-generation SEVs, and in the SAVR arm, 35% of the valves were Trifecta (Abbott) or Mitroflow (Sorin), which have both been shown to have durability issues. In contrast to other low/intermediate-risk TAVI versus SAVR trials, there was no adjudication of SVD by an independent echocardiography core laboratory. The next RCT to reach 10 years of follow-up will be PARTNER 2A, followed by CoreValve HR and SURTAVI, but these trials that recruited an elderly population with high/intermediate surgical risk will likely have the same limitation as the NOTION trial: a major attrition rate at 10 years. To obtain a more definitive conclusion regarding the long-term durability of TAVI and how it compares with SAVR, we will have to wait for the 10-year results of the PARTNER 3 and Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients (Evolut LR) trials that will be available in approximately 5 years from now. Additionally, there is a need for large real-life registries with long and complete clinical and echocardiographic follow-up to determine the long-term durability of the different TAVI valves.
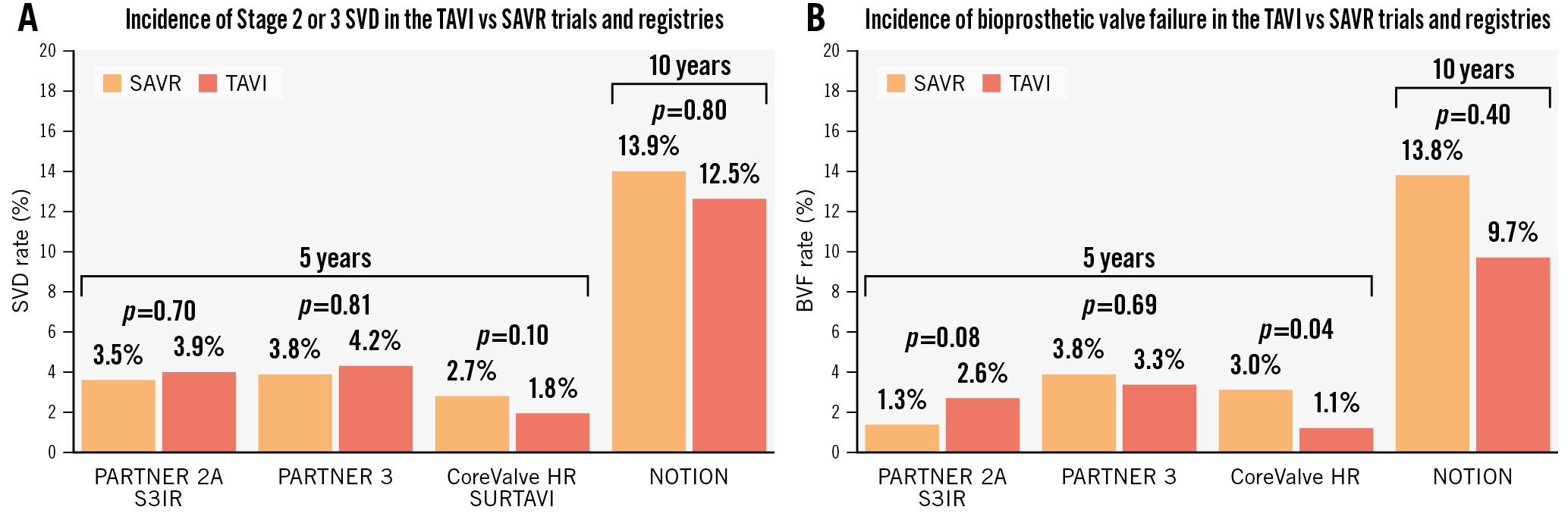
Figure 6. Incidence of Stage 2 or 3 SVD or of all-cause BVF according to VARC-3 in the TAVI versus SAVR RCTs and registries. Incidence of Stage 2 (moderate) or 3 (severe) SVD (A) and of all-cause BVF (B) according to VARC-3 definitions in the PARTNER 2A Trial and S3IR, PARTNER 3 Trial, CoreValve HR trial and SURTAVI Trial at 5 years and in the NOTION trial at 10 years. BVF: bioprosthetic valve failure; CoreValve HR: Medtronic CoreValve U.S. Pivotal Trial; RCT: randomised controlled trial; SAVR: surgical aortic valve replacement; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation; VARC-3: Valve Academic Research Consortium 3
Prevention and management of bioprosthetic valve failure
PREVENTION OF BIOPROSTHETIC VALVE DYSFUNCTION AND FAILURE
Currently, no pharmacological treatment exists to reduce or halt the progression of SVD, and the only option to treat BVF is to perform a redo-TAVI (TAVI-in-TAVI) procedure or surgery to explant and replace the failed THV with a new bioprosthetic valve. It is thus important to prevent SVD and any other type of BVD that may lead to BVF in order to optimise valve durability (Figure 1, Figure 4, Central illustration).
PROSTHESIS-PATIENT MISMATCH AND PARAVALVULAR REGURGITATION
PPM may cause BVF directly if it is severe or very severe and is associated with high residual gradients and persistent or recurrent symptoms of heart failure. In such a situation, which is rare following TAVI, one can consider (i) surgery to replace the severely mismatched THV or (ii) redo-TAVI with an overexpansion of the first THV in order to reduce the severity of PPM. Severe PPM may also predispose patients to leaflet thrombosis or SVD and thus to BVF. Furthermore, a study from the VIVID registry reported that pre-existing severe PPM of the failed surgical bioprosthesis is associated with an increased risk of mortality following a valve-in-valve procedure55. Hence, it is important to pay particular attention to the prevention of severe PPM at the time of the first TAVI or SAVR, especially in the presence of risk factors for PPM such as female sex, a small annulus, valve-in-valve in small failed bioprostheses, and large body size5657.
To prevent PPM, it is first important to anticipate the risk of severe PPM prior to the SAVR or TAVI procedure, using the predicted EOAi method, which can be achieved by calculating the predicted EOAi from the normal EOA reference value for the model and size of THV to be implanted and the patient’s body surface area5758. If the predicted EOAi is ≤0.65 cm2/m2 (or ≤0.55 cm2/m2 if the patient is obese), there is a risk of severe PPM. In such cases, an alternative procedure or type of prosthetic valve should be considered such as root enlargement for SAVR or selection of a type of bioprosthetic valve that has a larger predicted EOA for both SAVR and TAVI. Given that TAVI is associated with a lower risk of severe PPM, particularly in patients with a small annulus, TAVI may also be considered instead of SAVR in patients at risk of severe PPM with surgery.
Balloon predilatation or post-dilatation may also help to reduce the severity of PPM and the ensuing high residual gradients following TAVI59. In the context of valve-in-valve procedures in failed surgical bioprosthetic valves, the fracturing of the failed bioprosthetic valve stent and the implantation of a supra-annular SEV significantly reduces the incidence of severe PPM and might contribute to improving the durability of the valve-in-valve606162.
The operator should always gauge the risk-benefit ratio when considering an alternative or concomitant procedure or a different type of bioprosthetic valve in order to prevent severe PPM. An alteration in procedural strategy should not be chosen if it increases the risk of potentially more serious complications, such as aortic annulus rupture, coronary artery obstruction, serious bleeding, or paravalvular regurgitation. In this context, it should be emphasised that even moderate paravalvular regurgitation is likely to have a greater impact on clinical outcomes, compared to severe PPM, and this impact occurs more rapidly during follow-up. The prevention of paravalvular regurgitation is also essential to optimise valve durability given that this is the second cause, after SVD, of BVF and reintervention following TAVI1763. In the FRANCE TAVI registry, ≥moderate paravalvular regurgitation at discharge was associated with an increased risk of aortic valve reintervention (4.7% vs 2.2%; p<0.001)63. Finally, to reduce leaflet mechanical stress and associated SVD, it is important to optimise THV sizing, positioning, and deployment, and ensure circular deployment of the THV stent without overexpansion. Balloon post-dilatation may help to achieve more complete and circular deployment of the THV stent and thus contribute to preventing SVD33.
VALVE LEAFLET THROMBOSIS
Valve leaflet thrombosis that is clinically significant and not reversible with anticoagulation may directly cause BVF and thus require reintervention. Furthermore, an episode of valve leaflet thrombosis, subclinical or clinical, may also predispose patients to accelerated SVD even if successfully treated by anticoagulation (Figure 5, Central illustration). Subclinical leaflet thrombosis may occur in 5% to 25% of patients during the first year following TAVI or SAVR64656667. The incidence of leaflet thrombosis appears to be higher in intra-annular BEVs compared to supra-annular SEVs67. Hence, it is essential to prevent valve leaflet thrombosis following TAVI or SAVR. In patients undergoing TAVI with no indication for oral anticoagulation (OAC), non-vitamin K antagonist (VKA) oral anticoagulants (NOACs) such as apixaban, rivaroxaban, or edoxaban are effective in preventing the risk of valve leaflet thrombosis but not that of thromboembolic events, and they are associated with an increased risk of bleeding compared to single (SAPT) or dual antiplatelet therapy (DAPT)68,69]. In patients with an indication for OAC, NOACs have not been proven to offer any advantage over VKA in preventing thromboembolic events or reducing bleeding risk69. According to these results from recent RCTs, there thus appears to be no advantage of using NOACs rather than SAPT in patients with no indication for OAC, and of using NOACs rather than VKA in patients with an indication for OAC.
For now, the European (EG) and American guidelines (AG)7071 recommend lifelong SAPT in patients without a baseline indication for OAC to prevent thromboembolic events following TAVI (Class I [EG]/IIa [AG]) and SAPT or OAC for 3 to 6 months following SAVR (Class IIa [EG & AG]). OAC, preferably with VKA, is recommended in patients with a baseline indication for OAC following TAVI or SAVR (Class I [EG & AG]). VKA or DAPT is recommended in patients undergoing TAVI who have no indication for OAC but have a low risk of bleeding (Class IIb [AG]).
If clinically significant valve thrombosis − which is defined as showing evidence of leaflet thickening at echocardiography or CT (HALT) combined with (i) clinical sequelae of a thromboembolic event or symptoms of heart failure or (ii) Stage 2 or 3 haemodynamic valve deterioration (Figure 1, Table 2, Table 3) − occurs after TAVI or SAVR, OAC using VKA is recommended (Class IIa [EG & AG]) for a period of 3 to 6 months. If, after this period, the leaflet thickening and haemodynamic deterioration has resolved, the OAC can be stopped. If the haemodynamic deterioration has not resolved, OAC should be maintained for an additional 3 to 6 months, and if a recurrent episode of valve thrombosis occurs, lifelong OAC should be considered71. However, some studies have reported that long-term treatment with VKA may be associated with faster SVD of surgical bioprosthetic valves due to its inhibition of the matrix Gla protein46.
It is also important to ensure optimal positioning and deployment of the THV stent to avoid underexpansion or non-circular/irregular expansion of the stent to prevent leaflet thrombosis and potentially ensuing SVD313233. Optimal sizing and positioning of the THV as well as rational use of balloon post-dilatation should thus be considered for this purpose and to optimise valve durability.
MANAGEMENT AND OUTCOME OF THV DYSFUNCTION AND FAILURE
According to VARC-3 criteria, BVD can be divided into 4 categories: SVD, non-SVD, valve thrombosis and endocarditis (Figure 1, Figure 2)4. When BVD is suspected during echocardiographic follow-up, it is essential to perform comprehensive multimodal imaging to identify the aetiology and mechanism of BVD and BVF in order to determine the need for intervention and the most appropriate type of reintervention (Table 2).
Two types of procedure can be used for the treatment of THV failure: (i) implantation of a new THV in the failed index THV (i.e., redo-TAVI or TAVI-in-TAVI); (ii) surgical explantation of the failed THV and implantation of a bioprosthetic valve. Figure 7 presents an algorithm for the management of THV failure due to SVD and in particular the selection and planning of the type of intervention: standalone TAVI-in-TAVI, complex TAVI-in-TAVI with a concomitant procedure, or SAVR with THV explant and replacement.
TAVI-in-TAVI is associated with excellent outcomes in the short term727374, while a TAVI explant and replacement procedure is associated with higher rates of mortality and morbidity due to its more invasive and complex nature7374757677. Conversely, TAVI-in-TAVI is not appropriate to treat THV failure due to endocarditis, and THV explant is recommended in such cases. TAVI-in-TAVI may not be optimal for treating symptomatic severe PPM unless balloon overexpansion of the index THV is performed to accommodate a larger new THV.
TAVI is associated with an increased risk of coronary obstruction, and preprocedural CT imaging and planning is essential to anticipate and manage this risk (Figure 7). The snorkel/chimney stenting technique might not be feasible with the overlap of 2 THV stent frames, and the Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction (BASILICA) technique, which consists in transcatheter laceration of the index THV leaflets, is more challenging in failed THVs than in failed surgical bioprosthetic valves. Therefore, new techniques such as balloon-assisted BASILICA78 and ShortCut (Pi-Cardia)79 have emerged to prevent the risk of coronary obstruction at the time of redo-TAVI (Figure 7). Low positioning of the first THV and commissural alignment, especially if using a SEV, should also be considered to facilitate and optimise (i) future TAVI-in-TAVI and (ii) coronary access for future percutaneous coronary interventions.
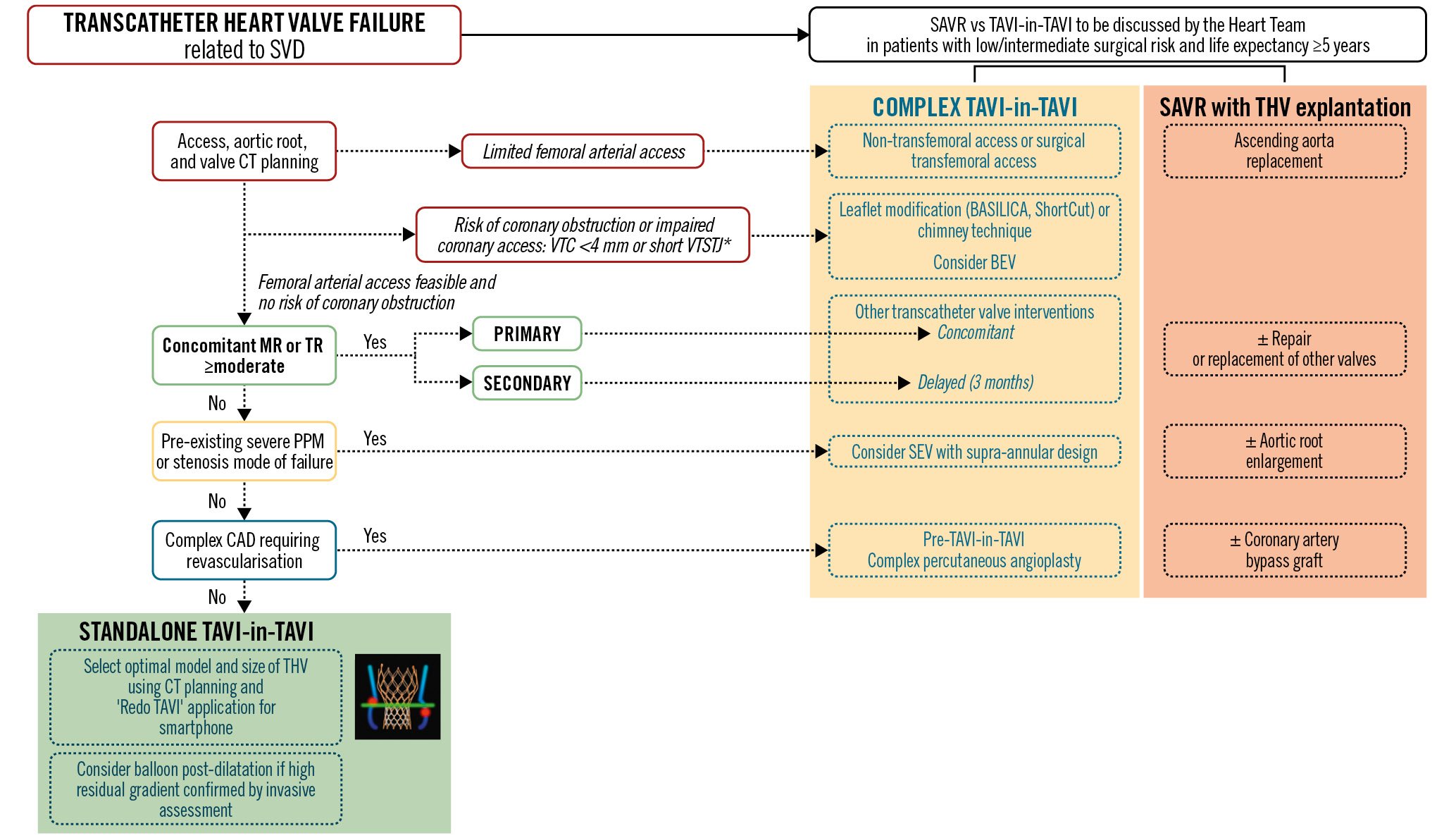
Figure 7. Management of structural THV failure. In a patient with transcatheter heart valve failure related to SVD and low-to-intermediate surgical risk, the first step is to discuss the case among the Heart Team in order to select standalone TAVI-in-TAVI, complex TAVI-in-TAVI, or SAVR with THV explantation. Arguments in favour of SAVR are if the patient requires ascending aorta replacement, aortic root enlargement, a repair or replacement of another valve, and/or coronary artery bypass grafting. If TAVI-in-TAVI is considered the preferred option, the next step is to assess the arterial access, the failed THV, the aortic root and the coronary ostia using CT imaging and planning. If there is limited or impossible femoral arterial access, non-transfemoral or surgical transfemoral access should be considered. If there is a risk of coronary obstruction or of impaired coronary access for future percutaneous coronary intervention as identified by a VTC <4 mm and/or a short VTSTJ, leaflet modification or the chimney technique should be considered, and a BEV may be preferable for the TAVI-in-TAVI. If femoral access is feasible and there is no risk of coronary obstruction, the next step is to assess the presence and aetiology (primary vs secondary) of significant MR or TR. If ≥moderate primary MR or TR is present, concomitant transcatheter interventions may be considered. If the MR or TR is secondary, it is preferable to consider a staged approach with standalone TAVI-in-TAVI first, and then the mitral and/or tricuspid transcatheter intervention 3 months later if the MR and/or TR and heart failure symptoms still persist. If pre-existing severe PPM of the failed THV and/or if severe THV stenosis are present, a SEV with supra-annular design should be considered for the TAVI-in-TAVI. In the presence of complex CAD requiring revascularisation, complex percutaneous angioplasty should be considered prior to the TAVI-in-TAVI procedure. The application ‘Redo TAVI’ (KRUTSCH Associates) can be used to select the optimal model and size of THV for the TAVI-in-TAVI. If a high residual gradient (mean gradient >20 mmHg), confirmed by invasive assessment, is present following the TAVI-in-TAVI procedure, a balloon post-dilatation may be considered. *VTSTJ <2.5 mm is considered high risk and 2.5-3.5 mm intermediate risk for coronary artery obstruction. The schematic representation of the VTC and VTSTJ measurements in a scenario of SEV-in-BEV is reproduced with permission100. BEV: balloon-expandable valve; CAD: coronary artery disease; CT: computed tomography; MR: mitral regurgitation; PPM: prosthesis-patient mismatch; SAVR: surgical aortic valve replacement; SEV: self-expanding valve; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; TR: tricuspid regurgitation; VTC: virtual THV to coronary artery distance; VTSTJ: virtual THV to sinotubular junction distance
Implications for lifetime management
The decision of TAVI versus SAVR and the selection of prosthetic valve are key for optimal patient lifetime management (Table 5). When performing AVR, the proven durability of the bioprosthetic valve should ideally match the expected life expectancy of the patient (Figure 8)8081. For example, a valve with a demonstrated durability of 7 years is adequate for an 80-year-old patient but is not appropriate for a 70-year-old patient, who would ideally require a valve durability of about 15 years. Until now, there have been robust data that support TAVI midterm durability up to 8 years, but the existing data are scarce and insufficient to confirm long-term durability to 10 years and beyond. When deciding between TAVI versus SAVR for the first AVR procedure in a patient’s lifetime, other factors should be taken into consideration, including the following (Table 4)81: (i) surgical risk: high surgical risk as well as moderate/severe frailty are strong arguments in favour of TAVI regardless of a patient’s age; (ii) bicuspid aortic valve with concomitant aortopathy definitely favours SAVR; (iii) concomitant complex and significant coronary artery disease or severe primary mitral regurgitation are arguments in favour of SAVR; (iv) a small annulus and high risk of severe PPM may favour TAVI.
Table 5. Criteria favouring TAVI or SAVR.
| Criteria | Favours TAVI | Favours SAVR | Comments |
|---|---|---|---|
| Age >75 y | +++ | + | Age <50 y favours Ross procedure or mechanical valve in SAVRAge >65 y favours bioprosthetic versus mechanical valve in SAVR |
| Long life expectancy | + | +++ | |
| High surgical risk | +++ | − | Moderate/severe frailty favours bioprosthetic valve in SAVR |
| Intermediate surgical risk | ++ | ++ | |
| Low surgical risk | ++ | +++ | |
| Moderate/severe frailty | +++ | − | |
| Bicuspid aortic valve | ++ | +++ | Small annulus/high risk of severe PPM may favour supra-annular SEV in TAVI |
| Small annulus/high risk of severe PPM | +++ | + | |
| Aortic dilation | − | +++ | Severe aortic valve calcification may favour BEVHigh likelihood of need for redo-TAVI and/or coronary intervention may favour BEV in TAVI |
| Severe calcification of aorta (porcelain aorta) | +++ | − | |
| Severe/complex CAD | + | +++ | |
| Severe primary MR | + | +++ | |
| Septal hypertrophy requiring myectomy | + | +++ | |
| Previous cardiac surgery | +++ | − | |
| Patient goals and preferences | +++ | +++ | |
| +: mildly favours; ++: moderately favours; +++: strongly favours; −: disfavours; BEV: balloon-expandable valve; CAD: coronary artery disease; MR: mitral regurgitation; PPM: prosthesis-patient mismatch; SAVR: surgical aortic valve replacement; SEV: self-expanding valve; TAVI: transcatheter aortic valve implantation; y: years | |||
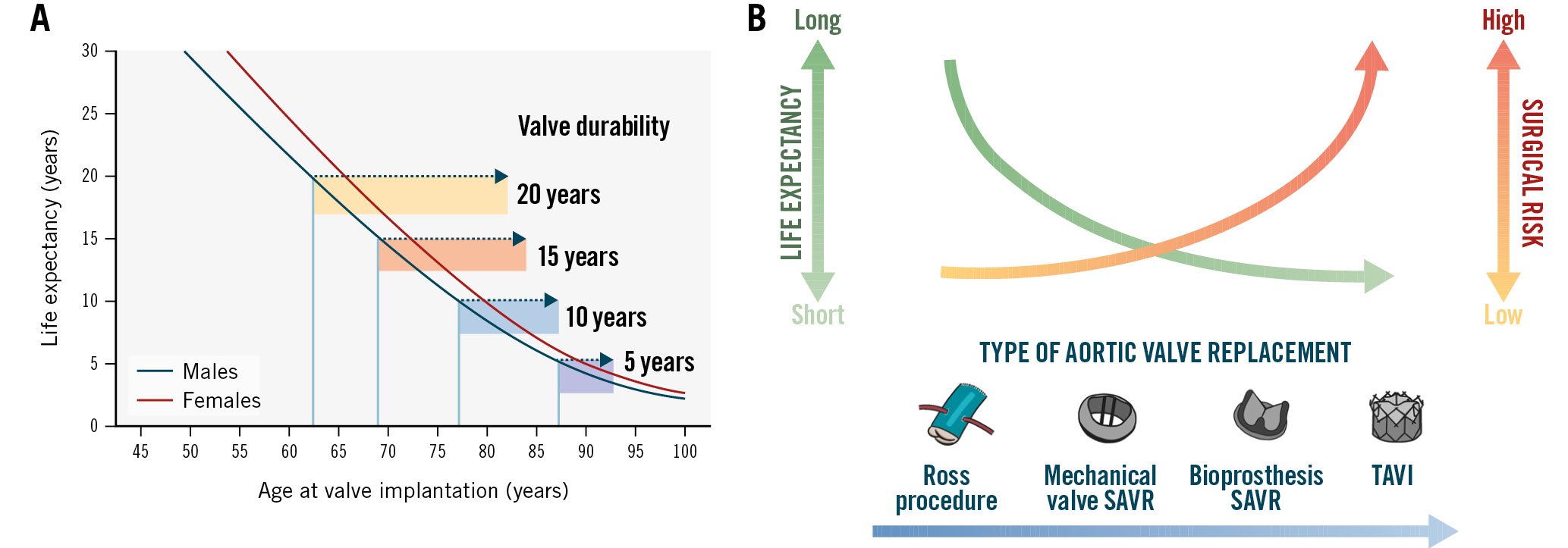
Figure 8. Balancing the patient’s life expectancy, surgical risk, and expected valve durability in the decision between TAVI versus SAVR. (A) Schematic of the ratio between life expectancy and valve durability. Adapted with permission80. (B) Type of AVR according to life expectancy and surgical risk. SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
Future perspectives
The ideal bioprosthetic valve should be abundantly available, immune-compatible, and capable of growth, self-repair, and life-long performance82. Several future perspectives in research and development may enhance the long-term durability of TAVI or SAVR valves in the coming years (Figure 9)83.
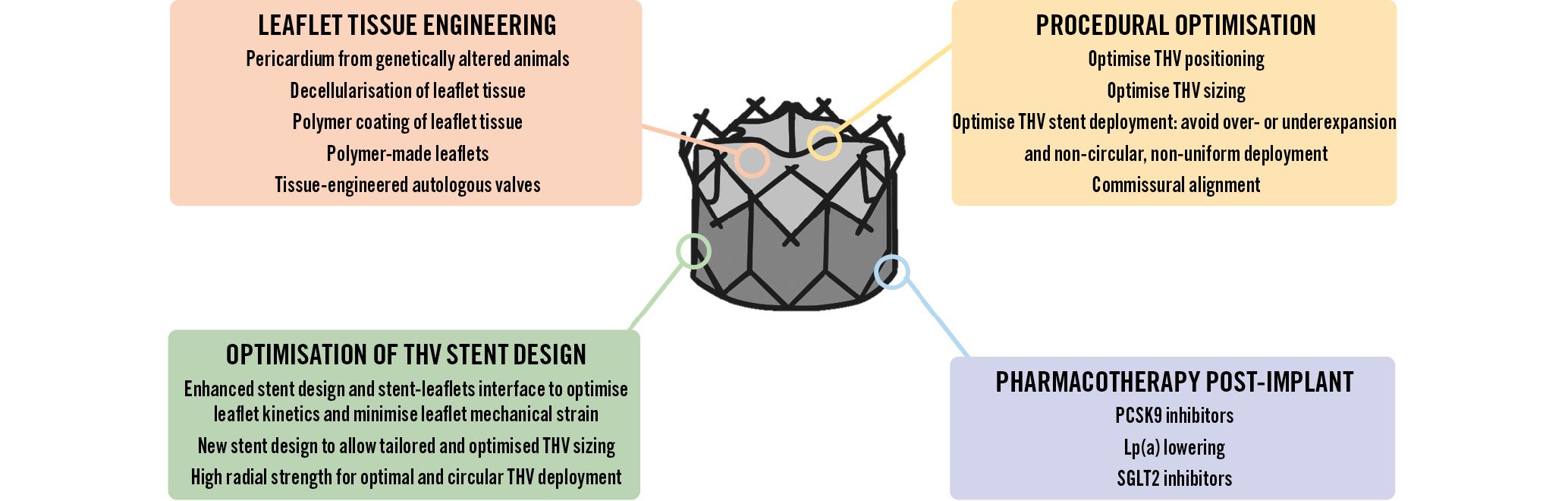
Figure 9. Future directions to optimise THV durability. Lp(a): lipoprotein(a); SGLT2: sodium-glucose cotransporter 2; PCSK9: proprotein convertase subtilisin/hexin type 9; THV: transcatheter heart valve
LEAFLET TISSUE ENGINEERING
Future advances in the durability of bioprosthetic valves may come from the utilisation of novel sources or processing of the pericardium used to fabricate the valve leaflets, including a novel biomimetic valve made from a single piece of native-shaped tissue designed to mimic the performance of a pre-disease human aortic valve (Figure 9)84. The pericardium from genetically-altered pigs is free from the alpha-gal antigen, which is the major antigen responsible for low-grade immune rejection despite glutaraldehyde treatment35. Research is also ongoing to decellularise and therefore reduce the antigenicity of human pericardium harvested from cadavers85. Another potentially promising future direction is to generate autologous valves by tissue engineering. The autologous cells are harvested from the patient and expanded in vitro and then seeded onto a biodegradable scaffold to generate the living autologous valve that is then implanted in the patient86.
Several new chemical processes have been proposed to (i) eliminate the residual antigenicity of bovine or porcine pericardial tissue, (ii) stabilise and reinforce the tissue, and (iii) protect against tissue mineralisation and disruption (Figure 9). New chemical processes preventing serum albumin infiltration and glycation may also contribute to prevent SVD39. Coating the surface of the leaflet tissues with polymer may be applied to prevent (i) the infiltration of lipoproteins and of host immune/inflammatory cells within the bioprosthetic valve leaflet, and (ii) the adhesion of platelets and thrombus to the surface of the leaflets87.
Research is ongoing to find an alternative to pericardium for the fabrication of valve leaflets including polymer and textile (Figure 9)828388. Polymer prosthetic valves have shown satisfactory hydrodynamics and in vitro durability along with reduced thrombogenic and calcification potential in limited in vivo studies. The polymer leaflets can be crimped to very small calibres with apparently no microstructure disruption, which is a major advantage for their application to TAVI.
OPTIMISATION OF THV STENT DESIGN
The development of THV stents with more radial force that allow more complete and circular deployment as well as the improvement of the stent-leaflet configuration and interaction may help to reduce leaflet mechanical stress and thus improve valve durability (Figure 4, Figure 9)50. New THV designs, the range of available sizes and TAVI techniques that optimise valve sizing, and the match between the size of the THV and the size of the patient’s aortic annulus can contribute to optimise THV deployment and thus minimise leaflet mechanical stress and the ensuing impact on valve durability.
PHARMACOTHERAPY POST-IMPLANT
Another perspective to prevent SVD is to treat the patients with some targeted medications after TAVI or SAVR (Figure 9). To this effect, pharmacotherapies targeting lipids, insulin resistance, and inflammation may help to prevent or slow SVD. In particular, RCTs are needed to determine if PCSK9 inhibition or lipoprotein(a)-lowering therapies are efficient and safe to prevent SVD following TAVI or SAVR.
Conclusions
SVD is the main factor limiting the durability of bioprosthetic valves following TAVI, but non-structural BVD such as PPM and paravalvular regurgitation as well as valve thrombosis or endocarditis may also lead to BVF and require reintervention (Central illustration). The incidence of BVF related to SVD or other causes is low (<5%) at midterm (5 to 8 years) follow-up and compares favourably with that of SAVR. The long-term follow-up data of RCTs conducted with the first generations of THVs also suggest similar valve durability in TAVI versus SAVR at 10 years, but these trials suffer from major survivorship bias, and the long-term durability of TAVI will need to be confirmed by the 10-year analysis of the PARTNER 3 and Evolut LR RCTs. It is unknown whether the different types of THV (BEV vs SEV, and intra-annular vs supra-annular) will achieve similar long-term durability, and several RCTs (SMART and BEST) are ongoing to investigate this issue. Future optimisation of THV durability may come from (i) the use of the pericardium of transgenic alpha-gal-free pigs or new chemical processes to protect the pericardial tissue, (ii) the use of polymer-coated or polymer-made valve leaflets, or (iii) pharmacological treatment post-implant to prevent valve thrombosis and SVD.
Conflict of interest statement
J. Ternacle has been a consultant for Abbott, Edwards Lifesciences, Pi-Cardia, Philips Healthcare, and GE HealthCare. M-A. Clavel has received funds from Edwards Lifesciences for computed tomography core laboratory analyses in the field of surgical bioprostheses; and research grants from Edwards Lifesciences, Medtronic, and Pi-Cardia, with no personal compensation. P. Pibarot has received funding from Edwards Lifesciences, Pi-Cardia, and Cardiac Success for echocardiography core laboratory analyses in the field of transcatheter valve therapies, and Medtronic for in vitro analyses, with no personal compensation. The other authors have no conflicts of interest to declare.

