
Introduction
“Valve-in-valve” transcatheter aortic valve implantation (ViV-TAVI) is an alternative to conventional redo surgery for bioprosthetic aortic valve replacement (AVR) dysfunction1 but ViV-TAVI for degenerate stentless bioprostheses is challenging2. We report the five-year follow-up of a single-centre consecutive series of patients undergoing ViV-TAVI for degenerate homograft AVR.
Methods
All patients receiving ViV-TAVI for degenerate homograft AVR between October 2009 and April 2019 were retrospectively analysed. Clinical, echo, and brain natriuretic peptide (BNP) levels were measured preprocedurally and after 1, 3, and 5 years. The Royal Brompton and Harefield Clinical Practice Committee approved the study protocol, the study was performed in compliance with UK Data Protection and Information Governance legislation, and all subjects gave written, informed consent to ViV-TAVI and study participation.
ViV-TAVI PROCEDURE
Our ViV-TAVI procedural methods for degenerate homografts lacking calcified leaflets (Figure 1) have been reported previously3.
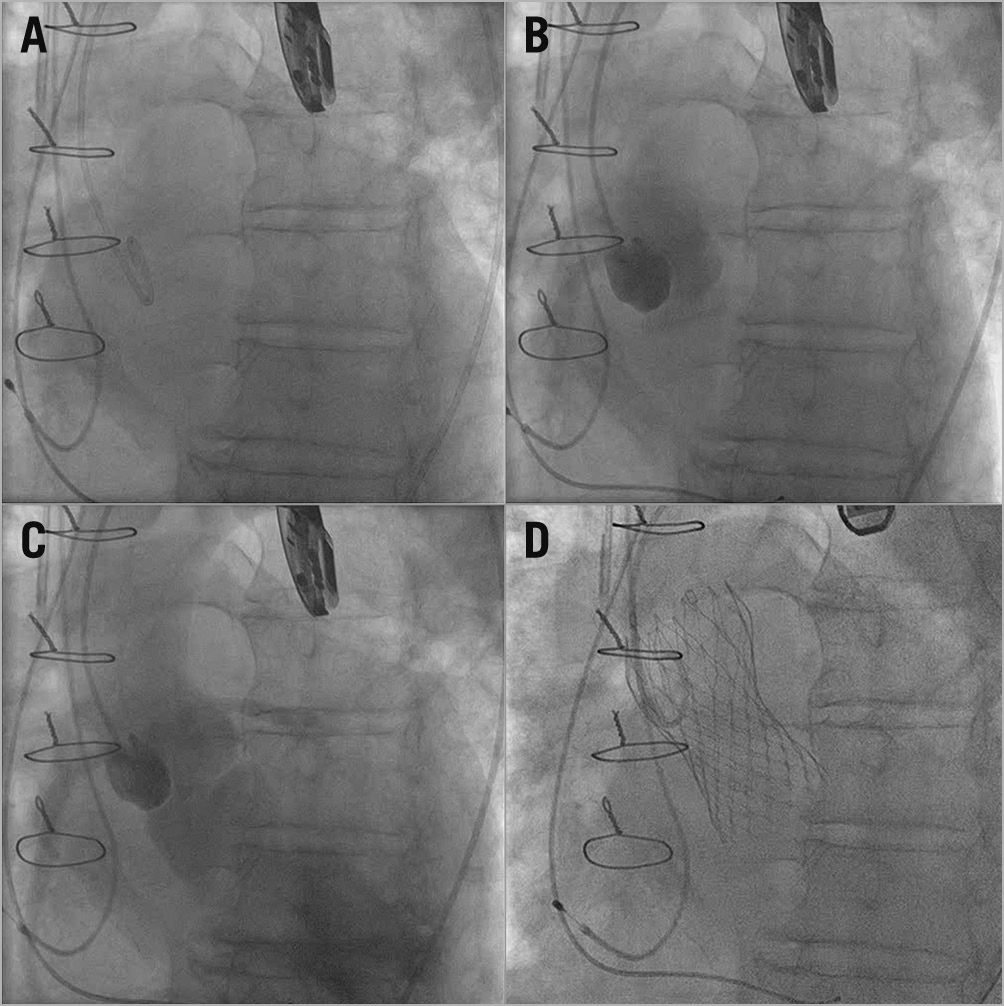
Figure 1. Homograft AVR. A) & B) No calcific landmarks. C) Severe transvalvular regurgitation. D) Final ViV-TAVI deployment.
Results
PATIENT CHARACTERISTICS
Twenty-seven patients with degenerate homograft AVR (aged 68±16 years, range 25-88 years, 19 male) underwent ViV-TAVI. Five were <50 years old (4 had each undergone 2 previous homograft AVRs, 1 was a 25-year-old on cardiac mechanical support). Median time from homograft AVR to ViV-TAVI was 15 years (range 5-22 years). All presented with severe symptomatic transvalvular aortic regurgitation (AR), 63% in New York Heart Association (NYHA) Class III and 37% in NYHA Class IV. Patients had multiple comorbidities (Table 1), EuroSCORE II 21±16% and STS-PROM score 15±10%. The left ventricular (LV) cavity was dilated and impaired, patients had raised LV filling pressure (E/E’ 19±8), mitral and tricuspid regurgitation, and pulmonary hypertension (42±5 mmHg) (Table 2). Mean (IQR) homograft label size was 22 mm (20-26 mm) and aortic annular perimeter on multi-detector computerised tomography was 73 mm (63-82 mm).
HOMOGRAFT ViV-TAVI PROCEDURE
The access route was transfemoral in 25 patients, left subclavian in 1, and direct aortic in 1. Before June 2014, a CoreValve® (Medtronic, Minneapolis, MN, USA) THV was implanted in 18 patients; after June 2014, 6 received an Evolut™ R THV (Medtronic) and 3 received a Direct Flow Medical® THV (Direct Flow Medical, Santa Rosa, CA, USA). Unconstrained THV perimeter (IQR) was 82 mm (72-97 mm), and mean THV device oversizing was 11.8% (range 2.7-23.4%).
SHORT-TERM OUTCOMES
There were no intraprocedural deaths, myocardial infarction, tamponade, stroke, or major vascular complications. One patient died within 30 days from a subarachnoid haemorrhage (30-day mortality 3.7%). Periprocedural device migration occurred in two patients requiring a second THV implantation during the same procedure: in both cases, the initial THV devices were oversized by 2-3%. There were no cases of device embolisation or coronary obstruction, and paravalvular leak (PVL) was absent/trivial/mild in 24. Thirteen patients were discharged on warfarin (pre-existing atrial fibrillation), the remainder on a single antiplatelet agent.
5-YEAR OUTCOME
Median follow-up was 2,046 days (range 9-3,684 days); one patient was lost to follow-up. Actuarial mortality at 1, 3, and 5 years was 11.4% (CI: 3.8-31.5%), 15.5% (CI: 6.1-36.2%), and 25.2% (CI: 12.0-48.1%), respectively (numbers at risk 22, 21, and 15) (Figure 2). Two patients died between 30 days and 1 year: the first (the salvage case on mechanical support [also the youngest patient]) died at 35 days from multi-organ failure; the second died after 238 days of bronchopneumonia. A further 3 patients died between 1 and 3 years (1 stroke, 2 respiratory); 2 had mild-moderate PVL at one-year follow-up, while the third patient with mild-moderate PVL at 1 year was lost to follow-up. No patients died between 3- and 5-year follow-up.
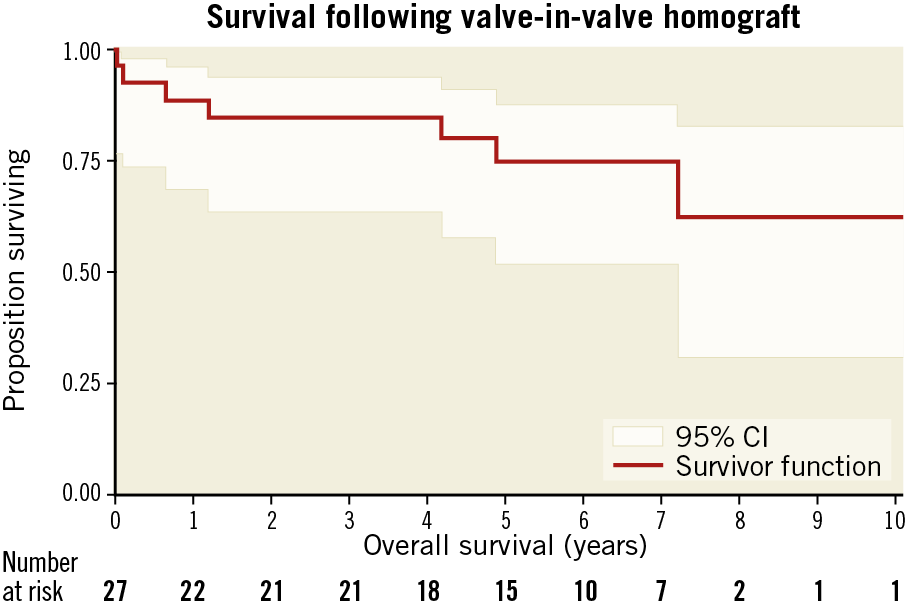
Figure 2. Kaplan-Meier curve for ViV-TAVI for failing homograft AVR.
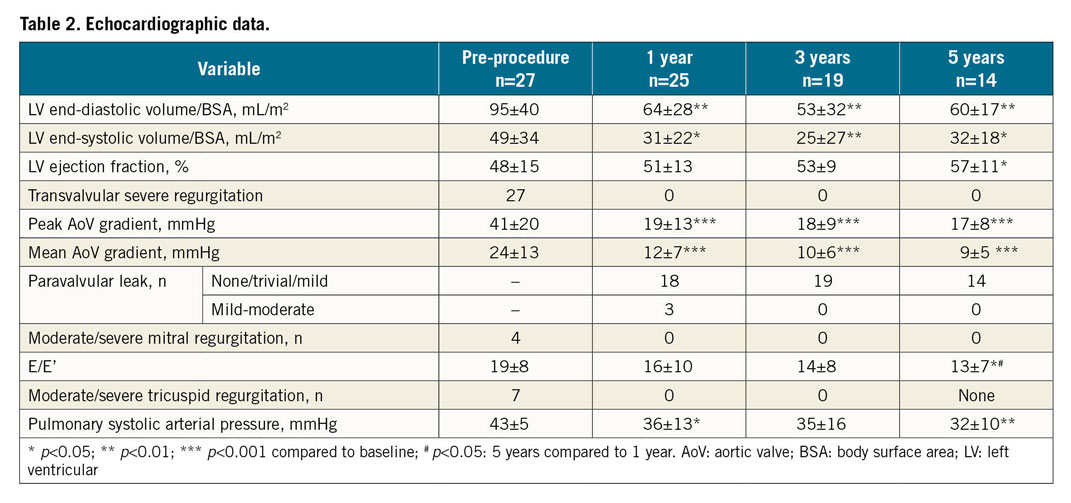
After five years, the patients’ NYHA class decreased from 63% NYHA Class III, 37% NYHA Class IV to 7% NYHA Class II, 93% Class I; BNP levels also decreased (500±570 ng/L to 188±131 ng/L, p=0.01). There was no echo evidence of late THV migration, increase in severity of PVL, device thrombosis, endocarditis, or structural valve dysfunction. No patient required reintervention. Left ventricular ejection fraction (LVEF) increased, LV cavity size and aortic pressure gradients decreased, aortic valve area did not change (p=0.81), no patient had more than mild mitral or tricuspid regurgitation, and E/E’ and pulmonary pressures decreased (Table 2).
Discussion
Actuarial mortality after ViV-TAVI for high-risk patients with degenerate homograft AVR at 1, 3, and 5 years was comparable to five-year mortality for stented and stentless ViV-TAVI4 and redo surgical AVR5. At five years, transcatheter devices were durable, and LV function and haemodynamics improved, highlighting that ViV-TAVI in a degenerated homograft can be performed safely with favourable midterm to long-term outcomes.
Limitations
This was a single-centre study, performed by select operators, using predominantly self-expanding THV, and hence is subject to the limitations of one unique centre’s pattern of practice. THV malpositioning and paravalvular leak severity were not analysed by a central core lab. Although it would have been of interest to investigate possible predictors for mortality at 1, 3, and 5 years in a Cox regression analysis that might guide patient selection, our sample size was too small and underpowered to detect any differences.
Conclusion
Despite technical challenges, five-year mortality of 25.2% following ViV-TAVI for failing homograft AVR in a high-risk population is comparable with redo surgical AVR. Long-term valve durability appears favourable and was associated with recovery of LV function and haemodynamics in this single-centre series.
|
Impact on daily practice At five years, ViV-TAVI in high-risk patients with degenerated homograft AVR has a mortality rate comparable with redo surgical AVR. The THV devices appear durable with no cases of late structural valve dysfunction. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

