Abstract
Aims: The aim of this study was to assess the feasibility and early outcomes of transcatheter aortic valve implantation (TAVI) in dysfunctional TAVI prostheses (redo TAVI).
Methods and results: Nineteen redo TAVI procedures were performed between October 2011 and November 2015 at two German centres. Mean age was 78 years, 13 (68%) were male, and the mean logistic EuroSCORE was 32%. Median time elapsed since index TAVI was 644 days (interquartile range 191-1,831). Failure mode of the index TAVI prosthesis was regurgitation (AR) in 16 patients (n=12 paravalvular AR, n=3 combined paravalvular/valvular AR, n=1 valvular AR) and stenosis in three patients. Device success was achieved in 89% (17/19). Median invasive post-interventional transprosthetic gradient was 3.0 mmHg. No severe prosthesis-patient mismatch (PPM) was observed. At one year, mean pressure gradient was 9±1.2 mmHg and no relevant PPM was documented in 90% of the cases. All-cause mortality at 30 days and one year was 11% and 33% (6/18, five non-cardiac deaths), respectively. Mean follow-up time was 404 days.
Conclusions: Redo TAVI appears to be feasible. Paravalvular regurgitation was the most common indication for a redo procedure. Rates of device success were high with low post-interventional gradients and no severe PPM. Good functional status of the prosthesis was maintained after 12 months, but mortality rates were high in this small comorbid patient population.
Introduction
Transcatheter aortic valve implantation (TAVI) is an established therapy for patients with severe symptomatic aortic valve stenosis at a prohibitive or high surgical risk1,2. Over the last decade, the number of implanted TAVI prostheses has rapidly increased3,4. Considering that indications for TAVI may be extended in the future to a broader population, concerns regarding the durability of TAVI prostheses have emerged5-7. Degeneration of biological valves is a well-known phenomenon, and the occurrence of primary valve failure has been described at a median of seven years after surgical valve replacement3,8-10. Few cases of TAVI prosthesis degeneration have been reported and, so far, treatment of dysfunctional TAVI prostheses has only been sporadically described5,6,11-15.
TAVI has been established as a valuable therapy for degenerated surgical bioprostheses. Data from the Valve-in-Valve International Data (VIVID) registry have demonstrated favourable results, with a one-year survival rate of 83% and no evident differences between self-expanding and balloon-expandable TAVI valves in terms of clinical outcomes16. Consequently, TAVI in TAVI can be a treatment option for dysfunctional TAVI prostheses. This retrospective cohort study provides a first insight into the clinical characteristics and outcome data of patients who underwent TAVI in TAVI (redo TAVI) procedures performed at two centres in Germany.
Methods
PATIENTS
Between 2008 and 2015, 1,600 TAVI procedures were performed at the Asklepios Klinik St. Georg, Hamburg, Germany, and, from 2007 to 2015, 701 TAVI procedures were performed at the Heart Center in Bad Segeberg, Germany. The first redo TAVI was performed in October 2011. Between October 2011 and November 2015, 19 patients with dysfunctional TAVI prostheses underwent a second TAVI (Hamburg, n=12; Bad Segeberg, n=7). We defined a redo TAVI as a second procedure using a further prosthesis implanted inside the initial one due to symptomatic significant stenosis or regurgitation requiring rehospitalisation or occurring at least 30 days after the first TAVI procedure. Patients who received two TAVI prostheses as a bail-out procedure during the index intervention were excluded from this study. For the current study, a retrospective analysis was performed to evaluate indications, valve function and clinical follow-up of all redo TAVI patients.
REDO TAVI PROCEDURE
Redo TAVI procedures were mainly performed by the percutaneous transfemoral technique (n=17). Careful attention was paid during crossing of the index prosthesis to avoid paravalvular crossing or crossing through one of the stent struts12. Therefore, after crossing the index prosthesis, a pigtail catheter was inserted into the left ventricle and rotated to ensure free movement. The implantation landmark of the new TAVI prosthesis was the native aortic annulus visualised by fluoroscopy/angiography. After implantation of the second prosthesis, the delivery catheter was removed and the stiff wire replaced by a pigtail catheter to assess the transvalvular pressure gradient. Finally, a standardised aortic root angiography (25 ml with 15 ml/s) was performed to assess the final result (Figure 1).
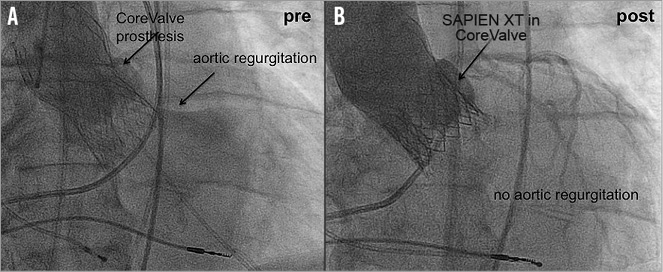
Figure 1. Pre- and post-implantation angiographic images of a redo TAVI procedure. A) CoreValve prosthesis with severe paravalvular aortic regurgitation. B) After redo TAVI with a SAPIEN XT valve into the CoreValve prosthesis with no residual aortic regurgitation.
For sizing of the second TAVI prosthesis, the diameter of the index prosthesis was assessed in accordance with the manufacturer’s reported diameter, and diameters were additionally evaluated by transoesophageal echocardiography and/or multislice computed tomography (CT). The distance to the coronaries and the related anatomy of the aortic root were assessed using multislice CT and/or invasive angiography in accordance with local institutional guidelines.
ENDPOINT DEFINITIONS
Endpoints were defined in accordance with the second report of the Valve Academic Research Consortium (VARC-2)17. Transvalvular pressure gradients were initially assessed invasively immediately after implantation of the second TAVI prosthesis, and follow-up measurements were performed with transthoracic echocardiography. Prosthesis-patient mismatch (PPM) was assessed in accordance with the VARC-2 criteria using the indexed effective orifice area.
STATISTICAL ANALYSIS
Continuous variables are described as means and standard deviations if normally distributed, or as medians plus interquartile range (IQR) or range if not. Categorical variables are described with absolute and relative frequencies. All data were entered into a table and analysed employing the built-in analysis of GraphPad Prism, Version 5 (GraphPad Software, San Diego, CA, USA).
Results
PATIENTS
The patient population had a mean age of 77.8±6.6 years with 13 male patients (68.4%). Mean logistic EuroSCORE was 31.6±20.1% (range 7.0-87.4%), while the median EuroSCORE II was 6.9% (IQR 3.7-15.0%). The index TAVI was not included as a patient-related risk factor in the calculation of the logistic EuroSCORE and EuroSCORE II. Patients were severely symptomatic at the time of presentation, with 95% (n=18) of the patients presenting in New York Heart Association (NYHA) functional Class III (n=8) and IV (n=10); one patient was in NYHA Class II. Baseline patient characteristics are given in Table 1.
INDICATIONS FOR REDO TAVI
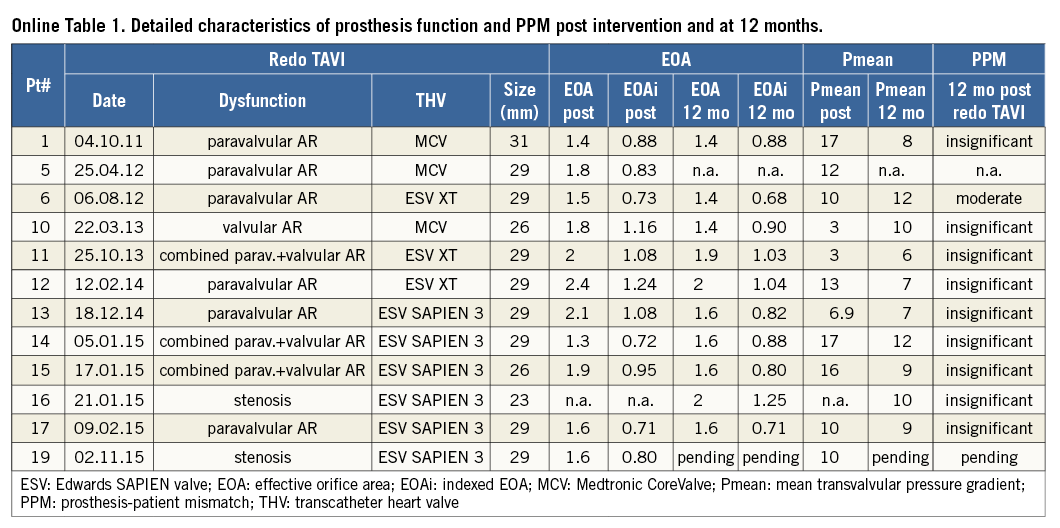
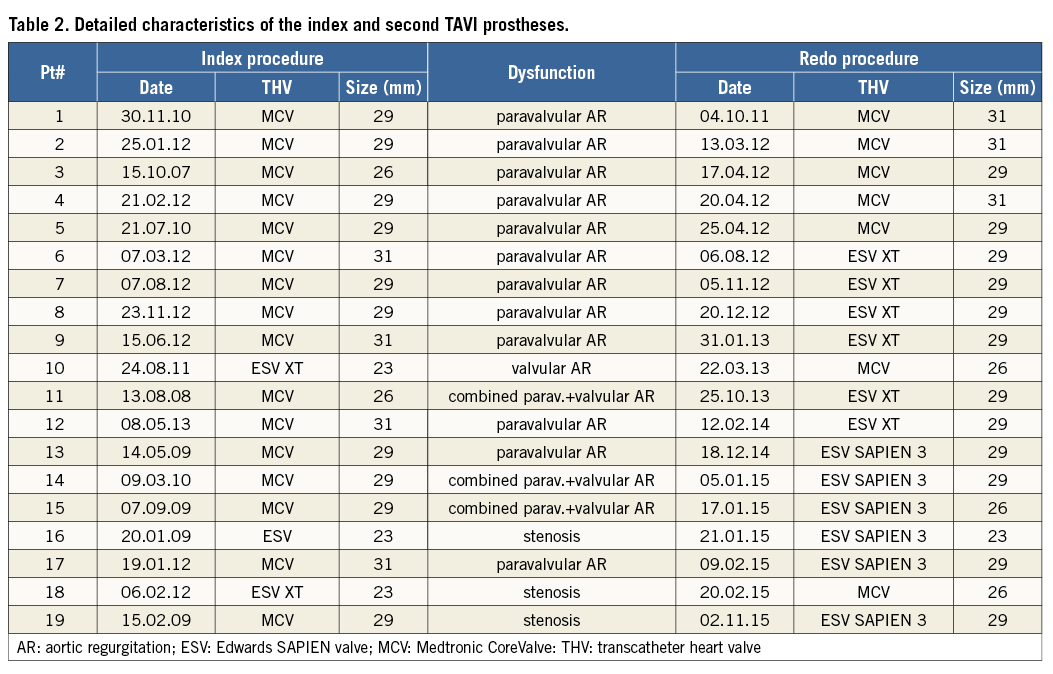
At the time of redo TAVI, a median of 644 (IQR 191-1,831) days had elapsed since the index TAVI. The earliest redo TAVI was performed 27 days after the first procedure. The mechanism of failure of the index prosthesis was aortic regurgitation (AR) in 16 patients (84%) and stenosis in three patients (16%). AR was mainly paravalvular in origin (n=12, 75%), and was definitely present after the first TAVI procedure in eight patients. Three patients had combined paravalvular and transvalvular AR, and only one patient had isolated transvalvular AR (Figure 2). Dysfunctional prostheses were the self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA; n=16) and the balloon-expandable SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA; n=3). For Redo TAVI procedures, the self-expanding CoreValve (n=7) and the balloon-expandable SAPIEN XT (n=6) and SAPIEN 3 (Edwards Lifesciences; n=6) valves were used. In the majority of the procedures (11/19), a balloon-expandable valve (BEV) was implanted into a self-expanding valve (SEV), but all other combinations were also performed (SEV in SEV, n=5; SEV in BEV, n=2; and BEV in BEV, n=1). Detailed characteristics of the index and second TAVI prostheses are shown in Table 2.
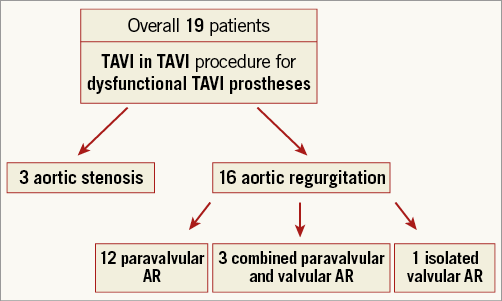
Figure 2. Patient flow chart.
PROCEDURAL DETAILS
All redo TAVI procedures were performed in a hybrid operating room. Vascular access was predominantly transfemoral (n=16, 84%); transapical access was used in two patients and transaortic access in one. Most procedures were performed under mild analgosedation (n=13, 68.4%), while general anaesthesia was used in six patients (31.6%). Reasons for general anaesthesia were cardiogenic shock, pre-existing mechanical ventilation, or access route.
Median procedure time was 100 (IQR 85-120) minutes. Median fluoroscopy time was 22 (IQR 14-28) minutes. Mean contrast dye amount was 130±62 ml. Predilation of the index prosthesis was not performed in any case, while post-dilation was performed in seven cases. Transseptal puncture and antegrade crossing and snaring of a J-wire was necessary in one case due to the inability to cross a stenotic TAVI prosthesis retrogradely (Medtronic CoreValve, 29 mm).
SAFETY AND DEVICE SUCCESS
Device success was achieved in 17 patients (89%). Causes of device failure were residual AR of grade 2+ in one patient and valve embolisation requiring an additional (third) valve in another patient. Residual AR as determined by post-procedural angiography was none or trace in 13 patients (68%), mild in five patients (26%), and moderate in one patient (5%). Coronary obstruction or conversion to open heart surgery did not occur. No deaths or strokes occurred within the first 72 hours. Only two minor vascular complications were reported. All-cause mortality at 30 days was 11% (two patients who were in cardiogenic shock before the procedure). One disabling stroke occurred at day three. The rate of new pacemaker implantation at 30 days was 11% (n=2). Reasons for implantation of a pacemaker were complete atrioventricular block in one case and slow atrial fibrillation in the other one.
POST-IMPLANTATION PRESSURE GRADIENTS, EFFECTIVE ORIFICE AREA AND PPM
The median invasive post-interventional transprosthetic mean pressure gradient (Pmean) was 3.0 (IQR 1-6.5) mmHg. Even though pre-interventional prosthesis dysfunction was mainly AR (16/19, 84%), the median post-interventional effective orifice area (EOA) increased from 1.8 (IQR 1.2-2.5) to 2.7 (IQR 2-3.5) cm². The indexed EOA (EOAi) increased from 0.9 (IQR 0.7-1.4) to 1.5 (IQR 1.08-1.7) cm²/m². No acute severe PPM following redo TAVI was observed. According to the VARC-2 definition, insignificant PPM was reported in 89% (16/18) of patients, while two patients had moderate PPM. Echocardiographic or invasive data to calculate PPM were not available in one patient. Detailed characteristics of the post-interventional prosthesis function and PPM are shown in Table 3.
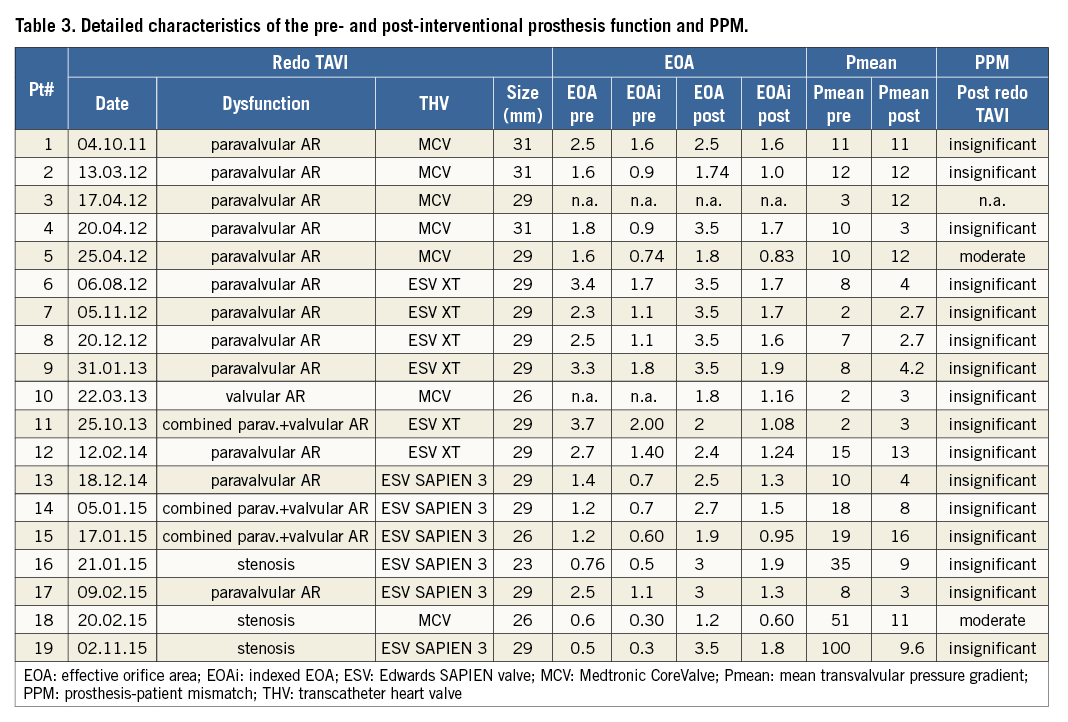
One-year echocardiography was available in 12 patients (six patients died and one patient was lost to echocardiographic follow-up). Mean pressure gradient (Pmean) at 12 months was 9 (median IQR 7.25-10) mmHg. Median EOA was 1.6 (IQR 1.45-1.83) cm² and EOAi was 0.88 (IQR 0.81-1.0) cm²/m². PPM was insignificant in nine patients and moderate in one patient. Detailed characteristics of the prosthesis function and PPM at 12 months are shown in Online Table 1.
Valve thrombosis was diagnosed in one patient 10 months after redo TAVI (SAPIEN 3 in CoreValve). This patient had been on dual antiplatelet therapy since the redo procedure. The diagnosis was based on an elevated pressure gradient (mean pressure gradient 32 mmHg) that resolved with oral anticoagulation (mean pressure gradient of 12 mmHg and an EOA of 1.6 cm2).
CLINICAL OUTCOME
One-year clinical follow-up was achieved in 95% of patients (18/19), since the one-year follow-up visit is still pending for one patient. The mean follow-up time was 404±340 days. At one year, six patients (33%) had died (one cardiac and five non-cardiac deaths). The Kaplan-Meier survival curve is shown in Figure 3. For the remaining patients (n=12), three were in NYHA Class I, six were in NYHA Class II and two were in NYHA Class III (NYHA class could not be feasibly assessed in one patient due to a disabling stroke). Three of the six patients who died during the first 12 months were in a critical condition (in cardiogenic shock or mechanically ventilated) before redo TAVI. Overall, the logistic EuroSCORE was higher in the group of patients who died during the first 12 months compared to the patients who were alive at that time point, even though statistical significance was not reached (mean logistic EuroSCORE: dead: 43.6±27.2%; alive: 26.1±11.1%; p=0.07).
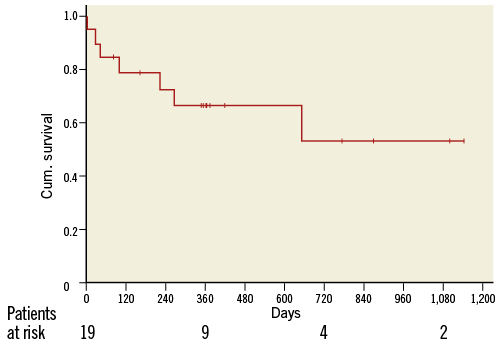
Figure 3. Kaplan-Meier survival curve.
Discussion
MAIN FINDINGS
The main findings of this study are as follows:
– Current indications for redo TAVI are mainly paravalvular regurgitation, but also early restenosis
– Redo TAVI for dysfunctional TAVI prostheses is feasible for both balloon-expandable and self-expanding index devices
– Redo TAVI is associated with high device success rates (89%)
– Redo TAVI is associated with low residual pressure gradients and no severe PPM up to one-year follow-up
INDICATIONS FOR REDO TAVI
Paravalvular AR negatively affects the prognosis after TAVI with a dramatic increase in mortality and morbidity in patients with more than mild AR18-20. In our cohort, the most common indication for redo TAVI was pre-existing or worsening paravalvular AR rather than leaflet degeneration or dysfunction. Paravalvular AR usually results from incomplete circumferential apposition of the TAVI prosthesis skirt within the aortic annulus, malpositioning or undersizing3,20-22, particularly with first-generation devices. Several corrective techniques have been proposed to overcome post-TAVI paravalvular AR. Even though data regarding these techniques are mainly derived from small series or case reports, balloon-post-dilation and valve-in-valve are the most common approaches to tackle this complication. This is different from valve-in-valve procedures where a TAVI prosthesis is implanted into a surgical valve with a rigid ring. In the latter situation, paravalvular AR cannot be treated with a TAVI prosthesis5,23,24.
FEASIBILITY AND SAFETY
The current study underscores the feasibility of redo TAVI procedures. In addition, redo TAVI appears to be rather safe, as the procedure was uneventful in 18 out of 19 patients (embolisation occurred in one patient due to misplacement of the prosthesis with too low an implantation depth). Overall, the device success rate was high (89%) with no conversion to open heart surgery, documented coronary obstruction or intraprocedural cardiopulmonary resuscitation. These results are comparable with initial data reported from the global VIVID registry for TAVI in degenerated surgical bioprostheses, where device success rates up to 93% have been described16,25. The safety of these procedures is important since these patients are at high risk for redo surgery, and periprocedural complications are associated with a high risk of mortality11-15,26-28.
DEVICE SUCCESS AND TRANSVALVULAR PRESSURE GRADIENT
The main reason for device failure in valve-in-valve procedures is an elevated post-interventional mean transvalvular gradient. In the VIVID registry, 27% of patients had a residual transvalvular gradient ≥20 mmHg16. On the other hand, in our study, no patient had an elevated transvalvular gradient after redo TAVI, which is an important observation. Hence, the phenomenon of elevated post-procedural gradients after TAVI in surgical bioprosthetic valves does not seem to be an issue with redo TAVI, in which residual gradients are reasonably low.
Data from the PARTNER trials showed no difference at five years in durability and valvular function between TAVI prostheses and surgically implanted ones7,12. At 12-month follow-up, the mean pressure gradients were still low after redo TAVI, which may be an important observation in the current debate on TAVI durability.
PROSTHESIS-PATIENT MISMATCH
PPM is common after SAVR and after valve-in-valve procedures. The incidence of PPM in the PARTNER trial cohort A was 60% (28% severe) in SAVR patients, as opposed to 46% (20% severe) in TAVI patients17,29. Data from the CoreValve US High Risk Pivotal Trial showed that severe PPM was more common in patients treated with SAVR (25.7%) than in patients treated with TAVI (6.2%)18-20,30. In the VIVID registry, the incidence of severe PPM was 32%18. Importantly, in the CoreValve US High Risk Pivotal Trial, patients with severe PPM were at a greater risk of death and acute kidney injury than patients without severe PPM30. Pibarot et al also showed that pre-existing severe PPM is associated with a significant adverse impact on survival29. On the other hand, in the VIVID registry, one-year survival was not significantly different between patients with and without severe PPM17. However, since severe PPM may have an impact on survival, the lack of significant PPM after redo TAVI is encouraging. One explanation might be the lower profile of the transcatheter heart valves compared to surgical valves. Another possible explanation for this finding might be the accurate annulus sizing which is fundamental prior to the initial TAVI procedure and which is not performed in patients prior to a SAVR, since sizing in SAVR is performed intraoperatively. In addition, TAVI prostheses are commonly oversized to ensure optimal anchoring and sealing of the paravalvular space, which might further explain the low rate of PPM after redo TAVI. The additional radial force of the second prosthesis with or without additional balloon dilatation may also limit the occurrence of PPM. Finally, the lack of a stiff surgical valve ring in THVs (which is commonly the aetiology for THV underexpansion in valve-in-valve procedures and the reason for elevated post-procedural gradients and PPM) may further explain the low PPM rates observed in this study.
VALVE TYPE SELECTION FOR REDO TAVI
In this study, various combinations for redo TAVI procedures are described (balloon-expandable valves [BEV] in BEV, self-expanding valves [SEV] in BEV, SEV in SEV and BEV in SEV). Since the number of patients is limited, we cannot give a definite recommendation regarding valve type selection. However, we believe that in patients with paravalvular regurgitation BEV may have an advantage if the index prosthesis is not fully expanded, since the radial force of the prosthesis might further expand the initial valve and reduce the paravalvular leak. This is essentially different compared to surgical bioprostheses, as further dilatation of a surgical valve ring is not usually possible. Nevertheless, the main indication for a redo TAVI in our cohort was a PVL due to a low implantation of the index prosthesis. An optimal implantation of the second prosthesis independent of the implantation mode (BEV or SEV) usually results in an optimal valve function.
FOLLOW-UP
In addition to the technical success and excellent haemodynamics observed after redo TAVI, clinical status as measured by the NYHA functional class has improved in all surviving patients. The relatively high mortality rate after one year (predominantly from non-cardiac causes) reflects the high-risk characteristics of the treated population and should be interpreted in this context. Patient selection is definitely an important pre-procedural aspect. Extremely comorbid patients remain at high risk for an adverse outcome. In high-risk patients with a long medical history or a long pre-interventional hospital stay the indication for a redo procedure always needs to be discussed by the local Heart Team.
Overall, with the current expansion of TAVI to intermediate and lower-risk patients and the expected occurrence of prosthesis degeneration with long-term follow-up, the observations of the current analysis may have promising clinical implications.
Limitations
This is an observational study with a limited number of patients. The main failure mode for the majority of patients was paravalvular regurgitation due to initial sizing or positioning issues rather than prosthesis degeneration/dysfunction. In addition, the data presented are derived from a retrospective analysis of prospectively collected data.
Conclusions
Redo TAVI appears to be feasible for dysfunctional balloon-expandable and self-expanding index TAVI devices. Current indications for redo TAVI are mainly paravalvular regurgitation, but restenosis and transvalvular regurgitation have also been observed and treated. Rates of device success are high and post-interventional gradients are extremely low, with no significant PPM. Good functional status of the second prosthesis was maintained in all patients up to one year.
| Impact on daily practice In patients with a dysfunctional TAVI prosthesis, a repeat TAVI procedure appears to be feasible and is associated with excellent haemodynamic outcomes. Further large-scale clinical trials are warranted to document long-term outcomes in this population. |
Conflict of interest statement
T. Schmidt reports lecture fees from Medtronic. C. Frerker reports lecture fees from Edwards Lifesciences. U. Schäfer reports receiving lecture fees from and is a proctor for Medtronic and Edwards Lifesciences and also reports receiving institutional research grants from Medtronic and Edwards Lifesciences. K-H. Kuck reports receiving research grants from Medtronic and is a consultant for Edwards Lifesciences. J. Jose has been supported by an EAPCI grant in Interventional Cardiology which has been partially sponsored by Medtronic. E. Holy is currently supported by an EAPCI grant in Interventional Cardiology which is partially sponsored by Edwards Lifesciences. G. Richardt reports receiving lecture fees from Edwards Lifesciences. M. Abdel-Wahab reports receiving institutional research grants from St. Jude Medical and Biotronik and is a proctor for Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data