Abstract
Background: Bioprosthetic valve fracture (BVF) is a technique to reduce gradients in valve-in-valve transcatheter aortic valve implantation (VIV-TAVI) procedures. The outcome of VIV-TAVI with BVF has not been compared with VIV-TAVI without BVF.
Aims: The aim of this study was to evaluate the outcome of VIV-TAVI with BVF compared to VIV-TAVI without BVF.
Methods: In total, 81 cases of BVF VIV-TAVI (BVF group) from 14 centres were compared to 79 cases of VIV-TAVI without BVF (control group).
Results: VARC-2-defined device success was 93% in the BVF group and 68.4% in the control group (p<0.001). The mean transvalvular gradient decreased from 37±13 mmHg to 10.8±5.9 mmHg (p<0.001) in the BVF group and from 35±16 mmHg to 15.8±6.8 mmHg (p<0.001) in the control group with a significantly higher final gradient in the control group (p<0.001). The transvalvular gradients did not change significantly over time. In-hospital major adverse events occurred in 3.7% in the BVF group and 7.6% in the control group (p=0.325). A linear mixed model identified BVF, self-expanding transcatheter heart valves (THVs) and other surgical aortic valve (SAV) types other than Mitroflow as predictors of lower transvalvular gradients.
Conclusions: Compared to VIV-TAVI alone, VIV-TAVI with BVF resulted in a significantly lower transvalvular gradient acutely and at follow-up. Independent predictors of lower gradients were the use of self-expanding THVs and the treatment of SAVs other than Mitroflow, irrespective of BVF performance. BVF significantly reduced the gradient independently from transcatheter or surgical valve type.
Introduction
Valve-in-valve transcatheter aortic valve implantation (VIV-TAVI) is a valuable therapeutic approach in patients with degenerated surgical aortic valve bioprostheses1. After surgical aortic valve (SAV) replacement, up to 45% of patients have prothesis-patient mismatch (PPM), which is particularly frequent in patients with small SAVs2,3. In this cohort VIV-TAVI may result in high residual gradients, which impact on survival1,4,5,6.
Bioprosthetic valve fracture (BVF) is a technique to reduce gradients in VIV-TAVI procedures by fracturing the sewing ring of the SAV with high-pressure non-compliant balloon inflation7,8,9,10. A comparison of acute as well as long-term outcome data between VIV-TAVI with BVF versus without BVF in patients with comparable baseline characteristics, including SAV type, is missing and defines the scope of the present study.
Material and methods
Fourteen international centres provided data on BVF VIV-TAVI procedures in patients with degenerated SAVs with fracturable or dilatable sewing rings. Data were collected retrospectively and prospectively. Patients with VIV-TAVIs performed during the same time period in SAVs, which were suitable for but did not undergo BVF, served as a control group.
Deteriorated SAVs were categorised as stenotic, regurgitant or mixed (stenosis and regurgitation ≥moderate). Indexed effective orifice area (iEOA) and PPM were calculated after surgical valve replacement. PPM was defined as mild (iEOA ≥0.85-1.0), moderate (iEOA 0.65-0.85) or severe (iEOA ≤0.65). The technique of BVF has been described elsewhere9. Timing of BVF (before or after transcatheter heart valve [THV] implantation) and choice of balloon size were per the operator’s discretion. BVF was performed with non-compliant balloons 1-6 mm larger than the SAV’s true internal diameter (ID). THVs were categorised as adequately or oversized for the patient’s SAV based on recommendations of the Valve in Valve app version 2.0 (UBQO Limited, London, United Kingdom). BVF was considered successful if the inflation pressure dropped suddenly without balloon perforation and the THV was fully expanded (in case of SAVs, which cannot be fractured but remodelled). Device success was defined as a correctly implanted THV without the use of a second valve and with a final mean gradient <20 mmHg, no moderate/severe aortic regurgitation and the absence of procedural death.
A VARC-2-defined early safety composite, clinical efficacy after 30 days and periprocedural complications including aortic root rupture, ventricular septal perforation, balloon rupture leading to clinical consequences, cardiac tamponade, coronary obstruction, among others, as well as reinterventions at follow-up, was evaluated.
STATISTICAL ANALYSIS
Continuous data are summarised as means±standard deviations or as medians (25th and 75th percentiles) as appropriate. Categorical data are presented as N (%). A linear mixed model was applied to associate mean gradient at discharge and follow-up to the treatment groups (BVF vs control), THV type (self-expanding vs balloon-expandable) and SAV type (Mitroflow vs non-Mitroflow). Estimates were adjusted with baseline mean transvalvular gradient. The time of measurement was included in the model. The response variable was log transformed to fulfil model assumptions. Parameter estimates and 95% confidence intervals of the parameter estimates are presented. Wilcoxon and Pearson/Fisher’s exact tests were conducted to compare variables between groups. All p-values were two-sided and a p-value <0.05 was considered significant. All calculations were performed with the statistical analysis software R (R Core Team, 2020; R Foundation for Statistical Computing, Vienna, Austria).
Results
BVF VIV-TAVI was performed in 81 patients in the time period between August 2015 and March 2020 and VIV-TAVI alone in 79 patients in the time period between July 2014 and March 2020. Baseline data are summarised in Table 1. Both groups were comparable, with the exception of more male patients (65 vs 42%, p=0.004), larger SAVs (24.1±2.4 vs 22.1±2.1, p<0.001) and true IDs (20.7±2.5 mm vs 19.1±1.8 mm, p<0.001) in the control group.
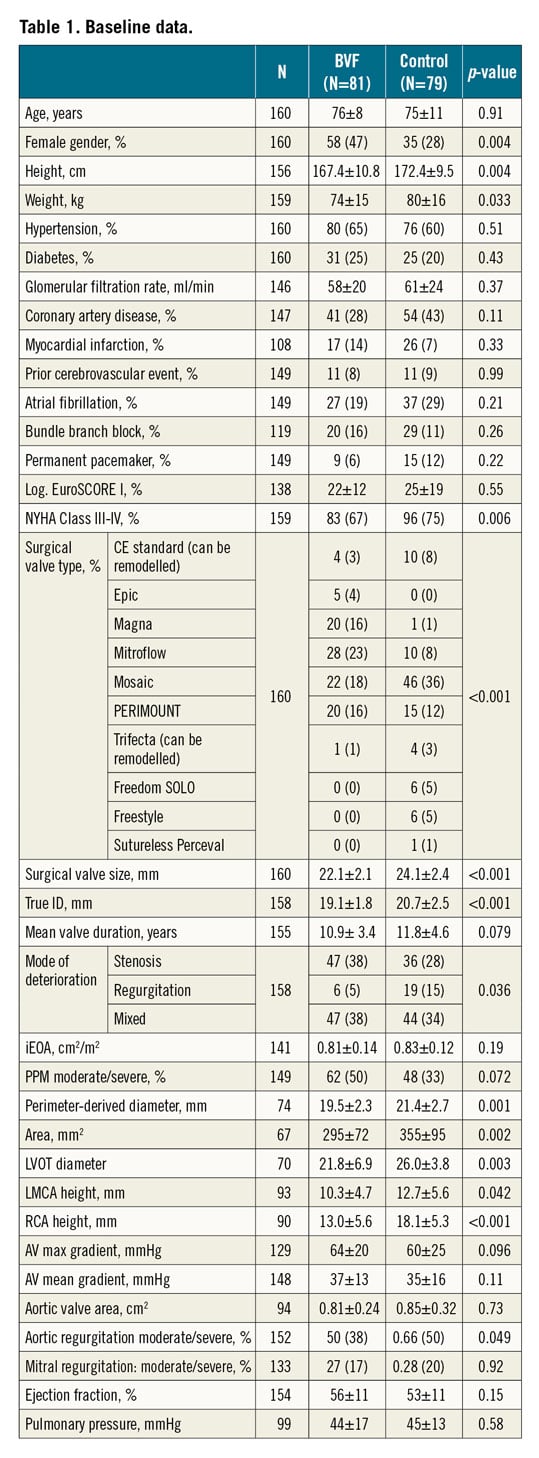
Ten types of surgical valve were treated, mainly Mosaic® (Medtronic, Minneapolis, MN, USA), Mitroflow (Sorin Group, Saluggia, Italy), PERIMOUNT (Edwards Lifesciences, Irvine, CA, USA) and Magna (Edwards Lifesciences). The most common mode of degeneration according to the standardised definition was stenosis and mixed (94% vs 78% in the control group, p=0.036). Mean interval to SAV failure in the BVF group was 10.9±3.4 vs 11.8±4.6 years (p=0.08). Moderate/severe PPM was present in the BVF group in 54%/9% vs 45%/3% (p=0.072). Baseline mean gradient in the BVF group was 37±13 mmHg vs 35±16 mmHg (p=0.11), and iEOA was 0.81±0.24 cm2/m2 vs 0.85±0.32 cm2/m2 (p=0.73). Moderate/severe aortic regurgitation was more frequent in the control group (66% vs 50%, p=0.049). The main access was transfemoral (p=0.34); cerebral protection was more often applied in the BVF group (31% vs 9%, p≤0.001) (Table 2). Balloon-expandable SAPIEN (Edwards Lifesciences) THVs were utilised slightly more often in the control group (29% vs 22%). Among the self-expanding THVs in both groups, the most prevalent was Evolut™ (Medtronic).
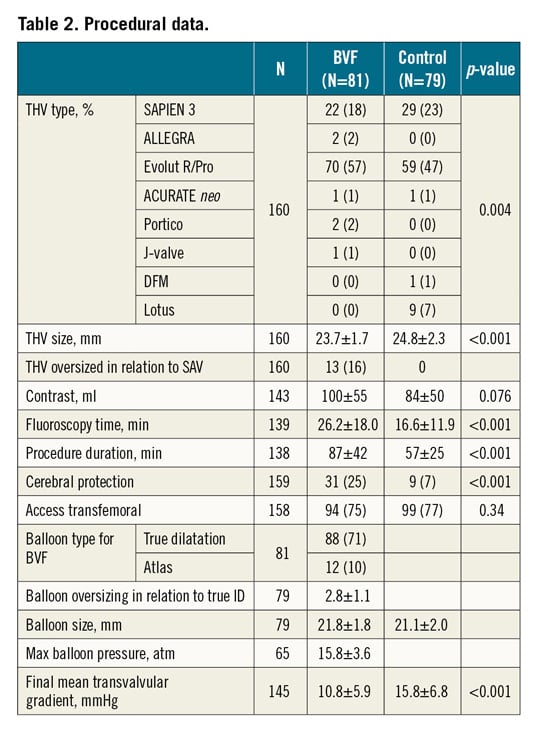
For BVF the TRUE® Dilatation balloon (Bard Peripheral Vascular Inc., Tempe, AZ, USA) was used for valve fracturing in 88% and Atlas® GOLD (Bard Peripheral Vascular Inc.) in 12%. BVF was performed in 89% after THV implantation, and in 11% before. Balloons were 2.8±1.1 mm (range 1-6 mm) oversized in relation to the true ID of the SAV and inflated with a pressure of 15.8±3.6 atm. In 84% THV sizing was in accordance with the recommendation of the Valve in Valve app; in 16% the THVs were oversized. Procedure duration was longer in the BVF group (87±42 min vs 57±25 min, p<0.001), as well as fluoroscopy time (26.2±18 min vs 16.6±11.9 min, p<0.001).
IN-HOSPITAL OUTCOME
Device success was achieved in 93% in the BVF group and in 68.4% in the control group (p<0.001). The main reason for procedure failure was a residual mean gradient ≥20 mmHg in both groups, which was found in 5 of 6 failures in the BVF group and in 22 of 25 failures in the control group (Table 3).
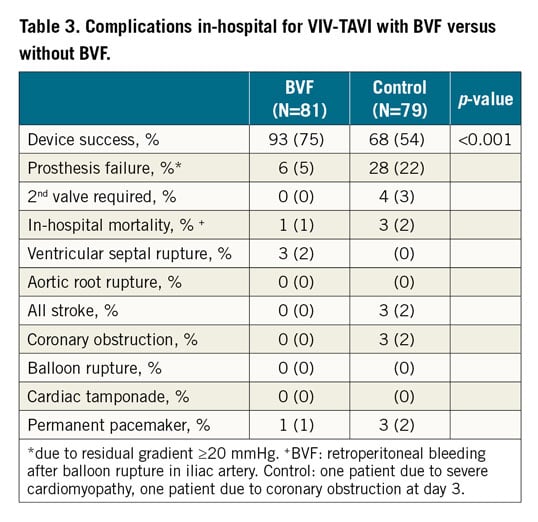
Failures due to high gradients were predominantly seen in Mitroflow valves (100% in the BVF group and 62.5% in the control group). Out of 5 failures in the BVF group, 3 patients received a self-expanding and two a balloon-expandable THV. Out of 22 failures in the control group, 14 patients received a self-expanding and 8 a balloon-expandable THV. Only in the control group was a second valve required in 3 cases (in 2 patients Evolut THVs were malpositioned, 1 received a second Evolut and 1 a SAPIEN. A third patient received a second SAPIEN after embolisation of the first into the left ventricle, which was retrieved surgically) (Table 3).
The mean gradient decreased from 37±13 mmHg to 10.8±5.9 mmHg (p<0.001) in the BVF group and from 35±16 mmHg to 15.8±6.8 mmHg (p<0.001) in the control group (Figure 1). The difference in the final mean gradient between the BVF group and the control group was significant (p<0.001). At discharge, moderate paravalvular aortic regurgitation was present in only one case in the control group.
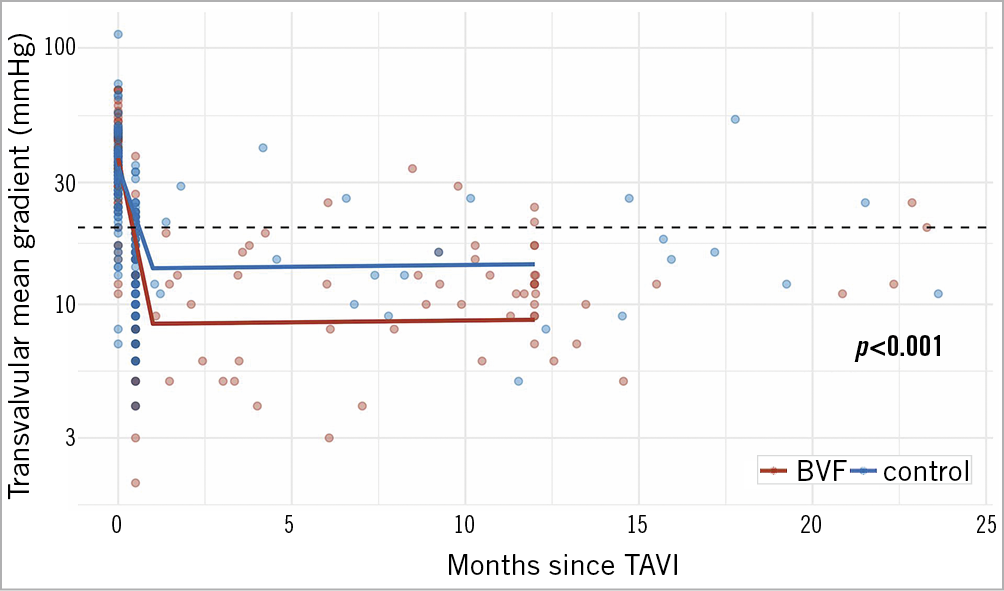
Figure 1. Reduction in mean transvalvular gradients by BVF (red line) versus non-BVF (blue line) in VIV-TAVI procedures acutely and over time.
Severe in-hospital complications occurred in 3.7% in the BVF group and 7.6% in the control group (p=0.325). In the BVF group, one patient died from an iliac artery perforation; in the control group, one died from severe cardiomyopathy and another from coronary obstruction at day 3. Other complications were two ventricular septal ruptures after BVF with balloons 4 mm larger than the true ID of the SAV, both without clinical consequences. In the control group, two strokes occurred and one coronary obstruction, which could be managed by percutaneous coronary intervention.
OUTCOME AT FOLLOW-UP
The clinical follow-up rate was 88.8% in the BVF group (9 patients lost to follow-up) with a mean follow-up time of 276 days (range 25-1,710 days) and 86% in the control group (11 patients lost to follow-up) with a mean follow-up time of 1,184 days (range 30-2,211 days).
In the BVF group one patient died of unknown cause, and one patient needed a second valve due to severe aortic regurgitation, which was not present at discharge but developed within 12 weeks. BVF during the index procedure was performed with a 2 mm oversized balloon.
Another patient was re-hospitalised due to heart failure and pneumonia. He had no THV dysfunction. In the control group 11 patients died; one death was valve related. Five patients required a surgical reintervention, 2 due to THV dysfunction, 3 due to endocarditis.
In the BVF group echocardiographic follow-up was obtainable in 59 of 71 patients (83.1%) with a mean follow-up time of 281 (range 25-709 days) in the BVF group and in 55 of 66 patients (83.3%) with a mean follow-up time of 831 (range 37-2,081 days) in the control group.
In both groups the mean gradient remained stable over time (BVF group: 10.8±5.9 mmHg at discharge, 12.4±6.3 mmHg at follow-up; control group: 15.8±6.8 at discharge and 18.4±9.4 mmHg at follow-up) (Figure 1).
LINEAR MIXED MODEL TO PREDICT MEAN TRANSVALVULAR GRADIENT FROM BASELINE DATA
The linear mixed model (Table 4) identified three predictors of a lower mean gradient: BVF compared to non-BVF (Figure 1), the use of self-expanding compared to balloon-expandable THVs (Figure 2), and other SAVs compared to the Mitroflow valve (Figure 3).
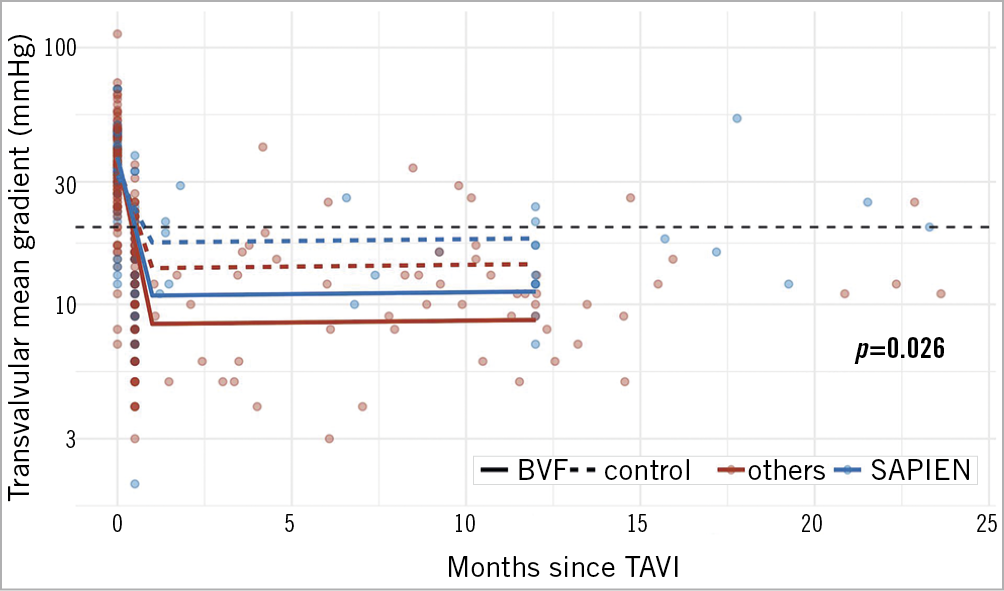
Figure 2. Mean gradients acutely and over time in VIV-TAVI after BVF (continuous line) or without BVF (dashed line) in SAPIEN THVs (blue) and in self-expanding THVs (red).
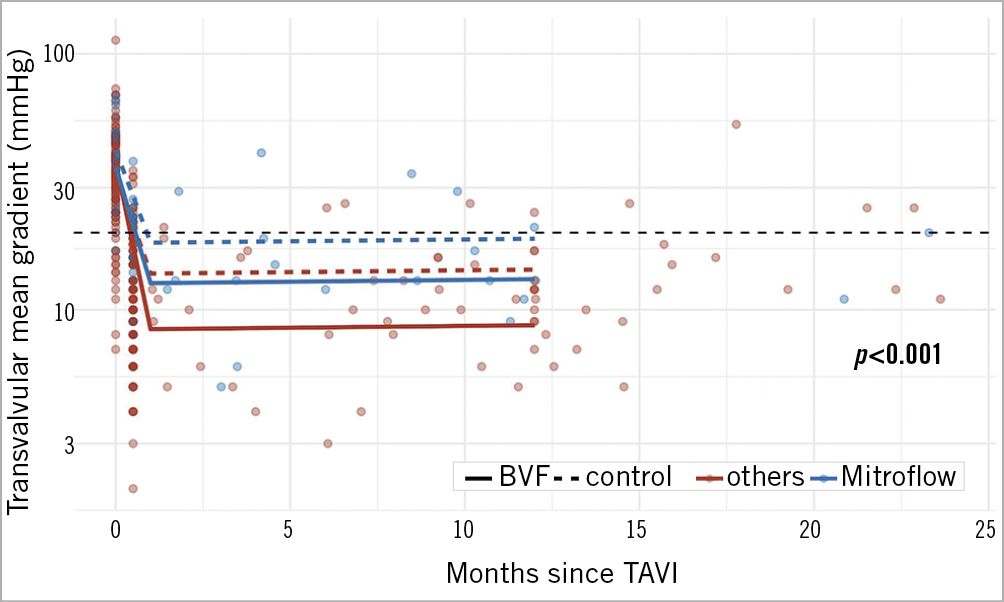
Figure 3. Mean gradients acutely and over time in VIV-TAVI after BVF (continuous line) or without BVF (dashed line) in Mitroflow SAVs (blue) and in non-Mitroflow SAVs (red).
This interaction of THV and SAV type was observable in both the BVF group and the control group (Figure 2, Figure 3). The lowest gradients were achieved with BVF in non-Mitroflow SAVs and the use of self-expanding THVs (Figure 4, left upper panel). The highest gradients in VIV procedures were found if a Mitroflow SAV was treated with a balloon-expandable THV without performing BVF (Figure 4, lower panel right).
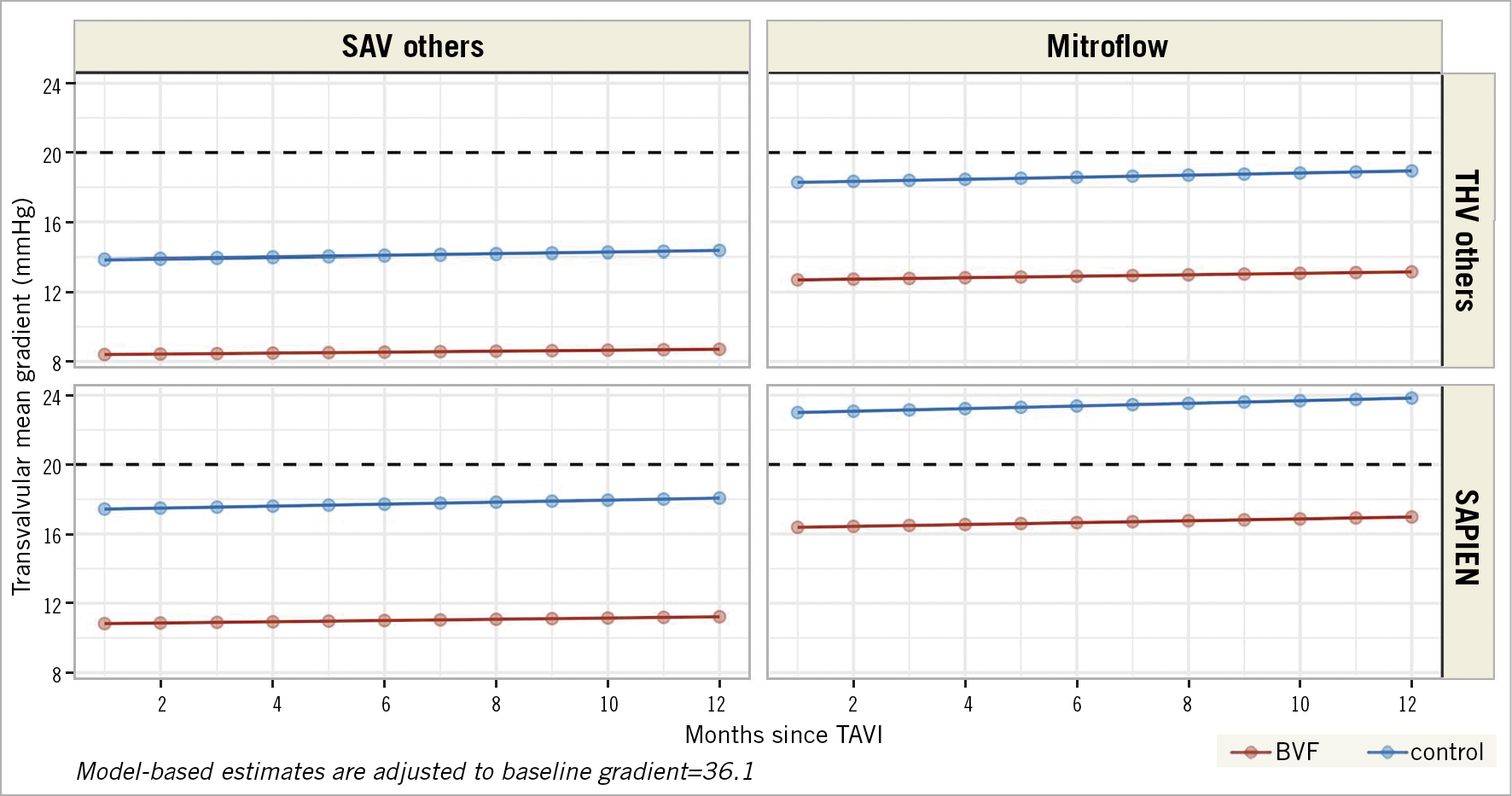
Figure 4. Model-based estimates of the mean transvalvular gradient over time after BVF (red lines) and without BVF (blue lines) for patients with self-expanding THVs in non-Mitroflow SAVs (left upper panel), for patients with non-Mitroflow SAVs and SAPIEN THVs (left lower panel), for patients with Mitroflow SAV and self-expanding THVs (right upper panel) and patients with Mitroflow SAVs and SAPIEN THVs (right lower panel). Model-based estimates are adjusted to the baseline gradient.
Discussion
The main findings of the present study are:
1. In patients with degenerated SAVs, BVF in VIV-TAVI resulted in a significant mean gradient reduction compared to VIV-TAVI alone.
2. The difference in the mean gradient between the two groups remained stable over time.
3. Independent predictors of lower gradients were the use of self-expanding THVs and the treatment of SAVs other than the Mitroflow, irrespective of BVF performance.
4. Compared to VIV-TAVI alone, BVF significantly reduced the gradient independently from THV or SAV type.
Although VIV-TAVI is an attractive option to avoid reoperation in failed SAVs, it has some major shortcomings. In small SAVs it can result in high gradients which impact on mortality1. Procedural results and long-term outcome of VIV-TAVI have been analysed in large registries1,4. The post-procedure mean gradient after VIV-TAVI reported by Dvir et al was 15.8±8.9 mmHg1, which was replicated in the current control group (15.8±6.8 mmHg). Also, the mean gradient at long-term follow-up of the control group (18.4±9.4 mmHg) is consistent with prior findings after one year (16.9 mmHg and 17.6 mmHg)1,4. As is evident from our data, but not emphasised in the registries mentioned, a mean gradient of such magnitude implies that a significant number of patients present with a mean gradient ≥20 mmHg, which per VARC definition is a device failure. In these patients in particular the risk of reintervention increases over time11.
BVF integrated in a VIV-TAVI procedure has been shown to be feasible in reducing transvalvular gradients12. The aim of the present study was to compare acute as well as long-term data of a VIV-TAVI group with a cohort of patients who underwent VIV-TAVI in conjunction with BVF. To the best of our knowledge, this analysis is the first to compare the clinical and haemodynamic outcome of VIV-TAVI with BVF versus VIV-TAVI alone. The control group comprised patients who were treated in a similar time period, who differed in baseline data only marginally and whose potentially crackable SAVs were not fractured.
In the control group, with significantly more male patients, the SAVs and true IDs were larger compared to the BVF group, which would attenuate the difference in the final mean transvalvular gradient between the groups. Despite the larger true IDs in the control group, however, the mean gradient after BVF was significantly lower, in fact in the same range as that shown by others12. For that reason, there was a striking difference in the VARC-defined device failure rate, which was driven mainly by a final mean gradient ≥20 mmHg, in favour of BVF (6.2% vs 27.8%, p<0.001). In consequence, these patients with higher gradients in our control group would have been candidates for BVF.
The gradient after VIV-TAVI remained stable at follow-up, which is in accordance with prior findings1,4. Until now, however, it was unknown whether gradient stability is also seen after BVF. For the first time, the present study shows that there is no significant change in the gradient after BVF over time. Additionally, we were able to show that the achieved difference in the gradient between the BVF group and the control group (5 mmHg) stays stable over time (6 mmHg). Because higher gradients are a risk factor for mortality and reintervention, BVF may potentially improve long-term survival and reduce the reintervention rate by correcting a pre-existing PPM6.
A key question is whether the achievement of favourable gradients by BVF comes with an increased procedural risk, as the safety of BVF procedures so far has only been examined in small studies. In this context, a comparison with a non-BVF VIV-TAVI group is of interest. We found a 30-day mortality rate of 1.2% in the BVF group, which corresponds to the findings of Allen et al12, and 2.5% in the control group, which corresponds to the data of the PARTNER 2 Valve-in-Valve Registry4. Of concern, however, are two ventricular septal perforations observed after BVF, both performed with 4 mm oversized balloons. Both patients had an uneventful clinical course without further treatment. Although we could not identify any unsuitable anatomy in these patients, based on this very limited experience, we would recommend not oversizing the balloon >3 mm; precaution should be taken with aggressive BVF in the presence of severe annular or left ventricular outflow tract (LVOT) calcification or narrow anatomy.
Another complication was the development of severe valvular aortic regurgitation in one patient three months after BVF, which was performed with a 2 mm oversized balloon after THV implantation and possibly resulted in leaflet damage. Although in our cohort BVF was performed after THV implantation in 89% of cases, the best timing of BVF (prior to or after TAVI) is still open for discussion12.
Coronary obstruction is an additional potential danger in VIV procedures. This complication is determined by virtual THV to coronary ostium distance (VTC), virtual THV to sinotubular junction distance (VTSTJ) and the leaflet in relationship to the coronary ostia and sinotubular junction (STJ). When cracking the valve, the VTC is even more narrowed, which increases the risk of coronary obstruction. In the BVF group, no such case occurred. In 3 patients, however, preventive measures were taken such as bioprosthetic or naïve aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) interventions (2 patients) and stent implantation using the chimney technique (1 patient). In contrast, we observed 2 coronary obstructions in the control group, which caused the death of one patient. Based on our limited experience, BVF does not increase the risk of coronary obstructions, but further investigations have to confirm this impression.
To look for independent predictors for lower final gradients, which could be helpful for future procedure planning and performance, a linear mixed model was applied. Three independent predictors were identified: the performance of BVF, the use of self-expanding THVs and the treatment of SAVs other than Mitroflow. Interestingly, these latter two predictors were valid for both the BVF group as well as the control group. The fact that balloon-expandable compared to self-expanding THVs in VIV-TAVI lead to higher gradients has already been shown11. We were now able to demonstrate that this also applies for BVF in VIV-TAVI, which has not been described before.
The Mitroflow as a risk factor for higher gradients is a novel finding. Different to other SAVs treated in this cohort, this valve has leaflets sutured outside the stent. Whether this particular valve design impacts on the gradient needs to be examined in larger series.
Eventually it would be desirable to develop an algorithm for how to treat degenerated SAVs and achieve an optimal result. Our findings may contribute to this proposal by providing the information that treatment of a Mitroflow SAV with a SAPIEN THV leads to the highest gradients, and treatment of a non-Mitroflow SAV with a self-expanding THV to the lowest (Central illustration). However, in any combination of SAVs with THVs, performance of BVF significantly reduces the gradient acutely and over time (Figure 4).
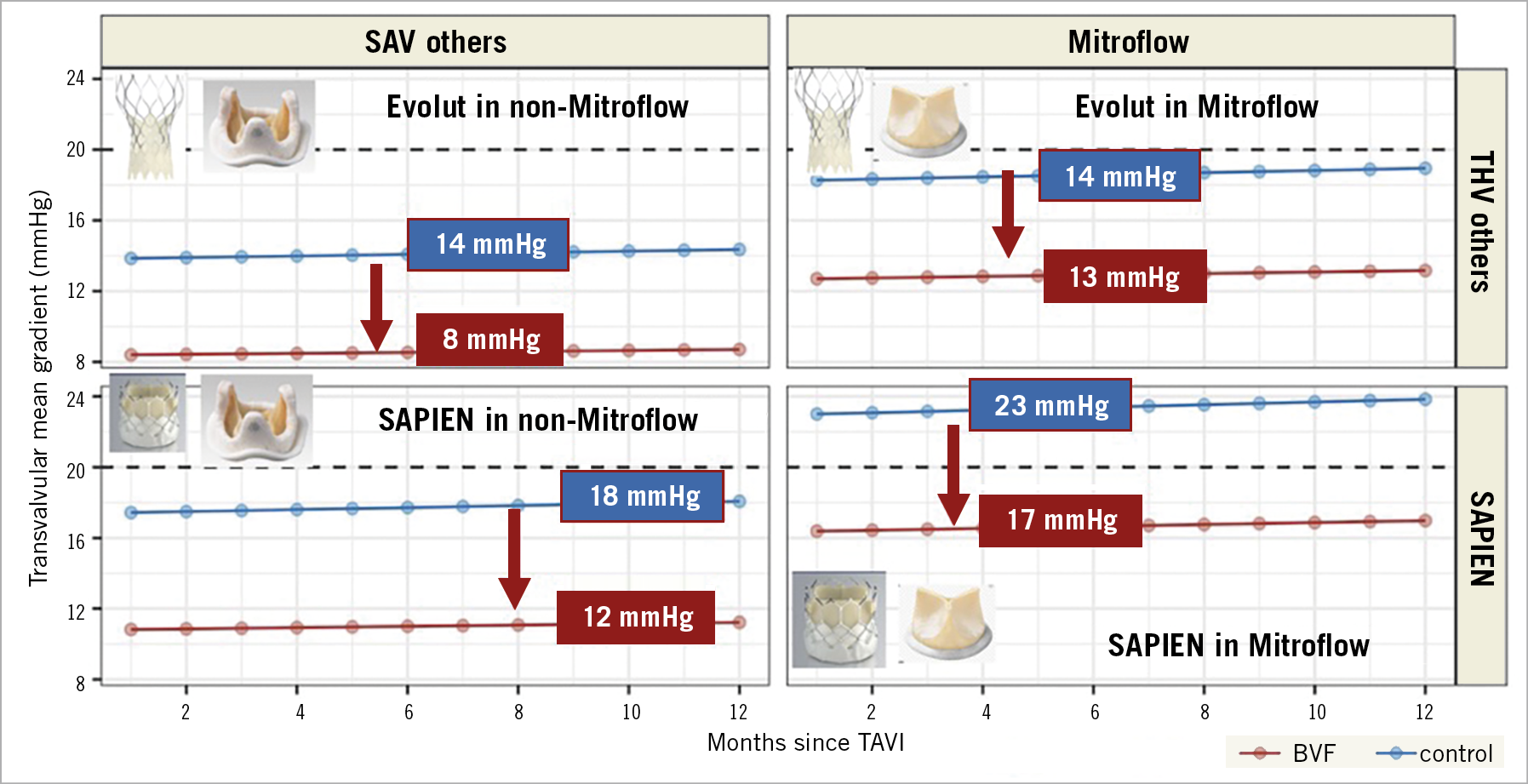
Central illustration. Summary of mean transvalvular gradients in VIV-TAVI with and without BVF. The highest transvalvular gradients are expected in patients with SAPIEN in Mitroflow without BVF, the lowest in patients with Evolut in non-Mitroflow valves with BVF. In any combination, BVF reduces by approximately 6 mmHg.
Limitations
This was an observational study with inherent limitations. There was no echocardiographic core lab, and data were not adjudicated by an independent committee. Timing of BVF and the degree of balloon oversizing were per the operator’s discretion. The control cohort was not randomised and differed in some aspects that may have extenuated the difference in the gradients between the groups.
Conclusions
In patients with degenerated SAVs, VIV-TAVI in conjunction with BVF resulted in a significantly lower gradient compared to VIV-TAVI alone. The gradient as well as the difference in gradient between the groups remained stable over time. Independent predictors of lower final gradients were the performance of BVF, the use of self-expanding THVs and the treatment of SAVs other than Mitroflow. BVF significantly reduced the gradient independently from THV or SAV type.
|
Impact on daily practice Regarding the final transvalvular gradient, the most unfavourable clinical scenario in VIV procedures would be the treatment of a Mitroflow SAV with a balloon-expandable THV without performing BVF. The lowest gradient can be achieved in SAVs other than Mitroflow treated with self-expanding THVs in conjunction with BVF. |
Conflict of interest statement
M. Abdel-Wahab is a consultant to Medtronic and Boston Scientific. F. Bedogni is a consultant and proctor of Medtronic, Abbott, BSI, and Meril. L. Conradi is a consultant to Edwards, Medtronic, Boston and Abbott. D Hildick-Smith is a proctor/advisor for Abbott, Boston, Edwards, and Medtronic. A. Latib is a consultant for Medtronic, Abbott, Edwards Lifesciences, ICS and Keystone Heart. N.M. Van Mieghem has received research grant support from Abbott Vascular, Boston Scientific, Edwards Lifesciences, Abiomed, Medtronic, PulseCath BV, and Daiichi Sankyon and advisory fees from Abbott, Boston Scientific, Abiomed, Medtronic, PulseCath BV, and Daiichi Sankyo. T. Pilgrim has received research grants to the institution from Boston Scientific, Edwards Lifesciences and Biotronik; speaker fees from Boston Scientific and Biotronik, declares consultancy for HighLifeSAS (CEC), and proctoring for Medtronic. M. Taramasso is a consultant for Abbott, Boston Scientific, 4tech, and CoreMedic, and has received honoraria from Edwards Lifesciences, Mitraltech, and SwissVortex. L. Testa is a medical proctor for Boston Scientific, Meril, Concept Medical, Abbott, and Philips, and is an advisory board member and/or has received speaker fees and/or institutional research grants from BSCI, Philips, Abbott, Medtronic, Terumo, and Concept Medical. J. Webb is a consultant to and receives research funding from Edwards Lifesciences, Abbott, and Boston Scientific. S. Windecker reports research and educational grants to the institution from Abbott, Amgen, AstraZeneca, BMS, Bayer, Biotronik, Boston Scientific, Cardinal Health, Cardiovalve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, Infraredx, Johnson & Johnson, Medicure, Medtronic, Novartis, Polares, OrPha Suisse, Pfizer, Regeneron, Sanofi-Aventis, Sinomed, Terumo, and V-Wave. S. Windecker also serves as unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave and Xeltis, but has not received personal payments by pharmaceutical companies or device manufacturers. He is also a member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. S. Windecker is an unpaid member of the Pfizer Research Award selection committee in Switzerland and of the Women as One Awards Committee. He is a member of the Clinical Study Group of the Deutsches Zentrum für Herz Kreislauf-Forschung and of the Advisory Board of the Australian Victorian Heart Institute. He is chairperson of the ESC Congress Program Committee and Deputy Editor of JACC CV Interventions. J. Schofer is a consultant for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

