
Abstract
Aims: Bioprosthetic valve fracture (BVF) may improve transvalvular gradients and transcatheter heart valve (THV) expansion during VIV interventions. However, the optimal timing of BVF is unknown. We assessed the impact of timing of BVF (before versus after) for valve-in-valve (VIV) intervention, on hydrodynamic function and THV expansion.
Methods and results: Three THV designs were assessed, a 23 mm SAPIEN 3 (S3), small ACURATE neo (ACn) and 23 mm Evolut R, deployed into 21 mm Mitroflow bioprosthetic surgical valves. We evaluated each THV in three groups: 1) no BVF, 2) BVF before VIV, and 3) BVF after VIV. Hydrodynamic testing was performed using a pulse duplicator to ISO 5840:2013 standard. Transvalvular gradients were lower when BVF was performed after VIV for the S3 (no BVF 15.5 mmHg, BVF before VIV 8.0 mmHg, BVF after VIV 5.6 mmHg), and the ACn (no BVF 9.8 mmHg, BVF before VIV 8.4 mmHg, BVF after VIV 5.1 mmHg). Transvalvular gradients were similar for the Evolut R, irrespective of performance of BVF or timing of BVF. BVF performed after VIV resulted in better expansion in all three THV designs. The ACn and Evolut R samples all had a mild degree of pinwheeling, and BVF timing did not impact on pinwheeling severity. The S3 samples had severe pinwheeling with no BVF, and significant improvement in pinwheeling when BVF was performed after VIV.
Conclusions: BVF performed after VIV was associated with superior THV expansion in all three THV designs tested, with lower residual transvalvular gradients in the S3 and ACn THVs. The Evolut R had similar hydrodynamic performance irrespective of BVF timing. Timing of BVF has potential implications on THV function.
Introduction
Valve-in-valve (VIV) intervention with transcatheter heart valves (THVs) is an established therapy for patients with failed surgical bioprothetic aortic valves1. However, in small-sized surgical valves, high gradients and patient-prosthesis mismatch may persist and lead to poor clinical outcomes2. To address this issue, bioprosthetic valve fracture (BVF) has emerged as a technique to optimise THV expansion, with a subsequent reduction in transvalvular gradients and an increase in effective orifice area (EOA)3,4. Early clinical experience has been favourable, but the long-term clinical implications as well as the optimal timing of BVF are currently unknown5. BVF performed prior to VIV intervention has associated risks of acute aortic insufficiency and haemodynamic compromise, whereas BVF performed after VIV intervention might damage THV leaflets, affecting both short- and long-term THV function.
This study assessed the effect of BVF timing, using three different THV designs, on acute hydrodynamic function and THV expansion, comparing BVF performed before versus after VIV intervention.
Methods
VALVES
VIV intervention was tested with a 23 mm SAPIEN 3 (S3) (Edwards Lifesciences, Irvine, CA, USA), a small ACURATE neo™ (ACn) (Boston Scientific, Marlborough, MA, USA) and a 23 mm Evolut™ R (Medtronic, Minneapolis, MN, USA) THV.
The S3 THV is a balloon-expandable THV made of a cobalt-chromium alloy frame, bovine pericardial leaflets and internal and external polyethylene terephthalate fabric seals at the inflow level of the valve. The 23 mm S3 valve has a stent frame height of 18 mm when fully expanded as per manufacturer specifications6. The ACn is a self-expanding THV with a nitinol frame and porcine pericardial leaflets positioned higher within the frame. There are inner and outer pericardial seals at the inflow level of the valve. There are three stabilisation arches for axial alignment in the ascending aorta, an upper crown and a lower crown. The total height of the ACn ranges between 48 and 51 mm with the stent body height being 18-19 mm. Three sizes (small, medium and large) are currently available to accommodate aortic annulus diameters of between 21 mm and 27 mm. The Evolut R has similar features of its prior iterations with a radiopaque self-expanding nitinol frame, supra-annular trileaflet porcine pericardial leaflets, and porcine pericardium fabric skirt. The 23 mm Evolut R has a skirt height and frame height of 13 mm and 45 mm, respectively. The inflow diameter is 23 mm and the outflow diameter is 34 mm7.
THVs were deployed into 21 mm sized Mitroflow aortic bioprostheses (Sorin Group USA Inc, Arvada, CO, USA). One Mitroflow valve was utilised to assess each test condition and each THV design. A total of nine Mitroflow valves were utilised. The Mitroflow bioprosthesis consists of an acetyl homopolymer stent frame with bovine pericardial sheets sutured externally to form the leaflets. The sewing ring covers the base of the frame and incorporates a non-rigid radiopaque silicone ring covered by a Dacron mesh8. The 21 mm Mitroflow valve has a true internal diameter of 17 mm9.
EX VIVO VALVE-IN-VALVE PROCEDURE
The THVs were positioned in the surgical bioprosthetic valve with the aim of achieving a “high” implant to maximise the EOA, and lowest residual transvalvular gradient10. The Mitroflow bioprosthesis has a visible radiopaque ring but the frame is radiolucent. Ex vivo VIV using the S3 THV was performed with the centre marker of the S3 THV positioned just above the level of the sewing ring of the Mitroflow valve. Ex vivo VIV with the ACn THV was performed by positioning the upper crown just above the top of the acetyl stent frame. Ex vivo VIV with the Evolut R was performed by positioning the THV at a depth of 2 mm in relation to the sewing ring of the Mitroflow valve (Figure 1).
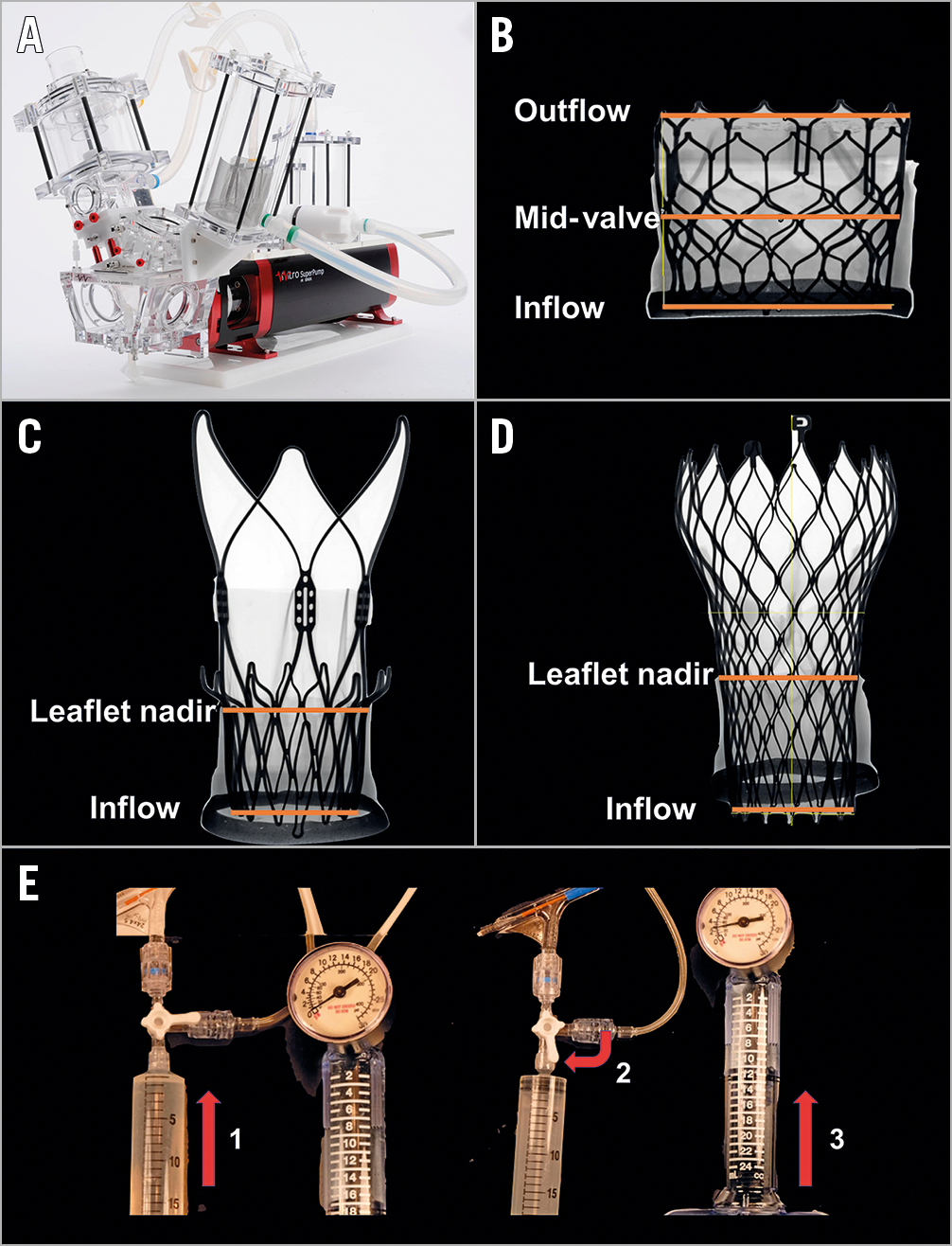
Figure 1. Bench testing methodology. A) Pulse duplicator used for hydrodynamic testing. B) Example of radiographic image of an S3 THV with orange lines indicating measurement made at inflow, mid-valve and outflow of valve. C) Example of radiographic image of an ACn THV with orange lines indicating measurements made at inflow and leaflet nadir of the valve. D) Example of radiographic image of an Evolut R THV with orange lines indicating measurements made at inflow and leaflet nadir of the valve. E) TRUE Dilatation balloon inflation technique. Set-up consisted of a large syringe, indeflator and a high-pressure stopcock. The TD balloon was filled with the syringe first (1) and then the stopcock was turned to the indeflator (2) which pressurised the balloon to the desired pressure (3).
EX VIVO BIOPROSTHETIC VALVE FRACTURE
BVF was performed using a 23 mm non-compliant TRUE® Dilatation balloon valvuloplasty catheter (Bard Peripheral Vascular Inc., Tempe, AZ, USA). A 23 mm sized TRUE Dilatation balloon was chosen based on a prior bench study from our group. This prior study demonstrated lower transvalvular gradients utilising a 23 mm TRUE Dilatation balloon compared to smaller-sized TRUE balloons when performing BVF following VIV intervention in 21 mm Mitroflow surgical valves, utilising either a SAPIEN or ACn THV11. Balloons were inflated using a set-up of a large syringe, indeflator and high-pressure stopcock. The balloon was filled with a hand injection of the syringe first and then the stopcock was opened to the indeflator to pressurise the balloon. The inflation pressure when BVF was achieved was recorded.
IMAGING
High-resolution photography was performed at the same magnification and same fixed camera height. Fluoroscopy was performed using a standard adult cardiac catheterisation laboratory (General Electric Healthcare, Chicago, IL, USA). Radiographic images were made using a Nikon XT H 225 ST microfocus X-ray tomography system (Nikon Metrology, Cambridge, Canada). The THV valves were visually inspected for macroscopic damage after BVF was performed.
MEASUREMENTS
Measurements were made using radiographic images for each sample. Diameter measurements were made using the centre of the THV strut as a marker. To account for potential elliptical THV deployment, two orthogonal measurements of diameter were made, and averaged.
SAPIEN 3 THVs had measurements at the inflow (IF), mid-valve (MV), and outflow (OF) of the THV. ACn THVs had measurements at the inflow (level of the adaptive polyethylene fabric seal) and nadir of the leaflets (at the level of the upper crown). Evolut R THVs had measurements at the inflow and nadir of the leaflets (Figure 1).
HYDRODYNAMIC ASSESSMENT
Hydrodynamic testing was performed for each sample, using a commercially available pulse duplicator (ViVitro Labs Inc, Victoria, Canada) (Figure 1). Valves were tested in accordance with International Standards Organization (ISO) 5840-3:2013 guidelines regarding in vitro pulsatile flow testing for heart valve substitutes implanted by transcatheter techniques12. Valves were placed in a holder fabricated from silicone with a durometer of scale Shore A hardness of 40±5. Justification for the selection of sample holder hardness was based on published data on acceptable tissue compliance matched with published data on the silicone material hardness scale13,14,15. Test fluid used was 0.9±0.2% sodium chloride test solution maintained at 37±2°C (one drop of Cosmocil® [Lonza, Basel, Switzerland] preservative per 1 L).
Valves were tested on the aortic side of the pulse duplicator with a spring-loaded disc valve (ViVitro Labs) on the mitral side of the pulse duplicator. Measurements were based on average results taken from 10 consecutive cycles. High-speed video was captured at each step condition. Pulsatile forward flow performance was tested at a nominal beat rate of 70±1 beats per minute, systolic duration of 35±5%, mean aortic pressure of 100±2 mmHg, and simulated cardiac outputs of 5±0.1 litres per minute. Mean gradient (mmHg), regurgitant fraction (%) and EOA (cm2) were assessed.
PINWHEELING INDEX
Pinwheeling, as defined by the ISO guideline for THV testing, refers to twisting of the leaflet free edges resulting from excessive leaflet redundancy12. A pinwheeling index (PWI) as described by Midha et al was utilised16. We quantified the degree of pinwheeling by tracing the contour of the leaflet free edges and compared it to the unconstrained, ideal configuration. The following equation was used to calculate a pinwheeling index:
Pinwheeling index (%) = [(Lactual – Lideal) / Lideal]×100
Lactual denotes the length of the leaflet from the valve frame to the coaptation centre. Lideal denotes the straight line distance between the endpoints of the leaflet free edge16. Determination of the pinwheeling index was performed using a custom-made Matlab code after image calibration.
STATISTICAL ANALYSIS
Hydrodynamic variables are reported as mean±standard deviation (SD).
Results
VALVE HYDRODYNAMICS
MEAN TRANSVALVULAR GRADIENT
BVF timing and impact on mean transvalvular gradient are reported in Figure 2. Compared to VIV alone, transvalvular gradients were lower with BVF, with the lowest gradients achieved when BVF was performed after VIV for the S3 (no BVF: 15.5±0.1 mmHg, BVF before VIV: 8.0±0.1 mmHg, BVF after VIV: 5.6±0.1 mmHg), and the ACn (no BVF: 9.8±0.2 mmHg, BVF before VIV: 8.4±8.4 mmHg, BVF after VIV: 5.1±0.1 mmHg). Transvalvular gradients were similar for the Evolut R, irrespective of performance of BVF or timing of BVF (no BVF: 15.1±0.2 mmHg, BVF before VIV: 13.8±0.2 mmHg, BVF after VIV: 13.3±0.1 mmHg).
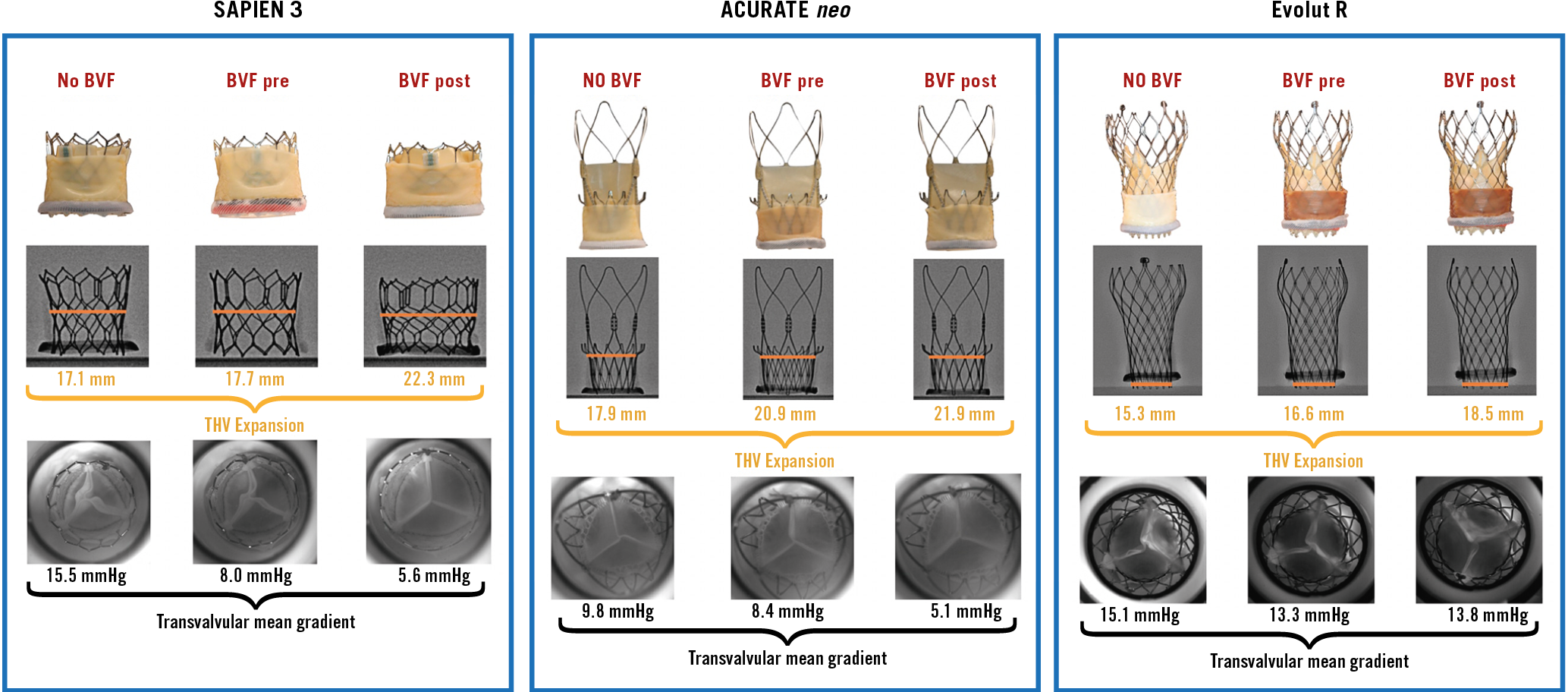
Figure 2. Multimodality imaging by BVF timing for VIV with the 23 mm S3, small ACn and 23 mm Evolut R in 21 mm Mitroflow bioprostheses. The orange lines represent THV dimensions at the mid-point of the S3, the leaflet nadir of the ACn and the inflow of the Evolut R.
EFFECTIVE ORIFICE AREA
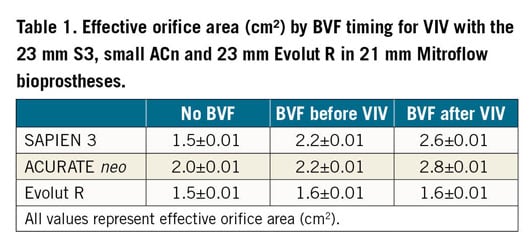
BVF timing and impact on EOA are reported in Table 1. BVF resulted in an increase in EOA for the S3 (no BVF: 1.5±0.01 cm2, BVF before VIV: 2.2±0.01 cm2, BVF after VIV: 2.6±0.01 cm2) and ACn (no BVF: 2.0±0.01 cm2, BVF before VIV: 2.2±0.01 cm2, BVF after VIV: 2.8±0.01 cm2) samples, with a greater increase in EOA achieved when BVF was performed after VIV. The performance of BVF or its timing did not have a significant impact on EOA for the Evolut R samples (no BVF: 1.5±0.01 cm2, BVF before VIV: 1.6±0.01 cm2, BVF after VIV: 1.6±0.01 cm2).
REGURGITANT FRACTION
BVF timing and impact on regurgitant fraction (RF) are reported in Figure 3. BVF resulted in a reduction in RF for the S3 (no BVF: 18.4%, BVF before VIV: 16.6%, BVF after VIV: 14.5%), with a greater reduction in RF when BVF was performed after VIV. BVF resulted in an increase in RF for the ACn samples (no BVF: 14.1%, BVF before VIV: 16.5%, BVF after VIV: 22.0%), with a greater increase in RF when BVF was performed after VIV. The performance of BVF or its timing did not have a significant impact on RF for the Evolut R samples (no BVF: 10.7%, BVF before VIV: 8.4%, BVF after VIV: 9.1%).
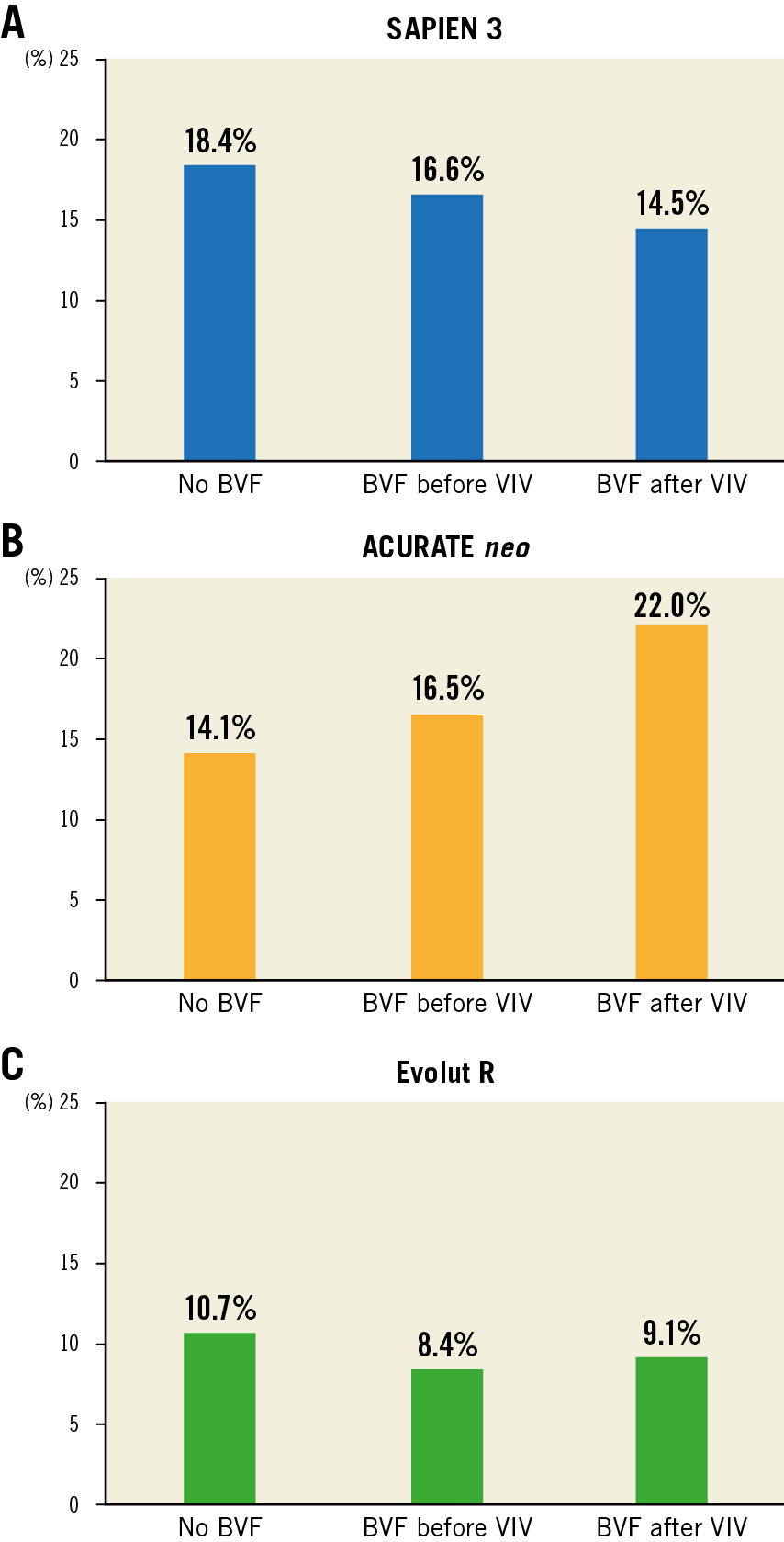
Figure 3. Regurgitant fraction by BVF timing for VIV with the 23 mm S3, small ACn and 23 mm Evolut R in 21 mm Mitroflow bioprostheses.
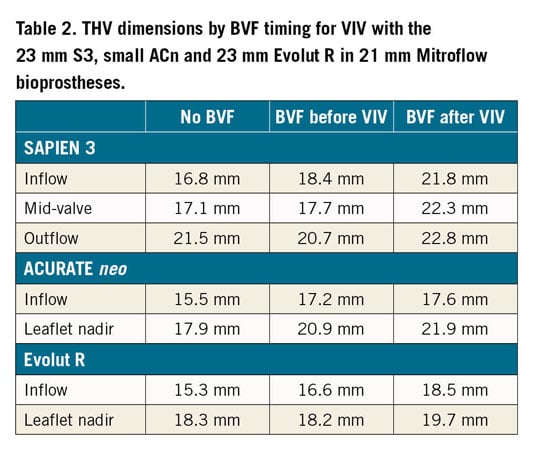
THV EXPANSION
BVF timing and impact on valve dimensions are reported in Table 2. Following VIV, all three THV samples tested were underexpanded. BVF improved expansion in all three THV designs. BVF was achieved at an inflation pressure of 12 atmospheres. In the S3 sample there was better expansion at the mid-valve and inflow of the THV, particularly if BVF was performed after VIV. In the ACn samples, BVF resulted in a similar degree of THV expansion at both the inflow and leaflet nadir, irrespective of timing. In the Evolut R sample, there was better expansion at both the inflow and leaflet nadir level of the THV when BVF was performed after VIV. After BVF was performed, there was no evidence of gross macroscopic damage to the three THV designs tested.
PINWHEELING
BVF timing and the impact on pinwheeling index (PWI) in the three THV designs tested are reported in Figure 4 and Moving image 1-Moving image 9. BVF performed before VIV did not result in a significant reduction in PWI in all three THV designs tested. BVF resulted in a significant reduction in pinwheeling if BVF was performed after VIV intervention in all three THV designs, particularly in the S3 sample. The S3 had the highest degree of pinwheeling with VIV alone; following BVF after VIV there was no significant degree of pinwheeling (no BVF, PWI 8.8%; BVF before VIV, PWI 7.7%; BVF after VIV, PWI 0.8%).
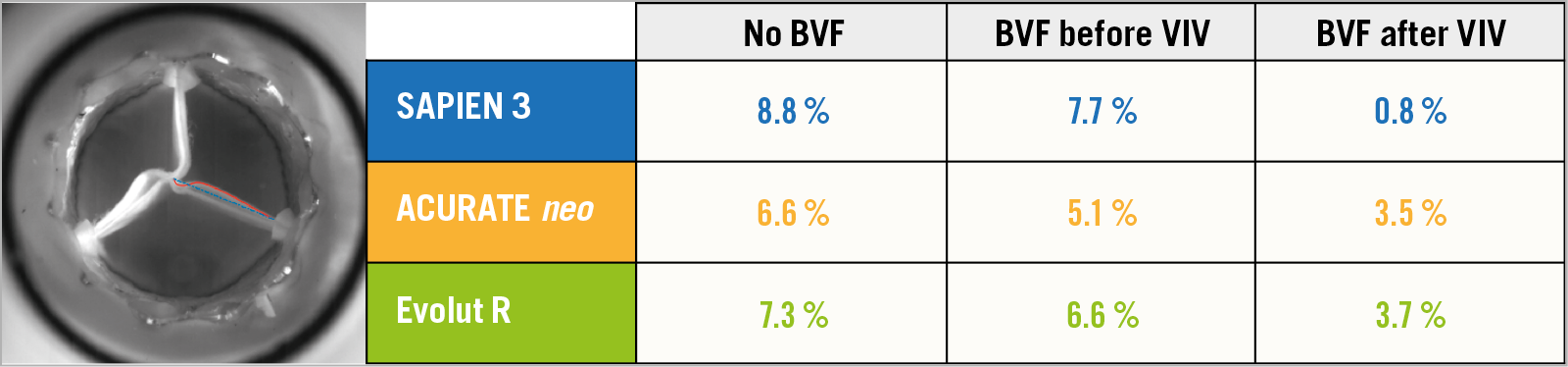
Figure 4. Pinwheeling index (%) by BVF timing for VIV with the 23 mm S3, small ACn and 23 mm Evolut R in 21 mm Mitroflow bioprostheses.
Discussion
This study demonstrates that BVF performed after VIV results in superior THV expansion. In the case of the S3 and ACn it was associated with a better reduction in transvalvular gradients. BVF or its timing did not have a significant impact on transvalvular gradients with the Evolut R. The implications of timing and the impact on various THV designs are important to consider when performing BVF.
The results of our bench study are consistent with small clinical series that also demonstrate superior THV function when BVF was performed after VIV, in both balloon-expandable and self-expanding THVs17. Importantly, this bench study also demonstrates that BVF and its timing impact on each THV design differently. The two self-expanding THVs assessed in this study each had a different response to BVF. This highlights the importance of assessing each THV design individually, rather than assuming that there is a “class effect” based on the mode of THV deployment. The difference between the two self-expanding THVs may be related to differences in THV design. Prior bench studies have demonstrated that, when the upper crown of the ACn is underexpanded, this leads to compromised THV function18. Performance of BVF leads to better expansion of the upper crown which subsequently led to improvement in transvalvular gradients with the ACn. In comparison, while there was better expansion at the inflow and leaflet nadir of the Evolut R with BVF, this did not impact on the THV at the level of leaflet coaptation or hydrodynamic performance. While BVF after VIV intervention may be desirable, other considerations are also of importance. BVF requires high-pressure balloon inflation with a non-compliant balloon, which might damage THV leaflets leading to both short- and long-term failure. There is a risk of acute leaflet dysfunction leading to aortic insufficiency and haemodynamic compromise19. Crimping of THVs has been shown to cause damage to the surface layers of leaflets20. High-pressure inflation with non-compliant balloons during BVF may similarly cause leaflet damage that may lead to accelerated leaflet degeneration and failure. To avoid these concerns, BVF may be performed before VIV intervention. However, there are also risks of acute aortic insufficiency when BVF is performed first, due to damage of the surgical valve leaflet. This study also demonstrates that expansion and hydrodynamic function with BVF before VIV can be less favourable, which may compromise long-term function.
The 23 mm Evolut samples had gradients that were higher than the S3 and ACn samples. Importantly, while the gradients were higher, they were still <20 mmHg which has been shown to be associated with favourable clinical outcomes following VIV intervention2. However, this study analysed haemodynamic data from VIV intervention in new, as opposed to degenerated, surgical valves; how these results translate to the haemodynamic results of VIV intervention and BVF in degenerated bioprosthetic valves is unknown. Nevertheless, prior clinical series have demonstrated similar gradients following VIV intervention with the 23 mm Evolut R in 21 mm Mitroflow valves5,21,22,23. Typically, gradients using a THV design with supra-annular positioned leaflets would have lower gradients compared to an intra-annular leaflet design. Factors related to THV design and positioning can influence gradients. In this study, the S3 THV was positioned high in the Mitroflow which may result in favourable gradients compared to a lower implant depth, where gradients may be higher10. While the VIV app recommends a 20 mm S3, a larger 23 mm S3 was utilised in this study that may have led to more favourable gradients. Utilisation of a larger 26 mm Evolut may result in superior gradients compared to a 23 mm Evolut.
Better THV expansion also resulted in less redundant leaflet tissue and pinwheeling, which may improve durability16. The degree of pinwheeling was minimal for the S3 when BVF was performed after VIV intervention. Importantly, BVF had a different effect on the two self-expanding valves with supra-annular leaflets in this study. Prior bench studies have demonstrated that optimum expansion of the ACn upper crown is important to facilitate favourable hydrodynamic function18. BVF resulted in better expansion at the level of the leaflet nadir, with an associated reduction in transvalvular gradients. With better THV expansion with the ACn there was an increasing RF. The mechanism for this is unclear. A potential reason for the increase in RF with the ACn and BVF may be related to the size of ACn used in this study. A small ACn was utilised in this study. While THV expansion was better after BVF, the small ACn may have been too small in a fractured 21 mm Mitroflow surgical valve, which may have led to increased leak between the THV and the surgical valve. A medium ACn may have resulted in better sealing in a fractured Mitroflow valve. Additionally, unlike the S3, the ACn does not have an outer sealing skirt which may have impacted on RF.
In some test conditions, the RF was higher than expected. In this study, the surgical valves were sealed to the silicone holders. Therefore, the mechanism for RF in this study was not related to paravalvular leak. The mechanism for leak may be related to either inter-valvular (between the surgical valve and THV) leak or central leak. In VIV intervention, there may be leaflet pinwheeling that can lead to a central coaptation defect leading to leak11.
Limitations
Bench testing may not entirely reflect how a THV will expand in a patient’s native annulus, within a degenerated surgical bioprosthesis, or valve deployment under physiological conditions. Future studies would need to assess the impact of BVF timing on long-term durability and clinical outcomes. Testing of BVF timing in different surgical valve designs would also be desirable. Accelerated wear testing can assess the impact of BVF timing on leaflet mechanical wear, but this mode of testing cannot assess calcification or stenosis as a mode of failure. Ultimately, large clinical series with long-term follow-up are required to understand the short and long implications of BVF. Clinical series are also required to understand the impact of BVF timing on complications such as aortic root rupture. In this study, VIV intervention with the ACn THV was performed by positioning the upper crown just above the top of the acetyl stent frame. However, this would be challenging to achieve clinically as the frame of the Mitroflow is radiolucent. Repetition of bench testing with more samples would be desirable.
Conclusions
BVF performed after VIV is associated with superior THV expansion in all three THV designs tested, and with lower residual transvalvular gradients with the SAPIEN 3 and ACURATE neo THVs. The Evolut R has similar hydrodynamic performance irrespective of BVF timing. Timing of BVF has potential implications on THV function.
|
Impact on daily practice Early clinical experience with bioprosthetic valve fracture has been favourable with an improvement in transcatheter heart valve function for patients undergoing valve-in-valve interventions. Whether bioprosthetic valve fracture should be performed before or after valve intervention, and its implications are poorly understood. This study demonstrates that BVF performed after VIV is associated with superior THV expansion in all three THV designs tested, and with lower residual transvalvular gradients with the SAPIEN 3 and ACURATE neo THVs. The Evolut R has similar hydrodynamic performance irrespective of BVF timing. Timing of BVF has potential implications on THV function. Larger clinical series are required to determine the impact of BVF timing on both short- and long-term THV function and durability. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
K.B. Allen has received research grants and speaker’s fees from Edwards Lifesciences and Medtronic; he is also a consultant to Abbott Vascular. K. Chhatriwalla is on the Speaker’s Bureau for Edwards Lifesciences, Medtronic and Abbott Vascular and is a proctor for Edwards Lifesciences and Medtronic. L.P. Dasi reports having patent applications filed on novel polymeric valves, vortex generators and superhydrophobic/omniphobic surfaces. P. Pibarot has received funding from Edwards Lifesciences for echocardiography core lab analyses, with no direct personal compensation. J. Sathananthan is a consultant to Edwards Lifesciences. J. Webb is a consultant to, and has received research funding from Edwards Lifesciences, Abbott, and ViVitro Labs. L. Søndergaard has received consultant fees and institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis. D.A. Wood is a consultant to and has received research funding from Edwards Lifesciences and Abbott. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. VIV with a 21 mm S3 in a 21 mm Mitroflow, and no BVF.
Moving image 2. VIV with a 21 mm S3 in a 21 mm Mitroflow, and BVF before VIV.
Moving image 3. VIV with a 21 mm S3 in a 21 mm Mitroflow, and BVF after VIV.
Moving image 4. VIV with a small ACn in a 21 mm Mitroflow, and no BVF.
Moving image 5. VIV with a small ACn in a 21 mm Mitroflow, and BVF before VIV.
Moving image 6. VIV with a small ACn in a 21 mm Mitroflow, and BVF after VIV.
Moving image 7. VIV with a 23 mm Evolut R in a 21 mm Mitroflow, and no BVF.
Moving image 8. VIV with a 23 mm Evolut R in a 21 mm Mitroflow, and BVF before VIV.
Moving image 9. VIV with a 23 mm Evolut R in a 21 mm Mitroflow, and BVF after VIV.

