
Abstract
Aims: We aimed to assess the prevalence and features of prosthesis-patient mismatch (PPM) following transcatheter aortic valve implantation (TAVI) and its prognostic impact considering baseline left ventricular ejection fraction (LVEF).
Methods and results: Data from 1,309 patients undergoing TAVI for severe aortic stenosis were derived from a single-centre dedicated TAVI registry. PPM was assessed according to echocardiography at discharge and was defined in accordance with VARC-2. Median follow-up time was 2.03 years. Moderate and severe PPM was detected in 22.9% and 12.9%, respectively. Patients with severe PPM had smaller annuli and more often received transcatheter heart valve (THV) sizes ≤23 mm. Supra-annular THV design showed the lowest rate of PPM. In patients with LVEF <40%, but not in those with LVEF ≥40%, severe PPM was associated with an increased three-year mortality rate (no vs. severe PPM for LVEF ≥40%: 34.6% vs. 29.5%, p=0.96; LVEF <40%: 45.1% vs. 68.0%, p=0.041) and was independently predictive of all-cause mortality according to multivariate analysis in these patients (HR 2.97; 95% CI: 1.58-5.59, p<0.001).
Conclusions: The presence of severe PPM depends on annular dimensions and THV size and design and is an independent predictor of mortality in patients with reduced LVEF. Hence, the risk of PPM should be considered within the process of THV selection.
Abbreviations
AS: aortic stenosis
BMI: body mass index
EOA: effective orifice area
GFR: glomerular filtration rate
LVEF: left ventricular ejection fraction
MI: myocardial infarction
MSCT: multislice computed tomography
PPM: prosthesis-patient mismatch
PVL: paravalvular leakage
STS PROM: Society of Thoracic Surgeons predicted risk of mortality
SVI: stroke volume index
TAVI: transcatheter aortic valve implantation
TTE/TEE: transthoracic/transoesophageal echocardiography
Introduction
Prosthesis-patient mismatch (PPM), defined as too small a prosthesis in relation to the patient’s body size as assessed by its indexed effective orifice area (iEOA), is associated with poor outcome after surgical aortic valve replacement (SAVR), in particular in patients with reduced left ventricular function1,2. Accordingly, for transcatheter aortic valve implantation (TAVI) also, at least moderate PPM is considered a device failure due to poor transcatheter heart valve (THV) performance, as is stated in the current Valve Academic Research Consortium (VARC) definition3. Currently, several different THV designs with varying haemodynamic properties are commercially available4. However, data regarding the prevalence and prognostic impact of PPM after TAVI using various THV designs are scarce, especially with respect to the left ventricular ejection fraction (LVEF) at baseline. Thus, the aim of the present study was to assess in a real-world patient population, firstly, clinical and procedural characteristics including different THV designs according to the presence of PPM after TAVI and, secondly, the prognostic impact of PPM taking baseline LVEF into consideration.
Methods
PATIENTS
All patients who underwent TAVI at our centre due to severe symptomatic native aortic stenosis (AS) between 2008 and 2017 and who had provided in-house echocardiographic data on PPM were included in the analysis (n=1,512). Patients with valve-in-valve procedures (THV in THV or surgical prosthesis), or TAVI for pure aortic regurgitation or in combination with percutaneous mitral valve treatment were excluded from the analysis, leaving 1,309 patients for the analysis. Median follow-up time was 2.03 years.
PROCEDURE
Preprocedural workup included transthoracic echocardiography (TTE), transoesophageal echocardiography (TEE) and coronary angiography. For annular sizing, contrast-enhanced multislice computed tomography (MSCT)-based measurements were consecutively performed in all patients from October 2011 onwards. TAVI was performed in a standard fashion as described previously4,5. Patients were treated with balloon-expandable intra-annular, self-expanding supra-annular, self-expanding intra-annular, mechanically expandable infra-annular, non-metallic and self-expanding cusp-fixated THVs. Further information on the THV types used in the study population is given in Supplementary Appendix 1.
DATA ACQUISITION AND FOLLOW-UP
The study was designed as a data analysis from a single-centre registry. Baseline, procedural, and follow-up data were prospectively collected in a dedicated database and retrospectively analysed. Echocardiographic data were derived from in-house TTE or TEE before TAVI and at discharge. Survival data were obtained either from in-house information, telephone follow-up of the patient or the referring physician, or by contacting civil registries. Patients provided informed consent to the procedure and data acquisition. The authors take full responsibility for data integrity.
DEFINITION OF PPM
PPM was assessed according to TTE at discharge and defined in accordance with VARC-2 criteria3, meaning that in patients with body mass index (BMI) <30 kg/m2 an iEOA >0.85 cm/m2 was considered as no PPM, an iEOA <0.85 ≥0.65 cm/m2 as moderate, and an iEOA <0.65 cm/m2 as severe PPM. In patients with BMI ≥30 kg/m2, no PPM was defined as an iEOA >0.70 cm/m2, moderate as an iEOA <0.70 ≥0.60 cm/m2, and severe as an iEOA <0.60 cm/m2. The EOA after TAVI was calculated by using the continuity equation, for which all variables including LVOT measurements were derived from echocardiography.
STATISTICAL ANALYSIS
Continuous variables were described as means±standard deviation (SD), categorical variables as absolute numbers and percentages. The t-test (for continuous variables) or the χ2 test (for categorical variables) was employed for comparisons among PPM groups. Prevalences of PPM were calculated for each year. Locally weighted scatter-plot smoothing was used to investigate time trends in these prevalences. To compare all-cause mortality among groups, Kaplan-Meier curves were generated. Log-rank tests were used to test for differences in all-cause mortality. To assess the association between PPM and three-year mortality dependent on LVEF, fractional polynomials for LVEF were modelled separately in each PPM group. Univariable Cox regression was used to estimate hazard ratios (HR) for moderate and severe PPM compared to no PPM. Multivariable Cox regression models were built to assess whether PPM was an independent predictor of mortality. The multivariable models were built in two steps: 1) variables considered as potential predictors of mortality (age, sex, Society of Thoracic Surgeons predicted risk of mortality [STS PROM], BMI category, hypertension, diabetes on insulin, prior myocardial infarction or stroke, atrial fibrillation, LVEF, transfemoral access, paravalvular leakage [PVL], Pmean at baseline and post TAVI, stroke volume index [SVI], permanent pacemaker implantation pre and post TAVI, and PPM) were first analysed in univariable Cox regression analysis; 2) variables that showed p-values <0.25 in the univariable analyses were used in a forward selection process using the Akaike information criterion as selection criterion. PPM was forced into the multivariable model (irrespective of the p-value).
A two-sided p-value of <0.05 was considered statistically significant. All statistical methods were implemented in R statistical software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org).
Results
BASELINE CHARACTERISTICS
Patients’ baseline characteristics are shown in Table 1. Patients were trichotomised according to the presence of no PPM (64.2%), moderate PPM (22.9%) or severe PPM (12.9%). There was no significant difference in terms of age, sex and STS PROM as well as comorbidities among patients with no PPM, moderate PPM, and severe PPM, despite a higher prevalence of atrial fibrillation, diabetes mellitus and prior myocardial infarction in patients with moderate or severe PPM. Patients in the severe PPM group presented with a higher BMI. Echocardiographic baseline parameters are given in Supplementary Table 1. Patients with severe PPM presented with smaller LVOT diameters, EOAs and SVIs at baseline. There was no difference in terms of baseline LVEF among PPM groups.
PROCEDURAL DATA AND ECHOCARDIOGRAPHIC OUTCOME
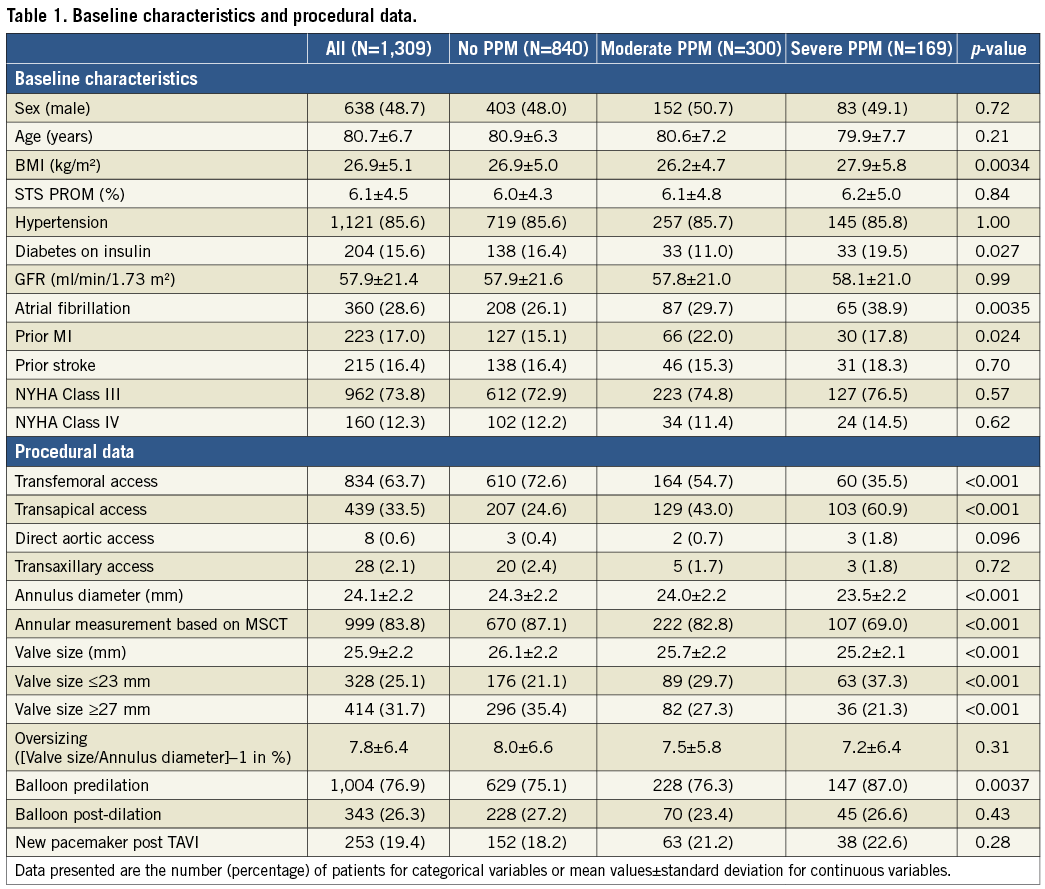
Procedural data are also shown in Table 1. Patients with severe PPM had smaller aortic annuli, received smaller valve sizes and were more often treated via transapical access and with pre-ballooning. There was no difference in the rate of post-ballooning among PPM groups. The rate of MSCT-based annular sizing was lower in the group of patients with severe PPM. Echocardiographic outcome at discharge is shown in Supplementary Table 2. Patients with severe PPM had higher mean transprosthetic gradients, lower Doppler velocity index and smaller EOA at discharge. No difference in the rate of more-than-mild PVL was present among PPM groups. Figure 1 illustrates the prevalence of PPM per THV design in the total study cohort. The majority of patients were treated with balloon-expandable intra-annular THVs, followed by self-expanding supra-annular THVs, self-expanding intra-annular THVs and self-expanding cusp-fixated THVs. Mechanically expandable infra-annular THVs were less commonly utilised and non-metallic THVs were rarely used. The lowest rate of severe PPM was present in self-expanding supra-annular THVs (4.3%), whereas the highest rate of severe PPM was detected in patients with self-expanding cusp-fixated (24.8%) and intra-annular THVs (23.9%). In patients receiving small THV sizes (≤23 mm), self-expanding intra-annular THV design showed the highest and supra-annular THV design the lowest rate of severe PPM, albeit the latter finding did not quite reach statistical significance (Supplementary Figure 1). Further information on PPM rates according to THV types used in the study population and the distribution of THV design according to annulus size are provided in Supplementary Appendix 1, Supplementary Figure 2 and Supplementary Figure 3. The risk of severe PPM decreased constantly over the study period, with an HR of 0.83 (95% CI: 0.77-0.90, p<0.001) per year (Supplementary Figure 4).
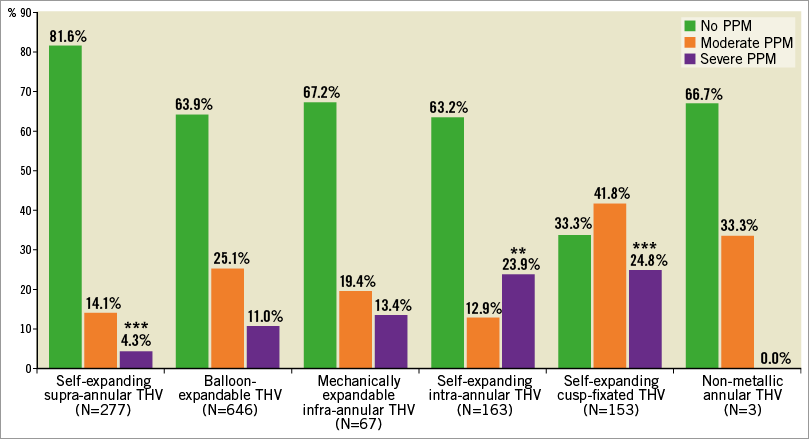
Figure 1. Prevalence of PPM according to all THV designs. ***p<0.001 for THV design vs. all other THV design groups. **p<0.01 for THV design vs. all other THV design groups.
OUTCOME ACCORDING TO PPM TAKING INTO CONSIDERATION LVEF AT BASELINE
There was no association of risk for all-cause mortality and PPM according to Kaplan-Meier analysis in the total patient cohort (severe vs. no PPM: HR 1.14, 95% CI: 0.84-1.55, p=0.41) (Figure 2A). Kaplan-Meier survival curves according to the presence of PPM after discriminating between patients with LVEF ≥40% and <40% are given in Figure 2B and Figure 2C, respectively. In patients with LVEF ≥40%, no association was found between PPM and mortality (severe vs. no PPM: HR 0.88, 95% CI: 0.60-1.30, p=0.52), whereas in patients presenting with an LVEF <40% severe PPM was associated with increased risk of mortality (severe vs. no PPM: HR 1.89, 95% CI: 1.13-3.16, p=0.016). As shown in Figure 3, with decreasing baseline LVEF the risk of all-cause mortality increased continuously in patients with severe PPM compared to those without PPM. Accordingly, in patients with baseline LVEF <40%, the rate of three-year mortality was 45.1% in patients without and 68.0% in those with severe PPM (p=0.041). Moreover, among patients with baseline LVEF <40%, severe PPM was found to be the strongest predictor of mortality according to multivariate analysis with an HR of 2.97 (95% CI: 1.58-5.59, p<0.001) (Supplementary Table 3). Further predictors of all-cause death in these patients were STS PROM, atrial fibrillation, and implantation of a new permanent pacemaker after TAVI. In contrast, a higher transprosthetic gradient at discharge was found to be protective against mortality. In the total patient cohort and in the subset of patients with baseline LVEF ≥40%, severe PPM showed no significant impact on mortality according to multivariate analysis (Supplementary Table 4, Supplementary Table 5).
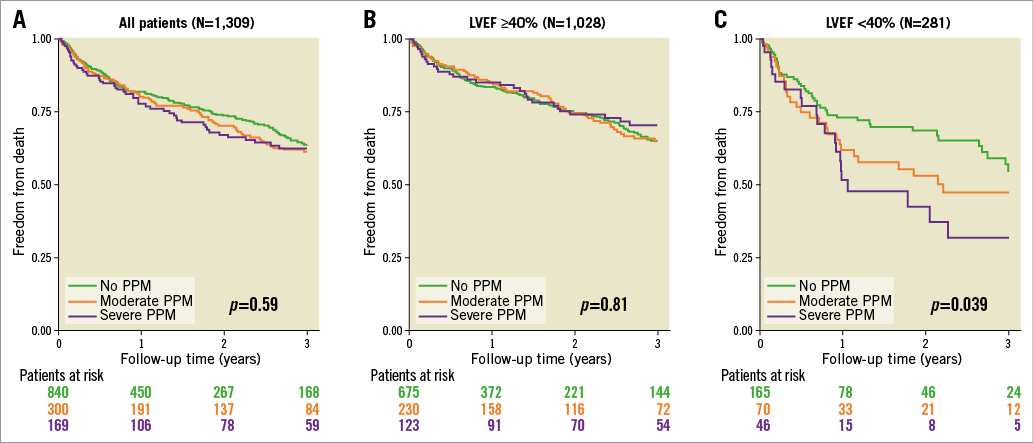
Figure 2. Mortality according to the presence of PPM. Kaplan-Meier survival curves according to the presence of no, moderate and severe PPM for the total patient cohort (A), patients with baseline LVEF ≥40% (B), and patients with baseline LVEF <40% (C).
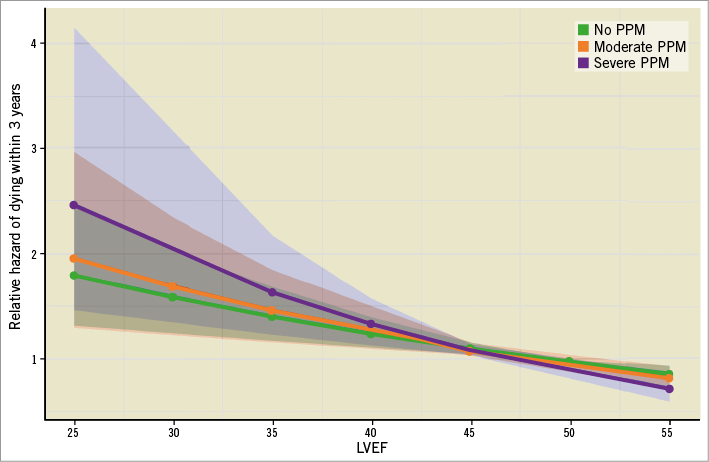
Figure 3. Risk of three-year mortality according to PPM depending on baseline LVEF. Light coloured areas display confidence intervals.
Discussion
The main findings of our study are as follows. 1) Moderate and severe PPM were detected in a substantial number of patients after TAVI but its incidence decreased over time. 2) Patients with PPM had higher BMI but smaller valve anatomies and received smaller valve sizes compared to those without PPM. 3) Supra-annular THVs showed the lowest rate of PPM. 4) In the total patient cohort PPM showed no impact on all-cause mortality. 5) However, among the subset of patients with LVEF <40%, severe PPM was strongly predictive of adverse outcome.
PREVALENCE AND CLINICAL FEATURES OF PPM AFTER TAVI
The prevalence of PPM after TAVI has so far been investigated in small, mostly single-centre studies6-10 as well as in randomised controlled trials, including the PARTNER trial A cohort11. In a recently published meta-analysis, severe PPM was found in 8% of TAVI patients12. However, these data only refer to first-generation devices. Our study is the first large-scale assessment of PPM after TAVI in a patient population in whom various currently available second-generation THVs were used in most of the cases. Interestingly, we found that the occurrence of PPM after TAVI decreased significantly over time and was, in fact, a rare finding in patients treated in recent years. The improved haemodynamic properties of currently available THVs, including various supra-annular designs, avoidance of significant valve undersizing by more accurate preprocedural planning using MSCT, as well as increased operator experience in terms of THV positioning, might have contributed to this finding6,13-15.
As shown in the present TAVI study, smaller annular and LVOT dimensions, smaller THV sizes and larger BMI are associated with severe PPM. However, in the current study, more than 20% of patients with severe PPM received valve sizes ≥27 mm, indicating that the risk for PPM is not restricted to small THV sizes. In fact, we were able to show that SVI, as an indicator for “low flow” across the aortic valve, is significantly lower in patients with severe PPM. Although the EOA is considered to be the most flow-independent parameter to assess the prosthetic valve function (e.g., in comparison to the transprosthetic gradient)3, there remains a flow dependency even of the EOA, as the flow across the prosthetic valve is directly incorporated in the continuity equation. Thus, for a given transprosthetic gradient, the calculated EOA will always be smaller in a patient with low flow compared to the same patient with normal flow. Accordingly, the mean transprosthetic gradient in the severe PPM group was 13.7 mmHg in the present study, which is far below the VARC-2 postulated cut-off of 20 mmHg for THV device success3. Interestingly, a higher mean transprosthetic gradient actually protected against adverse outcome among patients with impaired LV function (but not in the total patient cohort) in our study, probably because patients who are capable of generating higher gradients tend to have more contractile reserve. However, this flow dependency of both the EOA and, even more so, a single transprosthetic gradient, represents an inherent limitation of these parameters for the assessment of THV functionality.
RISK OF PPM ACCORDING TO THV DESIGN
As our data suggest, the risk of PPM might also be dependent on THV design. We found a higher rate of severe PPM in self-expanding cusp-fixated and intra-annular THVs and a significantly lower rate of severe PPM using supra-annular THV designs, indicating that the latter might be less prone to cause severe PPM. This is supported by previous data that also showed lower rates of severe PPM with a self-expanding supra-annular THV compared to a balloon-expandable THV in a propensity score-matched analysis of patients with small aortic annuli13. For surgical aortic valve prostheses, EOA references can be provided for all sizes of most of the currently used prosthetic valve types. Therefore, the occurrence of severe PPM after SAVR can be adequately predicted before insertion of a certain prosthetic valve type16. In contrast, such THV-specific reference values do not exist for TAVI, since the EOA after TAVI is directly linked to the individual annular dimension and the degree of THV expansion, making the risk of PPM after TAVI less predictable compared to SAVR. Thus, a limitation of our study was that data were not adjusted for valve size, annular dimensions, correct implantation position, and THV/annulus oversizing/undersizing. However, the impact of varying THV concepts with different haemodynamic properties on the risk of PPM is an important matter that should be considered in clinical practice and also addressed in future studies.
PROGNOSTIC IMPACT OF PPM ACCORDING TO BASELINE LVEF
The adverse impact of PPM following SAVR has been demonstrated in several studies, including a large meta-analysis incorporating data of more than 25,000 patients1. In contrast, PPM after TAVI has so far not clearly been linked to a higher mortality rate7,11,12. Severe PPM was found to be an independent predictor of death in only one single-centre analysis reporting outcome after CoreValve® (Medtronic, Minneapolis, MN, USA) implantation. In the present study, neither moderate nor severe PPM was associated with a significantly higher mortality rate in the total study cohort. Several reasons might have caused this differential impact of PPM on survival after SAVR and TAVI. Firstly, SAVR studies usually report longer follow-up data compared to TAVI studies; thus, the long-term effect of PPM after TAVI might not be fully elucidated. Secondly, as TAVI has been restricted to older patients with a higher rate of non-cardiac comorbidities, these factors might primarily determine patient outcome, thereby weakening the prognostic impact of PPM. Thirdly, patients with PPM present with larger BMI, which is known to be a powerful predictor of favourable outcome in TAVI patients17 and could counterbalance the putatively adverse effect of PPM.
The most important finding in the current study was that severe PPM after TAVI was independently and strongly associated with poorer outcome in patients with LVEF <40% compared to patients with LVEF ≥40%. An adverse effect of PPM in patients with impaired LV function has also been described for patients after SAVR2. So far, device iterations in the field of TAVI have been primarily directed to addressing the rate of PVL, because residual aortic regurgitation appeared to be the “Achilles’ heel” of the concept of TAVI in high surgical risk patients17. In the present study, which mainly comprises patients at intermediate surgical risk (STS PROM 6.2%), PVL was not an independent predictor of mortality. Also, other recent TAVI studies have found no or a less powerful impact of PVL on mortality in patients at lower surgical risk18,19. Thus, the present study emphasises that, to optimise the haemodynamic performance of a THV, not only the risk of PVL but also the potential risk of PPM should be counterbalanced, because both factors might be harmful to the patient, in particular in the vulnerable group of patients with reduced LV function. The detrimental effect of severe PPM in this subgroup of patients might be explained by a diminished left ventricular contractile reserve that renders those hearts more susceptible to an increased afterload caused by PPM.
Limitations
There are limitations inherent to the present study. First, the study has a retrospective design. Secondly, echocardiographic data were not adjudicated by an independent core lab. Thirdly, the study does not include echocardiographic follow-up data. Thus, THV durability according to PPM was not assessed in the present study. Fourthly, the study does not provide long-term mortality data or outcome parameters other than all-cause mortality. On the other hand, a particular strength of the current study is that a broad variety of different THV designs was used in large numbers by a single Heart Team.
Conclusions
The present study is the first large-scale analysis of PPM after TAVI including various THV types and assessing its impact on outcome with respect to baseline LVEF. Severe PPM was found in a significant number of patients and its occurrence was related to THV design and size. Moreover, we were able to identify the group of patients with LVEF <40% in whom mortality was strongly and independently predicted by the presence of severe PPM. As a consequence, patients at higher risk for and vulnerability to PPM should be identified and strategies should be implemented into clinical practice to protect against severe PPM, since this might improve outcome in AS patients presenting with impaired LV function.
| Impact on daily practice Severe PPM is found in >10% of patients after TAVI. Its presence depends on annular dimensions and THV design. Because severe PPM is an independent predictor of mortality in patients with poor LVEF, it should be recognised as an adverse finding after TAVI. Strategies to avoid it should be implemented into clinical practice. |
Conflict of interest statement
N. Schofer received travel compensation from Edwards Lifesciences and St. Jude Medical, speakers’ honoraria and travel compensation from Boston Scientific. F. Deuschl received travel compensation from Edwards Lifesciences, St. Jude Medical, Symetis and Boston Scientific. M. Seiffert served as consultant for JenaValve, received travel compensation from Edwards Lifesciences, Symetis, Boston Scientific and Biotronik and received speaking honoraria from Medtronic. J. Schirmer is a proctor for Symetis and JenaValve. L. Conradi is a proctor for and received speakers’ honoraria and travel compensation from JenaValve, Edwards Lifesciences and Boston Scientific, received speakers’ honoraria and travel compensation from Medtronic and is a consultant for Edwards Lifesciences. U. Schäfer is a consultant and proctor for Edwards Lifesciences, Abbott, Biotronik, JenaValve, Medtronic, Symetis and Boston Scientific, received speakers’ honoraria and travel compensation from JenaValve, Edwards Lifesciences, Abbott, Medtronic, Symetis and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. THV devices used in the study population.
Supplementary Figure 1. Distribution of prosthesis-patient mismatch according to transcatheter heart valve design for valve sizes ≤23 mm.
Supplementary Figure 2. Distribution of prosthesis-patient mismatch according to transcatheter heart valve type.
Supplementary Figure 3. Distribution of THV design according to annular size.
Supplementary Figure 4. Prevalence of PPM by year.
Supplementary Table 1. Echocardiographic baseline parameters.
Supplementary Table 2. Echocardiographic outcome at discharge.
Supplementary Table 3. Predictors of all-cause mortality in patients with baseline LVEF <40%.
Supplementary Table 4. Predictors of all-cause mortality in the total patient cohort.
Supplementary Table 5. Predictors of all-cause mortality in patients with baseline LVEF ≥40%.
To read the full content of this article, please download the PDF.

