Abstract
Background: The mechanically-expandable transcatheter valve is no longer commercially available, yet clinical and echocardiographic surveillance is imperative for thousands of patients who received transcatheter aortic valve implantation (TAVI) with this platform.
Aims: We aimed to determine the incidence and mechanism of bioprosthetic valve dysfunction (BVD) following TAVI with mechanically-expandable valves.
Methods: From 2013 to 2020, all 234 patients who underwent TAVI with the LOTUS valve were included. BVD was categorised as (i) structural valve deterioration (SVD), (ii) non-structural valve dysfunction (NSVD), (iii) clinical valve thrombosis and (iv) endocarditis, according to the Valve Academic Research Consortium-3 criteria.
Results: The mean age was 79±7 years, 60% were male, and the mean Society of Thoracic Surgeons score was 4.2±2.9%. The technical success rate was 94% and the 30-day device success rate was 78%. All-cause mortality at 1 year was 15%; median follow-up duration was 36 (IQR 18-60) months during which 47% of patients died. One hundred and three patients had ≥1 type of BVD (44%), which predominantly consisted of NSVD (39%, mostly because of ≥moderate patient-prosthesis mismatch). BVD during follow-up included endocarditis (3.4%), clinical valve thrombosis (3.4%) and SVD (1.3%). Both endocarditis and clinically apparent valve thrombosis occurred early and late after TAVI and resulted in valve-related deaths in 38% and 13% of patients, respectively. Overall, ≥moderate haemodynamic valve deterioration occurred in 5.5% and bioprosthetic failure in 7.3%, leading to valve-related deaths in 36% of cases.
Conclusions: BVD represents a relevant health issue after TAVI with a mechanically-expandable valve. Serious but reversible causes of BVD include endocarditis and clinically apparent valve thrombosis, both carrying a time-independent hazard post-TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) revolutionised the management of aortic valve stenosis and is forecast to be the dominant treatment modality in most patients1. The 3 competing transcatheter valve systems are balloon-expandable, self-expanding and mechanically-expandable technologies. However, in 2020 Boston Scientific voluntarily withdrew their mechanically-expandable LOTUS heart valve from the market2. Even though the device is no longer available, close follow-up of patients previously treated with a LOTUS valve is needed to monitor valve performance and preserve health outcomes. The controlled implantation of the LOTUS valve, with fully repositionable/retrievable features, high radial force and sealing fabric, made it an attractive platform for more complex anatomies, including bicuspid aortic valve morphologies and severe aortic valve calcification3. However, its relatively bulky metallic frame could potentially increase the risk of valve thrombosis4. For these reasons the objective of this study was to report the incidence and mechanism of bioprosthetic valve dysfunction (BVD), subsequent valve haemodynamics and clinical outcome in a large single-centre series of patients treated with the LOTUS valve.
Materials and methods
Patients
From September 2013 until November 2020, a total of 234 patients underwent TAVI with the LOTUS valve in the Erasmus University Medical Center, Rotterdam, the Netherlands. All patients underwent multidisciplinary assessment and a structured in-hospital and post-discharge follow-up using a prospective collection of predefined variables. Echocardiographic assessment was performed at baseline, predischarge or at 30 days, and 1 and 3 years. Survival status was checked with the Dutch Civil Registry every year. Institutional review board approval was obtained, and all patients provided informed consent at the end of the pre-TAVI outpatient clinic visit for the TAVI procedure and anonymous data collection for research purposes. The study complies with the Declaration of Helsinki.
Device and procedure
The LOTUS valve system consists of a stent frame with woven nitinol wire supporting 3 bovine pericardial leaflets in an intra-annular design and is deployed through mechanical expansion. The device is fully repositionable and retrievable allowing complete deployment and functional assessment of the transcatheter heart valve (THV) before the final release5. The LOTUS Edge system (Boston Scientific) (n=68 in this cohort) integrated several design improvements on the former LOTUS design (n=166). All procedures were performed via a transfemoral approach under general anaesthesia until 2015 and local anaesthesia thereafter. Pre- and post-dilatation was performed at the discretion of the operator.
Study endpoints and definitions
The primary objective was to investigate the type of BVD and any associated haemodynamic valve deterioration (HVD) and/or bioprosthetic valve failure (BVF) in accordance with the latest Valve Academic Research Consortium-3 (VARC-3) criteria6. The types of BVD were categorised as: (i) structural valve deterioration (SVD: intrinsic, permanent changes to the prosthetic valve), (ii) non-structural valve dysfunction (NSVD: any abnormality not intrinsic to the prosthetic valve resulting in valve dysfunction, such as patient-prosthesis mismatch or inappropriate positioning), (iii) clinically significant thrombosis and (iv) endocarditis. Prosthesis-patient mismatch (PPM) was classified as moderate (index aortic valve area 0.65 to 0.85 cm2/m2, if BMI ≥30 kg/m2 0.56–0.70 cm2/m2) or severe (index aortic valve area <0.65 cm2/m2, if BMI ≥30 kg/m2 ≤0.55 cm2/m2). In 6 out of 8 patients with suspected clinical valve thrombosis, a confirmatory multislice computed tomography (MSCT) was performed to assess for reduced leaflet motion (RLM) and/or hypoattenuated leaflet thickening (HALT) in accordance with the Society of Cardiovascular Computed Tomography consensus document7. For another 23 patients without suspected clinical valve thrombosis, the available MSCT studies obtained in the framework of post-TAVI surveillance were also analysed for presence of RLM/HALT or other signs of early BVD. In patients with BVD, the severity of HVD was classified as stage 1 (morphological valve deterioration without haemodynamic change), stage 2 (moderate haemodynamic deterioration) or stage 3 (severe haemodynamic deterioration). The clinical consequence of BVD was classified as BVF stage 1 (symptoms or echocardiographic signs of pressure/volume overload), stage 2 (valve reintervention) or stage 3 (valve-related death). The VARC-3 proposed device-related composite endpoints to further include:
(i) technical success, defined as freedom from mortality at exit from the procedure room with successful access, delivery and retrieval of the valve delivery system, correct valve positioning, and no need for surgery or intervention related to the device or to a major vascular or cardiac-related complication;
(ii) device success at 30 days, defined as freedom from mortality with technical success and no need for surgery or intervention related to the device or to a major vascular- or cardiac-related complication, and with the intended performance of the valve (mean gradient <20 mmHg, peak velocity <3 m/s, Doppler velocity index ≥0.25, and less than moderate aortic regurgitation).
Native aortic valve anatomy was considered complex in the presence of one of the following conditions: severe aortic valve calcification, moderate-severe left ventricular outflow tract (LVOT) calcification, bicuspid aortic valve morphology, degenerated surgical bioprosthesis or pure aortic regurgitation3. Semi-quantitative computed tomography evaluation categorised the aortic valve and LVOT calcifications as mild, moderate or severe.
Statistical analysis
Categorical variables were presented as frequencies and percentages and were compared with the chi-square test or the Fisher’s exact test. Normal and skewed continuous variables were presented as means (±standard deviation) and medians (interquartile range [IQR]), respectively. Continuous variables were compared using the Student’s t-test or Mann-Whitney U-test when appropriate. BVD, HVD and BVF were reported as cumulative incidence; to account for the competing risk of death, HVD and BVF were also analysed using a cumulative incidence function. Two-sided p-values <0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Science for Windows (IBM), version 21.
Results
Baseline patient characteristics of the entire cohort are presented in Table 1.
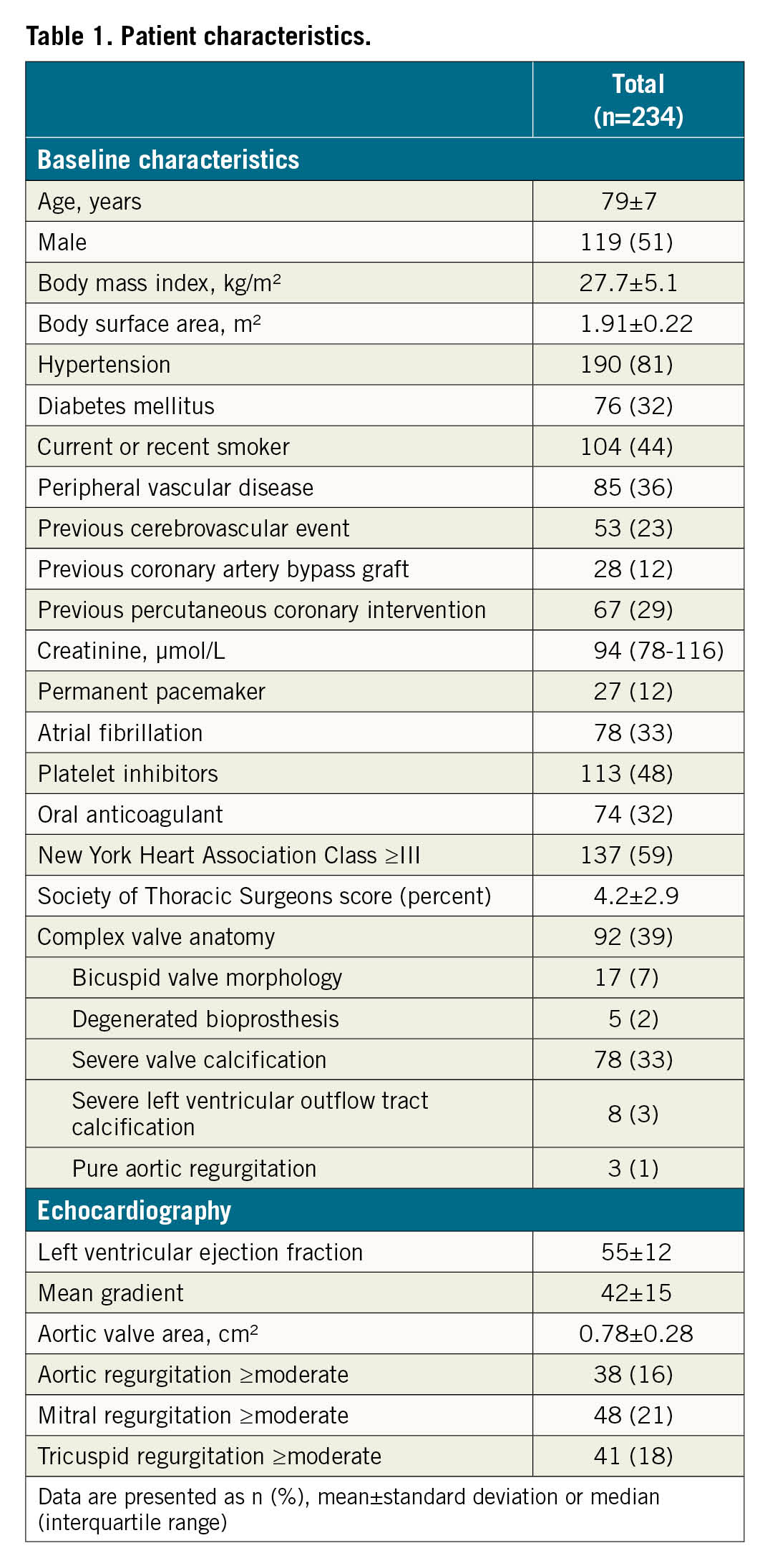
The mean age was 79±7 years, 60% were male and the mean Society of Thoracic Surgeons (STS) Predicted Risk of Mortality was 4.2±2.9 percent. Thirty-nine percent of patients were considered to have complex valvular anatomy. Technical success was 94% and 30-day device success was 78% (Table 2). Reasons for not achieving device success were unfavourable haemodynamic valve performance (11%), absence of technical success (6%) and/or death <30 days (6%). All-cause mortality at 1 year was 15%. The median follow-up duration was 36 (IQR 18-60) months, during which 47% of patients died.
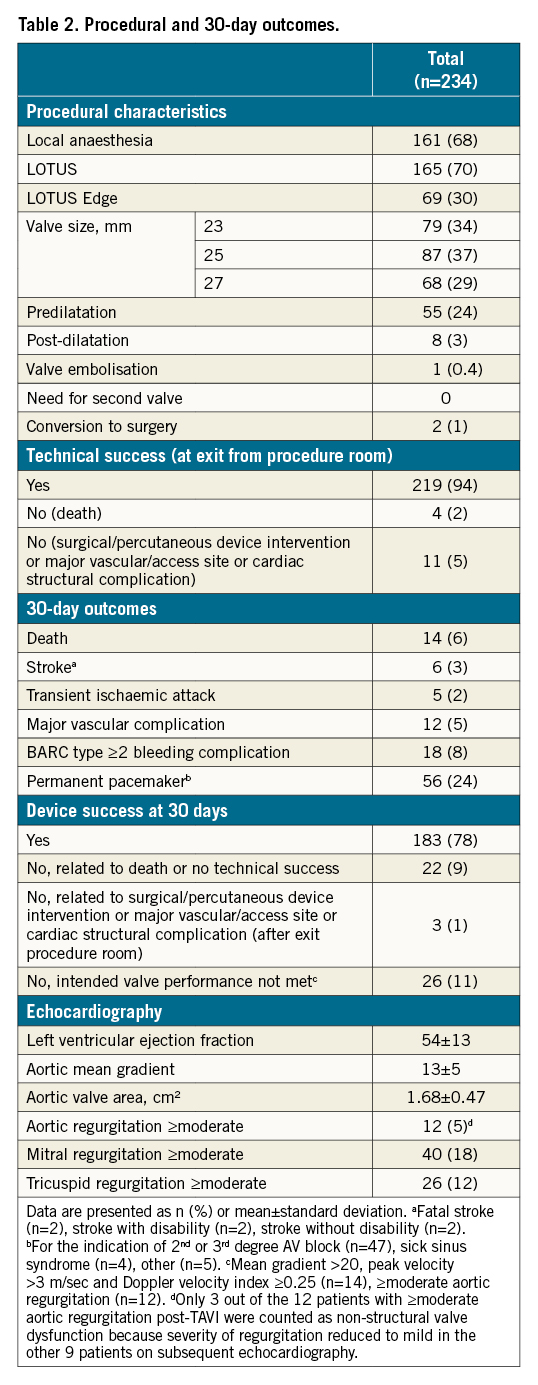
Overall haemodynamic valve performance during follow-up
Post-TAVI echocardiography data were available in all but 3 patients because of death during the procedure due to a fatal annular rupture, left ventricular wire perforation and major vascular complication in the abdominal aorta. A 1-year echocardiographic assessment was available in 90% of 1-year survivors. From baseline to post-TAVI, the effective orifice area (EOA) and mean gradient improved significantly (EOA: 0.77 vs 1.67 cm2; mean gradient: 41 vs 13 mmHg; p<0.001) and remained stable at 1 year (EOA: 1.72 cm2; mean gradient: 12 mmHg). PPM was present at the first echocardiogram post-procedure in 38% of patients, with 29% with moderate and 9% with severe PPM.
Bioprosthetic valve dysfunction
Patient-level details of the type, timing and management of bioprosthetic valve dysfunction and failure are presented in Supplementary Table 1 and summarised in the Central illustration. One hundred and three patients had ≥1 type of BVD (44%; 91 patients had 1 and 12 patients had 2 types of BVD). The main contributor to this endpoint was NSVD (39%), secondary to PPM in almost all cases (29% moderate PPM, 9% severe PPM and/or 3% other). Other contributors – in descending order of incidence - were endocarditis (3.4%), clinical valve thrombosis (3.4%) and SVD (1.3%).
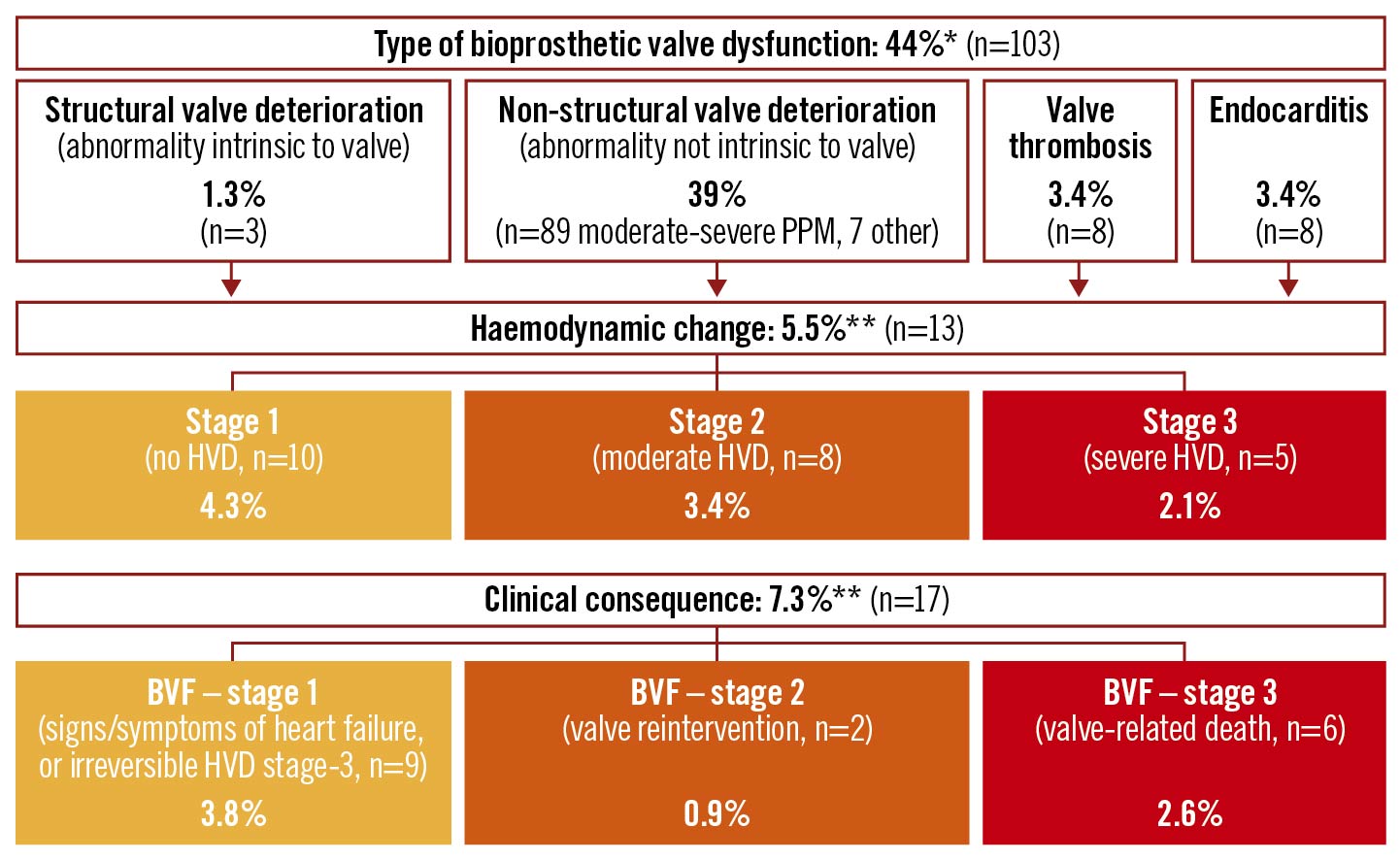
Central illustration. Type, haemodynamic change and clinical consequences of bioprosthetic valve dysfunction during a median of 3 years after mechanically-expandable heart valve implantation.
*A total of 103 patients had BVD (44%), of whom 91 patients had one type of BVD and 12 patients two types of BVD (i.e., moderate or severe PPM and another type of BVD). **The BVD resulted in a haemodynamic change (≥stage 2 HVD) in 5.5% and bioprosthetic valve failure in 7.3% (≥stage 1 BVF) during a median follow-up of 36 (IQR: 18-60) months (cumulative incidence). By cumulative incidence function, haemodynamic change (HVD stage ≥2) was found in 3.9% and bioprosthetic valve failure in 4.5% (BVF stage ≥1) at 36 months. BVD: bioprosthetic valve dysfunction; BVF: bioprosthetic valve failure; HVD: haemodynamic valve deterioration; NSVD: non-structural valve dysfunction; PPM: patient-prosthesis mismatch; PVL: paravalvular leakage
Overall, clinically relevant valve thrombosis was diagnosed in 8 patients, of which 4 (50%) occurred within 1 year and the other 4 (50%) between 1 and 5 years after TAVI. MSCT confirmed HALT and/or RLM in all but 2 patients in whom MSCT was not performed. None of the patients were on anticoagulants at the time of the event, except one who was on a reduced dose of direct oral anticoagulant therapy for atrial fibrillation. Initiation of anticoagulants (vitamin K antagonist or unfractionated heparin) returned the bioprosthetic haemodynamics to baseline in all but 1 patient (1 valve-related death). There was no HALT or RLM in the 23 patients who underwent an MSCT in the framework of post-TAVI surveillance (median: 3.5 months).
Bioprosthetic valve failure
Any stage of BVF occurred in 7.3%. In cumulative incidence function, the rate of any BVF was 4.5% at 3 years. Stage 3 BVF (valve-related death) occurred in 6 patients (2.6%) due to uncontrolled infection secondary to endocarditis (n=3), cardiogenic shock due to clinical valve thrombosis (n=1), cardiogenic shock after valve dislodgement (n=1) and death upon induction of anaesthesia for a TAVI-in-TAVI procedure to treat structural degeneration of the LOTUS valve (n=1). Stage 2 BVF (valve reintervention) occurred in 2 patients. In 1 case, haemodynamic valve deterioration that was secondary to endocarditis required surgical valve replacement (n=1), whereas another patient with rapid structural valve degeneration underwent successful TAVI-in-TAVI therapy with a 23 mm balloon-expandable valve.
Discussion
The main findings of this longitudinal follow-up study of the performance of bioprostheses after TAVI with the mechanically-expandable LOTUS valve are as follows (Central illustration): 1) ≥1 type of BVD occurred in 44% of the patients at a median of 36 (IQR 18-60) months post-TAVI. This was predominantly driven by moderate PPM. NSVD occurred in 39% of patients, endocarditis in 3.4%, clinical valve thrombosis in 3.4% and SVD in 1.3%. 2) The cumulative incidence of BVF was 7.3% and resulted in valve-related death in one third of cases.
The retirement of the Boston Scientific LOTUS platform in 2020 diminished research related to the mechanically-expandable TAVI technology. Although early safety and efficacy of the LOTUS valve has been established before38910, longer-term data are lacking. Dedicated studies investigating LOTUS BVD during follow-up are particularly scarce. Bridging this knowledge gap is essential to ensure a good outcome in LOTUS recipients. Also, it is conceivable that certain device-host interactions that are common in the LOTUS population may catalyse early BVD. Of note, the fully repositionable/recapturable yet bulky metallic LOTUS system was frequently reserved for more complex anatomies, possibly with resultant suboptimal frame expansion and haemodynamic stress, and subsequent early BVD11. The herein reported 44% BVD rate is challenging to compare with previous studies because of differences in patient-risk profiles, follow-up duration and endpoint definitions. Table 3 provides an overview of prior studies that reported HVD and BVF rates using VARC-3 equivalent definitions (definitions according to consensus criteria by the European Association of Percutaneous Cardiovascular Interventions12). The NOTION trial reported a 62% BVD rate (moderate/severe PPM in 44%) in a low-risk population at 8 years after CoreValve (Medtronic) implantation13. The risk of (VARC-3 defined) ≥stage 2 haemodynamic valve deterioration varies between 3.2-17.9% at 3-6 years after balloon-expandable TAVI1415161718192021 and between 1.5-9.5% at 4-8 years after self-expanding TAVI1617192022232425 (Table 3). Although these data imply better haemodynamic sustainability of the self-expanding valve, it is not associated with a lower incidence of BVF when compared with balloon-expandable valves (reported BVF rate 2.5-8% after self-expanding and 0.6-2.8% after balloon-expandable TAVI, Table 3). Also, data from the PARTNER 2 trial in aggregate with the SAPIEN 3 registry indicate that contemporary balloon-expandable device systems carry a 3-fold lower risk of haemodynamic deterioration as compared to older-generation balloon-expandable valves15. In the present study, we found a 5.5% ≥stage 2 HVD and a 3.5% ≥stage 2 BVF risk in a substantially shorter time-window (median 3 years). The LOTUS mechanically-expandable valve may be more susceptible to bioprosthetic dysfunction in comparison to other platforms. Therefore, the herein reported insights on BVD incidence and mechanisms may help inform best practices for LOTUS recipients who currently take part in post-TAVI care programs.
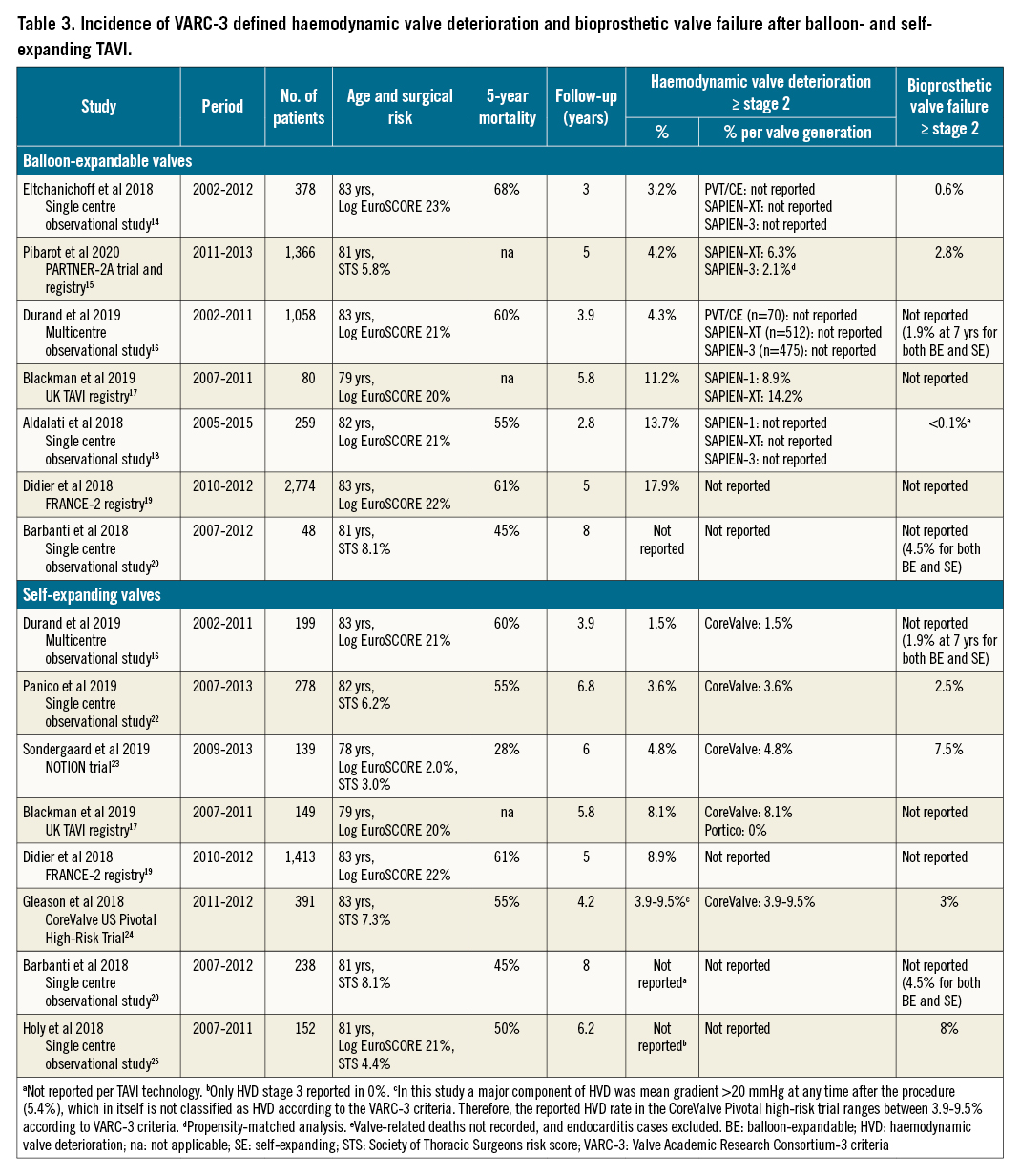
The main contributor to BVD was NSVD, which is a bioprosthetic abnormality due to extrinsic factors such as paravalvular aortic regurgitation (PAR), device malpositioning or PPM. In our study, almost all cases of NSVD consisted of moderate or severe PPM (38%); the rate of significant PAR and device malpositioning was low, reflecting the favourable mechanically-expandable technology allowing for complete deployment and functional assessment of the valve prior to the final release. The rate of ≥moderate PPM in our series was similar as compared to the NOTION trial (38 vs 44%, respectively). When using the same PPM definition as in the NOTION trial (≤0.85 cm2/m2 not corrected for BMI), the rate of ≥moderate PPM in this study would be 46%13. This is a somewhat surprising finding since patients in the NOTION trial were at low risk and received a self-expanding valve with a supra-annular design which is typically associated with lower rates of PPM26. Although NSVD results from any abnormality not intrinsic to the bioprosthesis, the presence of NSVD may accelerate intrinsic bioprosthetic degeneration and, thus, SVD627. SVD is an irreversible process manifested by gradual degeneration such as leaflet calcification, thickening and pannus formation that ultimately leads to BVF with aortic regurgitation and/or stenosis, and haemodynamic compromise. Clinically manifested SVD is uncommon within 5 years after valve implantation2428. In our study, SVD occurred in 1.3% of cases, although this should be interpreted with caution because of the low number of patients with >5 years of follow-up.
Clinical valve thrombosis was detected in 3.4% of the patients, which corroborates other TAVI series (ranging between 0.6 and 2.8%, and up to 4.5% after balloon-expandable valve implantation2930). The general factors that mediate clinical valve thrombosis are diverse and include, among others, prothrombotic states, high platelet activity and inadequate antiplatelet therapy. In the subset of LOTUS recipients, additional device- and host-specific factors might inflate the thrombosis risk. The bulky metallic frame interacting with excess calcium deposits in the aortic valvular complex may lead to areas of stagnant flow due to underexpansion and/or poor endothelialisation of the stent frame2129. Also, repetitive repositioning and recapturing during LOTUS implantation attempts may impose microscopic leaflet injury potentially creating a prothrombotic milieu. The finding that clinical valve thrombosis occurred both early and late after TAVI and was detected by routine echocardiography prior to the development of symptoms in 2 cases signifies the importance of frequent clinical and echocardiographic post-TAVI surveillance. A correct diagnosis early in the disease process allows for timely administration of corrective anticoagulant therapy. Conversely, failure to detect the erratic disease state during early phases may cause rapid clinical deterioration and (valve-related) death. Although oral anticoagulant therapy prevents the development of both subclinical and clinically overt valve thrombosis, further studies are needed to identify candidates for such a strategy.
Infective endocarditis is an important reversible cause of bioprosthetic dysfunction. Our cumulative incidence of 3.4% corroborates surgical (4.9-5.9%) and transcatheter series (4.4-5.8%)2331 and also equals the 1.1% per person-year incidence previously reported in a large collaborative study32.
Limitations
This study reports on the incidence, type and timing of bioprosthetic dysfunction as recorded by clinical and echocardiographic assessment at predefined time points after TAVI. It is possible that patients who did not undergo a clinical and echocardiographic assessment during follow-up (10% at 1 year) might have had bioprosthetic dysfunction. The fact that all 234 study subjects underwent TAVI over the course of a long time period (8 years) may have introduced heterogeneity into the study population which could have affected study results. As mentioned above, few patients exceeded the 5-year follow-up threshold, beyond which degenerative changes of the bioprosthesis may become clinically relevant, and, therefore, no conclusions on the rate of SVD can be drawn from this study. Nevertheless, this study design, streamlined to the recently released VARC-3 criteria, provides valuable insight into the mode and incidence of bioprosthetic dysfunction in LOTUS recipients that will inform best practices after LOTUS TAVI.
Conclusions
Our study suggests that patients treated with the mechanically-expandable LOTUS transcatheter heart valve are at considerable risk for bioprosthetic dysfunction at follow-up. Cardiologists and physicians involved in post-TAVI care of LOTUS recipients should have a heightened awareness for clinical and echocardiographic signs of bioprosthetic dysfunction. These measures will help provide a timely diagnosis of potentially hazardous yet reversible causes of bioprosthetic dysfunction.
Impact on daily practice
The mechanically-expandable LOTUS transcatheter heart valve is no longer commercially available, yet clinical and echocardiographic surveillance in patients previously treated with a LOTUS valve is crucial to maintain health outcomes. Our study indicates that LOTUS valve dysfunction is a common health issue. Potentially dangerous and reversible causes of bioprosthetic dysfunction carry a time-independent hazard after TAVI and, occasionally, can be detected by echocardiography prior to the onset of symptoms. Therefore, clinicians should be at heightened awareness for BVD and maintain a close follow-up early and late after LOTUS valve implantation.
Conflict of interest statement
N. Van Mieghem received research grants from Abbott Vascular, Boston Scientific, Medtronic, Edwards Lifesciences, Pie Medical, Daiichi Sankyo, PulseCath BV, and Abiomed. J. Daemen received institutional grant/research support from AstraZeneca, Abbott Vascular, Boston Scientific, ACIST Medical, Medtronic, MicroPort, Pie Medical, and ReCor Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

