
Abstract
Aims: The aim of this meta-analysis was to evaluate the evidence regarding the rates of procedural success and the incidence of adverse outcomes following valve-in-valve (VIV) transcatheter aortic valve replacement (TAVR) in patients with failed bioprosthetic aortic valves.
Methods and results: A systematic search of major electronic databases was conducted for studies relevant to patients with failed bioprosthetic aortic valves undergoing VIV-TAVR. The primary outcome was procedural success. A total of 5,553 patients from 24 studies were included. The mean Society of Thoracic Surgeons (STS) score was 7.84±5.14. The procedural success rate was high (97%, 95% confidence interval [CI]: 94-98%). At 30 days, all-cause mortality was 5% (95% CI: 3-6%), stroke 2% (95% CI: 1-2%), myocardial infarction 1% (95% CI: 1-2%), permanent pacemaker placement 6% (95% CI: 5-8%), and aortic regurgitation 7% (95% CI: 5-10%). At one year, the incidence of all-cause mortality was 12% (95% CI: 10-14%), stroke 3% (95% CI: 2-4%), myocardial infarction 1% (95% CI: 0-2%), and permanent pacemaker placement 7% (95% CI: 5-11%). At three years, the incidence of all-cause mortality was 29% (95% CI: 25-34%) and stroke 6% (95% CI: 5-9%).
Conclusions: VIV-TAVR appears to be associated with high procedural success rates and low adverse outcomes during the short-term and midterm follow-up period.
Introduction
Bioprosthetic valves implanted with surgery or with the transcatheter approach have been shown to have a durability of up to 10 years post implantation1. With the improved durability of newer generations of bioprosthetic valves and the accompanying lower bleeding and thromboembolic risks over mechanical valves, they are becoming an attractive alternative to treat severe aortic stenosis in younger patients <60 years old2,3. As a direct consequence, the rates of redo aortic valve replacement procedures are expected to increase substantially in the near future, given the longer life expectancy of patients receiving aortic valve replacement4.
Transcatheter aortic valve replacement (TAVR) has emerged as an acceptable therapy for most of the spectrum of patients with severe aortic stenosis (i.e., from high-risk inoperable patients to low-risk patients)5,6,7. Redo surgical aortic valve replacement (SAVR) carries a higher risk of procedural complications and intraoperative mortality, and thus valve-in-valve (VIV) TAVR has emerged as an alternative to redo SAVR in patients with high operative mortality risk; however, the studies were mostly small and single-centred8.
Therefore, we aimed to conduct a systematic review and meta-analysis evaluating the available evidence regarding the short-term and midterm procedural outcomes of VIV-TAVR in patients with failed bioprosthetic aortic valves.
Methods
DATA SOURCES
The PubMed, Cochrane, and Embase electronic databases were searched from inception until January 2020 for observational cohort studies and randomised controlled trials reporting clinical outcomes in patients with VIV-TAVR. This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was prospectively registered on the PROSPERO international prospective register of systematic reviews: CRD42019136105.
The study screening and selection process (PRISMA diagram) is presented in Supplementary Figure 1. Details of the search strategy are shown in Supplementary Table 1.
INCLUSION AND EXCLUSION CRITERIA
A study was included if it satisfied the following criteria: i) a randomised trial, prospective cohort, or a retrospective cohort, ii) reporting outcomes of interest in patients undergoing VIV-TAVR. Studies that were published as conference abstracts, case reports, narrative reviews, studies with <20 patients, studies that were designed as case series or studies that included the same patient population were excluded from this analysis.
DATA EXTRACTION
Two authors (M.M. Gad and A.A. Mahmoud) independently screened the titles and abstracts of the searched studies, screened full-text studies, and extracted study and population characteristics and outcomes of interest.
DEFINITION OF OUTCOMES
The primary outcome was the procedural success as defined by the individual study (Supplementary Table 2). The secondary outcomes were all-cause mortality, myocardial infarction, stroke, the incidence of aortic regurgitation, placement of a permanent pacemaker, and mean gradient across the valve at 30 days, one year, and three years.
ASSESSMENT OF STUDY QUALITY
The “Risk Of Bias In Non-randomised Studies - of Interventions” (ROBINS-I) scale was used to evaluate the risk of bias in the included studies9 (Supplementary Table 3).
STATISTICAL ANALYSIS
Single-arm proportion-weighted meta-analysis calculation was performed using the inverse variance method using the “meta” function of R statistical software10. Summary estimates were calculated using the DerSimonian and Laird random effects model11. The I2 statistic was used to assess the heterogeneity between the included studies. Continuity correction of 0.5 in studies with zero cell frequencies was utilised by the statistical package. Pre-specified subgroup analyses were performed based on device type (balloon-expandable versus self-expanding), country (USA-based versus outside of the USA), study design (retrospective versus prospective), and procedure used in prior valve replacement (SAVR versus TAVR).
Results
DATA SYNTHESIS
Among 1,914 records initially screened by title and abstract, 24 studies satisfied our final inclusion and exclusion criteria, comprising a total of 5,553 patients with a bioprosthetic aortic valve undergoing VIV-TAVR12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35. Six studies were not included, although they satisfied our inclusion criteria. One was excluded because a longer-term follow-up study was published using the same cohort36. The remainder of excluded studies were subsets of larger cohorts of patients published in another study37,38,39,40. Ten studies were prospective12,13,14,16,20,26,29,32,33,34, and the remainder of the studies were retrospective cohorts15,17,18,19,21,22,23,24,25,27,28,30,31,35 (Supplementary Table 4). The details of our systematic search are reported in the PRISMA figure (Supplementary Figure 1).
STUDY AND PATIENT CHARACTERISTICS
The weighted mean age of patients was 76.63±8.78 years and 61.8% were male. The baseline pooled mean ejection fraction (EF) was 52.14±11.36%. The pooled mean Society of Thoracic Surgeons (STS) score was 7.84±5.14. Balloon-expandable VIV-TAVR was performed exclusively in three studies20,31,34, and six studies reported using self-expanding valves only13,15,19,21,26,28. One study reported balloon-expandable and self-expanding valve outcomes separately23. The duration of the follow-up ranged from 30 days to three years. Overall, most of the studies were deemed to be of high quality based on the ROBINS-I scale (Supplementary Table 3). The baseline characteristics of the studies are reported in Supplementary Table 4.
PROCEDURAL SUCCESS
A total of 22 studies reported procedural success of VIV-TAVR12,15,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35. VIV-TAVR had a high success rate: the weighted success rate was 97% (95% CI: 94-98%) (Figure 1). The outcome was characterised by a high degree of statistical heterogeneity (I2=91%). The trim-and-fill method was used to identify possible publication bias and to adjust for it (Supplementary Figure 2A). Sensitivity analysis was carried out excluding studies with possible overlap of the patient population (Supplementary Figure 2B). VIV-TAVR had a higher success rate when the previous valve was placed surgically (97%, 95% CI: 95-99%) compared to transcutaneous replacement (91%, 95% CI: 79-97%) (Supplementary Figure 2C). Meta-regression was used to create a model correlating procedural success with baseline STS score and age, with no statistically significant findings (Supplementary Figure 2D, Supplementary Figure 2E). Publication bias was evaluated, and the results are presented in Supplementary Figure 2F and Supplementary Figure 2G. A Bayes estimator for heterogeneity is presented in Supplementary Figure 2H. There was no evidence of subgroup differences in the success rates based on the valve type (p-interaction=0.28), study location (p-interaction=0.32), or study design (p-interaction=0.58) (Supplementary Figure 3).
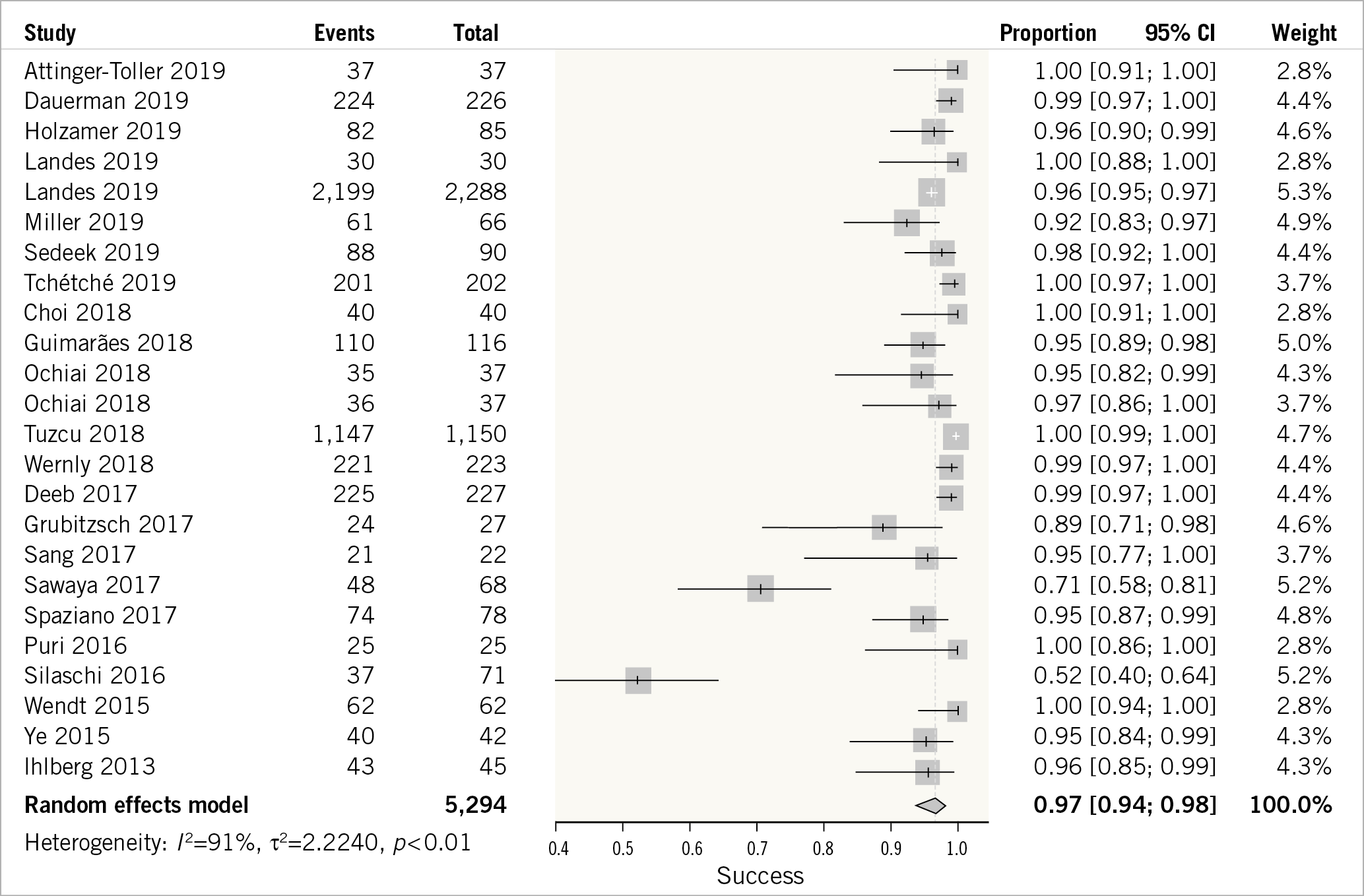
Figure 1. Forest plot showing valve-in-valve transcutaneous aortic valve repair procedural success.
SECONDARY OUTCOMES AT 30 DAYS
ALL-CAUSE MORTALITY AT 30 DAYS
A total of 22 studies reported 30-day all-cause mortality of VIV-TAVR12,14,15,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35. The 30-day mortality incidence was low (5%, 95% CI: 3-6%) (Figure 2A). The outcome was characterised by a high degree of statistical heterogeneity (I²=55%). There was no evidence of subgroup differences in the success rates based on the valve type (p-interaction=0.19), study location (p-interaction=0.29), or study design (p-interaction=0.49) (Supplementary Figure 4).
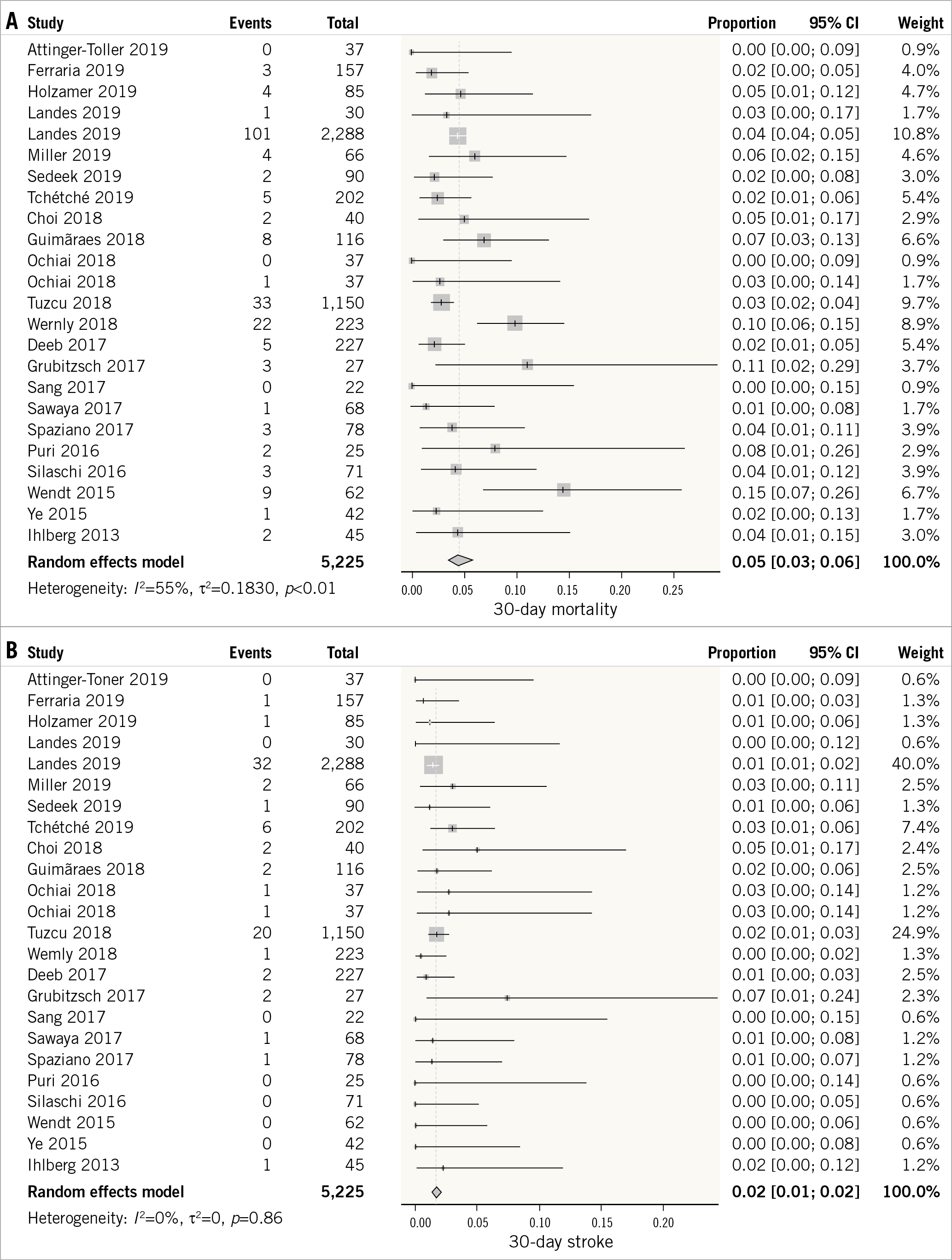
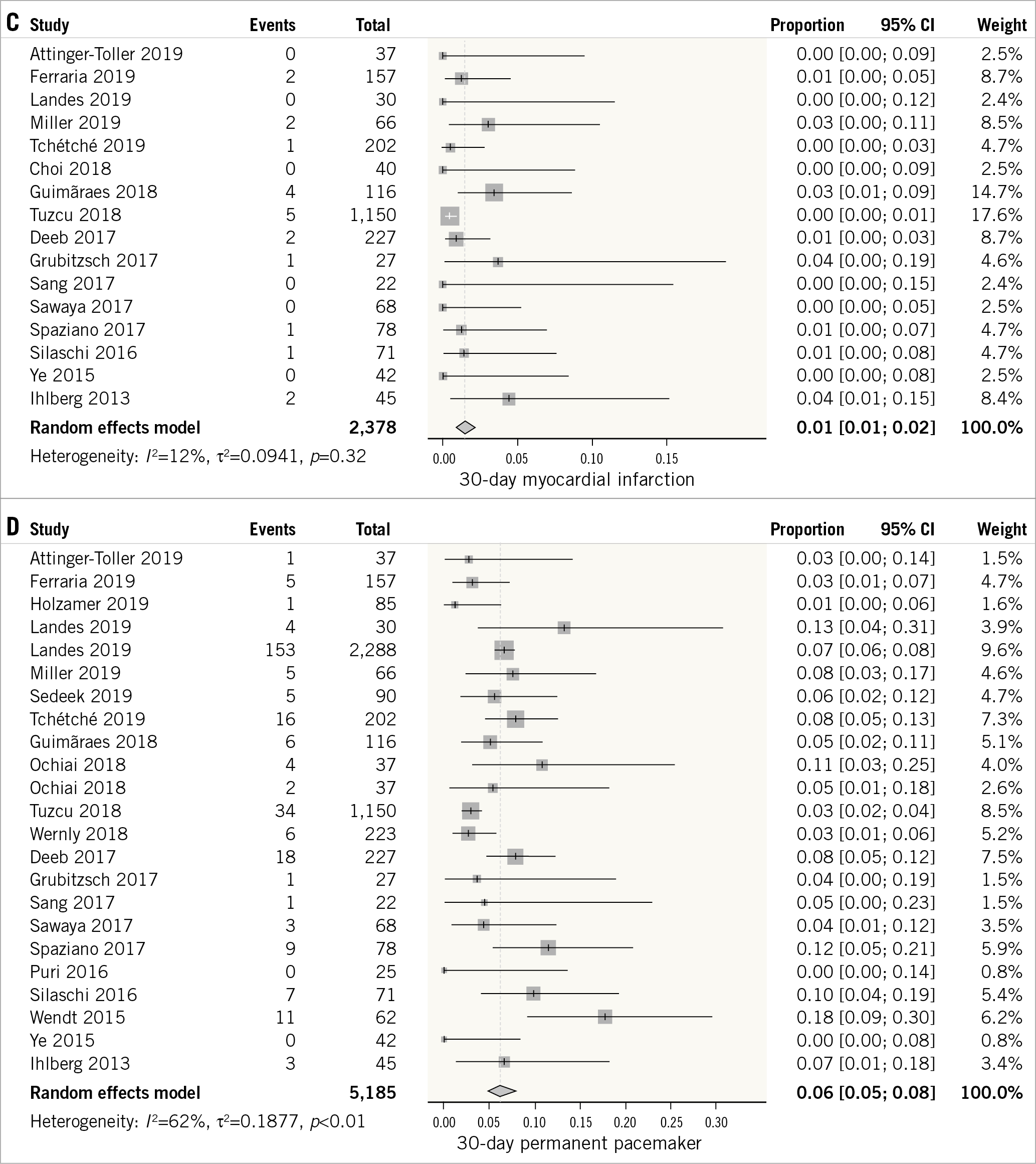
Figure 2. Forest plot showing the incidence of 30-day all-cause mortality (A), stroke (B), myocardial infarction (C), and permanent pacemaker placement (D).
STROKE AT 30 DAYS
A total of 22 studies reported the incidence of stroke at 30 days of VIV-TAVR12,14,15,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35. The outcome was characterised by a low degree of heterogeneity (I2=0%). The overall weighted incidence of stroke at 30 days was low (2%, 95% CI: 1-2%) (Figure 2B).
MYOCARDIAL INFARCTION AT 30 DAYS
Sixteen studies evaluated the incidence of myocardial infarction at 30 days12,14,16,17,19,21,22,24,26,27,28,29,30,32,34,35. The outcome had a low degree of heterogeneity between the studies (I2=12%). The overall weighted incidence of myocardial infarction was low (1%, 95% CI: 1-2%) (Figure 2C).
PERMANENT PACEMAKER PLACEMENT AT 30 DAYS
Twenty-one studies evaluated the outcome of permanent pacemaker placement at 30 days12,14,15,16,17,18,19,22,23,24,25,26,27,28,29,30,31,32,33,34,35. The outcome was characterised by a high degree of heterogeneity between the studies (I2=62%). The overall incidence of pacemaker placement at 30 days was 6% (95% CI: 5-8%) (Figure 2D).
POST-PROCEDURE AORTIC REGURGITATION AND MEAN PRESSURE GRADIENT ACROSS THE VALVE AT 30 DAYS
Eighteen studies reported the outcome of post-procedure aortic regurgitation at 30 days12,16,17,18,19,21,22,23,24,25,26,27,28,29,31,32,34,35. The outcome was characterised by a high degree of heterogeneity (I2=77%). The incidence of aortic regurgitation was 7% (95% CI: 5-10%) (Supplementary Figure 5A). The mean pressure gradient across the valve was reported by 17 studies12,14,15,16,17,18,19,22,23,24,26,27,28,30,31,32,35. The outcome was characterised by a very high degree of heterogeneity across the included studies (I2=91%). The weighted mean pressure gradient across the valve was 16.16 mmHg (95% CI: 15.30-17.02 mmHg) (Supplementary Figure 5B).
SECONDARY OUTCOMES AT ONE YEAR
ALL-CAUSE MORTALITY AT ONE YEAR
Sixteen studies reported the outcome of all-cause mortality at one year14,16,17,19,21,24,25,26,27,28,29,30,32,33,34,35. The outcome was characterised by a moderate degree of heterogeneity (I2=51%). The overall incidence of mortality at one year following VIV-TAVR was 12% (95% CI: 10-14%) (Figure 3A).
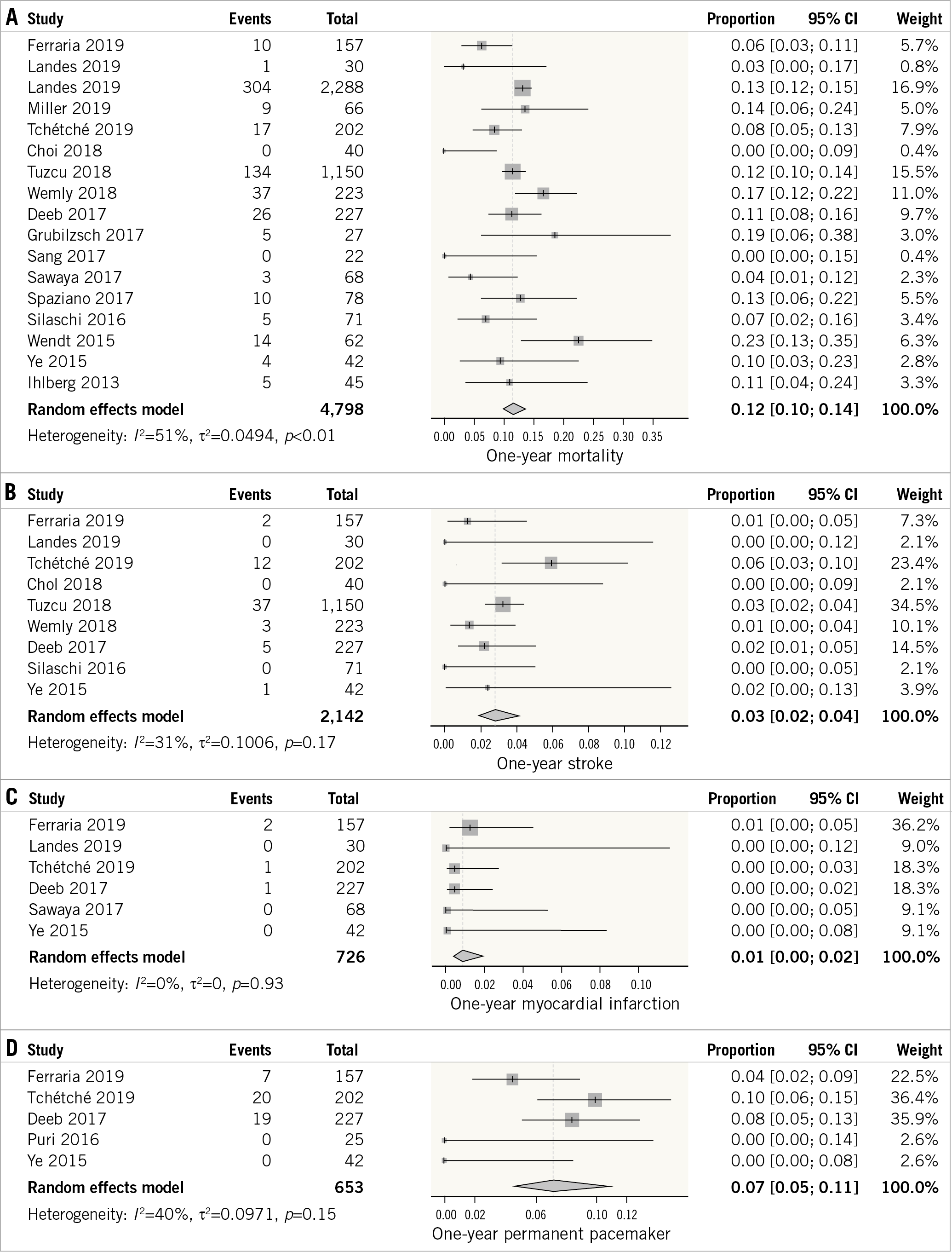
Figure 3. Forest plot showing the incidence of one-year all-cause mortality (A), stroke (B), myocardial infarction (C), and permanent pacemaker placement (D).
STROKE AT ONE YEAR
The outcome of stroke was reported by nine studies14,16,19,21,24,25,26,32,34. The outcome was characterised by a low degree of heterogeneity (I²=31%). The overall weighted incidence of stroke at one year remained low (3%, 95% CI: 2-4%) (Figure 3B).
MYOCARDIAL INFARCTION AT ONE YEAR
Six studies reported the outcome of myocardial infarction at one year following VIV-TAVR14,16,19,26,29,34. The outcome was characterised by a low degree of heterogeneity (I²=0%). The overall weighted incidence of myocardial infarction remained low (1%, 95% CI: 0-2%) (Figure 3C).
PERMANENT PACEMAKER PLACEMENT AT ONE YEAR
The outcome of permanent pacemaker placement was reported by five studies14,19,26,31,34. The outcome was characterised by a moderate degree of heterogeneity (I²=40%). The incidence of permanent pacemaker placement at one year was 7% (95% CI: 5-11%) (Figure 3D).
OUTCOMES AT THREE YEARS
Two studies reported the outcomes of VIV-TAVR patients beyond one year13,20. At three years, the incidence of all-cause mortality was 29% (95% CI: 25-34%) and the incidence of stroke was 6% (95% CI: 5-9%) (Figure 4).
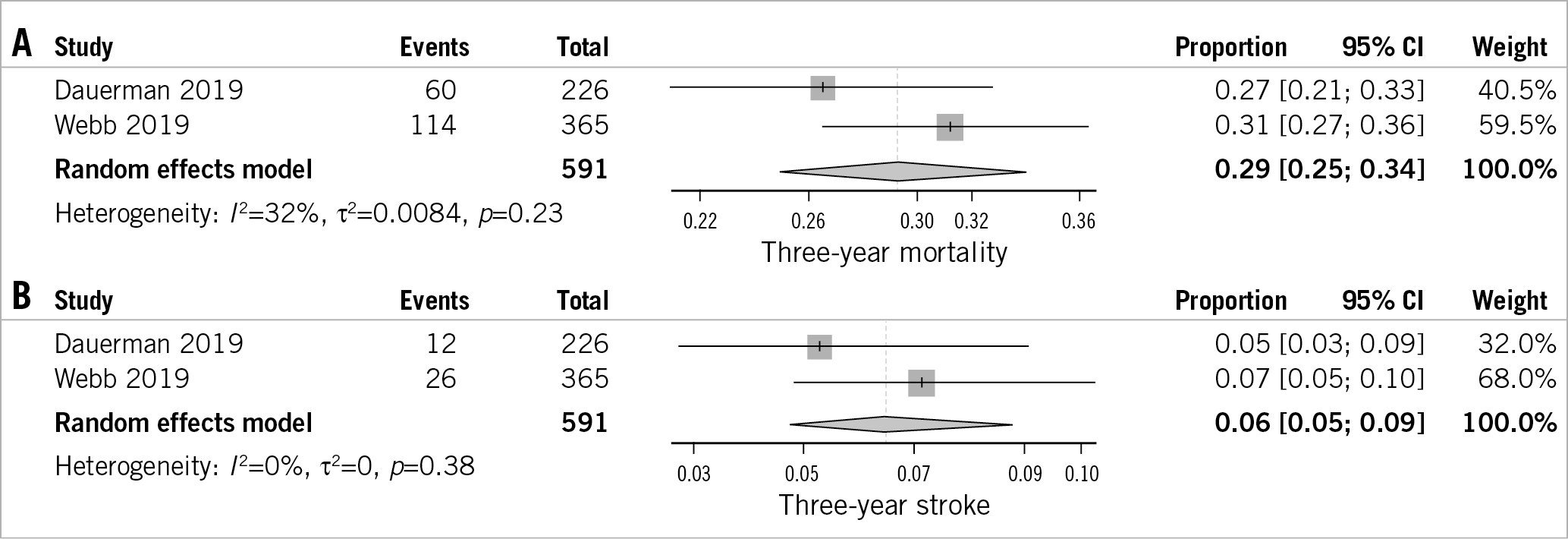
Figure 4. Forest plot showing the incidence of three-year all-cause mortality (A), and stroke (B).
Discussion
This meta-analysis of 24 studies, including 5,553 patients undergoing VIV-TAVR, demonstrated that VIV-TAVR is associated with favourable short-term and midterm outcomes. The use of VIV-TAVR was associated with high procedural success rates. The incidence of 30-day mortality was 5%, at one year 12%, and at three years 29%. The incidence of stroke and that of myocardial infarction were low at 30 days following the procedure and remained low at one year. The indicators of valve stability, such as clinically significant aortic regurgitation and mean pressure gradient across the valve, remained low at 30 days.
The procedural success rates varied significantly among the included studies with evidence of a high degree of heterogeneity in the outcome rates. One possible explanation for such a discrepancy could be the variation in the procedural volume from one study to another. The correlation between TAVR volume and improved outcomes was recently explored in a large study by Vemulapalli et al, showing improved outcomes in centres with high TAVR volume compared to those with low volume41. It is also expected that the procedural success rates would increase over time, given the continuing advances in TAVR technology with the development of newer-generation valves with better deliverability, lower profile and improved designs to lower the rates of paravalvular leak and mean gradient across the valves42,43,44,45.
Although we included a cohort of patients with high surgical morbidity and mortality risk (mean STS score 7.84), the incidences of mortality and stroke reported in our meta-analysis were not exceedingly high (around 5% for 30-day mortality and 2% for stroke). In some subgroups the incidence of mortality was similar to that reported in trials of de novo TAVR in intermediate-risk patients (~3%)6. The incidences of these outcomes remained low even after one year, with incidences similar to the lower risk cohort of the PARTNER 2 trial6.
Limitations
Although the current meta-analysis represents the largest study to date exploring the outcomes of VIV-TAVR, it is not free from limitations. Some of the limitations of the current meta-analysis include a high degree of heterogeneity in many of the outcomes explored. We attempted to mitigate such an effect by analysing the random-effects incidences, using the trim-and-fill method, and by conducting various subgroup analyses to explore the reasons for the heterogeneity. The lack of patient-level data hindered the exploration of the impact of the STS preoperative risk score on various outcomes of interest. Despite these limitations, our study addresses a relevant knowledge gap for operators and for counselling patients regarding the rates of procedure success and outcomes with VIV-TAVR.
Conclusions
VIV-TAVR appears to be associated with high procedural success rates and favourable short-term to midterm outcomes in patients with failed bioprosthetic valves, with an acceptable rate of adverse events compared with TAVR in intermediate- to high-risk patients. Future studies are encouraged to confirm the durability of the VIV procedure in the long term.
|
Impact on daily practice This meta-analysis demonstrates that valve-in-valve transaortic valve replacement performed by experienced operators has a high success rate and is associated with a low risk of adverse events in the short and mid term. |
Conflict of interest statement
D. Dvir is a consultant to Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

