
Abstract
Aims: Transcatheter valve-in-valve (VIV) implantation is usually discouraged in small surgical tissue valves. We report our first ten cases of fracturing small dysfunctional Mitroflow bioprostheses by high-pressure balloon dilatation to increase the internal diameter of the surgical valve before VIV (BF-VIV).
Methods and results: BF-VIV was performed in 10 patients (mean age 84±4 years) with failing Mitroflow valves size 19 mm (n=3, threshold of fracture 15 atm) and 21 mm (n=7, threshold of fracture 13 atm). An Edwards SAPIEN 3 or XT 20 mm or 23 mm transcatheter valve was implanted inside the fractured Mitroflow bioprosthesis. The procedure improved aortic valve area (0.7±0.3 vs. 1.1±0.3 cm2, p=0.001), reduced peak aortic valve gradient (66±27 vs. 29±7 mmHg, p=0.002), resolved aortic regurgitation and improved patients’ NYHA functional class (p=0.005). One patient had a minor stroke with complete resolution of symptoms and another patient required a pacemaker due to AV block. All patients were still alive at the end of follow-up (438±255 days).
Conclusions: Initial experience with transcatheter BF-VIV suggests that this method is feasible and safe, and that it improves aortic valve haemodynamics and clinical functional capacity. BF-VIV is a promising alternative to repeat surgery in patients with small failing Mitroflow bioprostheses.
Abbreviations
AVA: aortic valve area
BF-VIV: balloon fracturing valve-in-valve
CPS: cardiopulmonary support
SVD: structural valve degeneration
VIV: valve-in-valve
Introduction
The major disadvantage of surgical biological heart valves is related to their limited durability and predisposition to structural valve degeneration (SVD). Early failure of the Mitroflow aortic bioprosthesis (Sorin Group [now LivaNova], Milan, Italy) leading to reduced survival has been reported, and early SVD is more frequent in small Mitroflow valves size 19 mm (20% at five years) and 21 mm (5% at five years)1,2. The surgical risk may be high in patients with SVD due to advanced age and comorbidities. Therefore, transcatheter aortic valve-in-valve (VIV) implantation is used increasingly for treating SVD as an alternative to repeat surgery3,4. The results of transcatheter aortic VIV implantation in SVD are promising with a one-year survival rate of 83% in a large multicentre registry, but less favourable among patients with small bioprostheses (≤21 mm)5. In general, VIV implantation is discouraged in small surgical bioprosthetic valves due to unacceptable post-implant valve haemodynamics6. The small effective orifice in such valves will be further reduced with implantation of a transcatheter valve into the non-distensible valve ring, thereby creating a patient-prosthesis mismatch and a high post-procedural aortic valve gradient. With more than 100,000 Mitroflow surgical valves implanted worldwide and most of them size 19 and 21 mm7, it is expected that a large cohort of patients will need reoperation due to early SVD in the future.
We recently reported a new technique of fracturing the ring of failed Mitroflow bioprosthetic aortic valves size 19 and 21 mm with a high-pressure balloon to increase the prosthetic valve orifice area before transcathether VIV implantation8. Herein we report the results from the first 10 patients treated with high-pressure balloon fracturing of degenerated small Mitroflow bioprostheses before transcatheter VIV implantation (BF-VIV) at our centre.
Methods
PATIENTS AND STUDY DESIGN
This is a retrospective analysis of the first 10 patients undergoing high-pressure balloon fracturing of degenerated small Mitroflow bioprostheses (size 19 mm, n=3, and 21 mm, n=7) before transcatheter VIV implantation. All procedures were carried out at the Heart Centre, Aarhus University Hospital, Denmark. Data were acquired from the preoperative, perioperative and in-hospital periods, as well as 30 days post procedure and at the end of follow-up. The first implant was in January 2015 and the latest implant was in January 2017. Information on vital status was obtained from The Danish Civil Registration System at the end of follow-up on 1 March 2017. Mean follow-up time was 438±255 days. All 10 patients were included for data analysis and no patients were lost to follow-up.
All patients were evaluated preoperatively and at 30 days post procedure by transthoracic echocardiography, transoesophageal echocardiography, cardiac CT, and clinical assessment. Based on preoperative evaluation, patients were assessed for repeat surgery by a multidisciplinary team of dedicated cardiac surgeons and cardiologists. If the surgical risk was deemed too high according to EuroSCORE II, a calcified aorta or frailty, a TAVI multidisciplinary team of cardiologists, heart surgeons and anaesthesiologists assessed eligibility for prosthetic valve fracturing and VIV implantation.
The Danish Health Authority approved data acquisition from patient files, and informed consent was obtained from each patient before transcatheter VIV treatment.
FRACTURING SMALL MITROFLOW VALVES BEFORE VIV
The procedure was performed under local anaesthesia and conscious sedation in transfemoral cases (n=8) and under general anaesthesia for apical access (n=2). A cardiopulmonary support system (CPS) with cannulas in a femoral vein (17 Fr) and artery (15 Fr) was inserted for back-up in all cases. In two cases, a 0.014-inch guidewire was inserted into the left coronary artery for access in the event of coronary ostial compression. The failing Mitroflow valve was crossed either retrogradely from the aorta or antegradely from the left ventricular apex. An Atlas® GOLD balloon (20 mm/4 cm for Mitroflow 19 and 22 mm/4 cm for Mitroflow 21; Bard Peripheral Vascular, Inc., Tempe, AZ, USA) was gradually inflated during rapid RV pacing (180 bpm). Initially there was an indentation in the balloon from the stenotic part of the Mitroflow prosthesis and then a waist from the valve ring. Fracture of the Mitroflow valve ring occurred at a balloon pressure of 11-16 atm, manifested by a sudden drop in inflation pressure, resolution of the waist in the balloon and expansion of the balloon to its full diameter. Balloon fracturing was followed by standard VIV implantation of an Edwards SAPIEN 3 (S3) or SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA). The CPS was run with a support flow of 1.5-2 l/min during the BF-VIV (Figure 1, Moving image 1-Moving image 4).
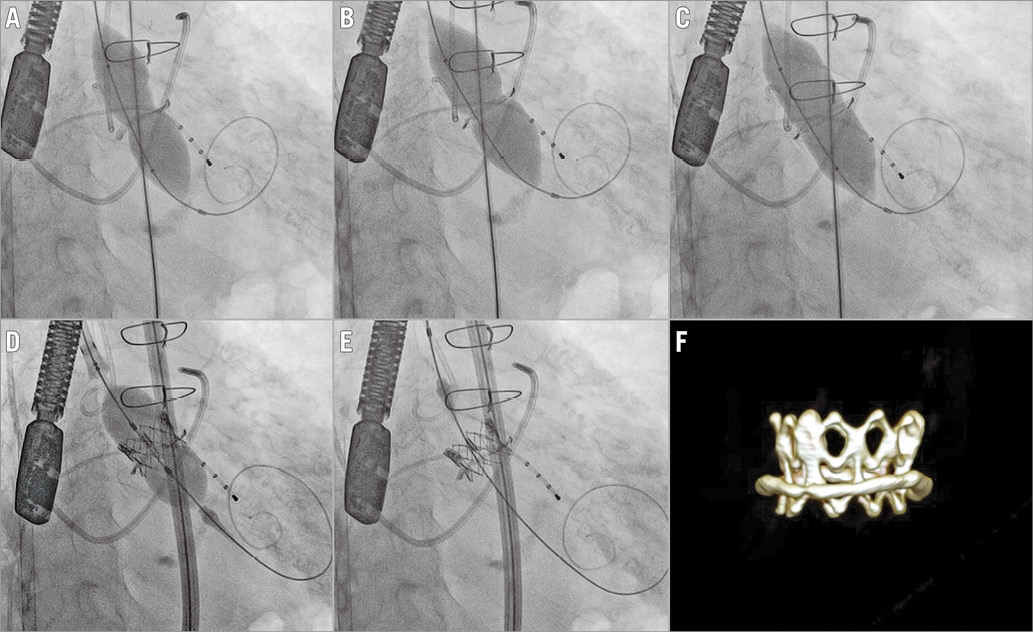
Figure 1. Fracturing a degenerated (stenosis and regurgitation) Mitroflow bioprosthesis size 19 with a high-pressure balloon before transcathether implantation of a SAPIEN XT size 20 in an 88-year-old woman. A) Inflating the Atlas GOLD 20 mm high-pressure balloon reveals a stenotic Mitroflow valve. B) With higher pressure, the balloon breaks the stenosis and a waist is then seen from the intact Mitroflow valve ring. C) With increasing pressure the Mitroflow fractures at 14 atm with an audible click, a drop in balloon pressure and resolution of the waist in the high-pressure balloon. D) & E) A SAPIEN XT size 20 is implanted in the fractured Mitroflow size 19. F) The position is confirmed by cardiac CT at 30-day follow-up.
STATISTICS
Results are presented as mean±standard deviation for continuous data and as number (percentage) for categorical data. To compare pre- and post-procedure data, a paired t-test was used for data that were normally distributed and a non-parametric Wilcoxon matched pairs signed-rank test was used for data that were non-normally distributed. A Shapiro-Wilk normality test was used to test for normality distribution. Contingency data were tested by chi-square and Fisher’s exact test. Due to low numbers in the subgroups (Mitroflow 19 and 21 mm), statistical analysis was only performed in the pooled group (“All”); no statistical analysis was performed to compare the Mitroflow 19 and 21 groups. A p-value <0.05 was considered statistically significant. GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis.
Results
PATIENT DEMOGRAPHICS (Table 1)
Ten patients underwent high-pressure balloon fracturing of small dysfunctional Mitroflow bioprostheses before transcatheter VIV implantation. Three patients had a degenerated Mitroflow size 19 mm and seven patients had a degenerated Mitroflow size 21 mm. Mean age of the patients was 84±4 years. Half of the patients were men, but all patients with Mitroflow size 19 were women. Predicted operative mortality risk assessed by EuroSCORE II was 15.2±15.2%; comorbidities were diabetes mellitus 30%, peripheral vascular disease 10%, chronic renal failure 50%, and ischaemic heart disease 30%. Mean time since surgical insertion of the Mitroflow valve was 11.7±1.6 years, and the mechanism of failure was stenosis in 50%, regurgitation in 10% and a combination of both stenosis and regurgitation in 40% of the patients.
PROCEDURE CHARACTERISTICS (Table 2)
Mean time used for the procedure was 128±58 min. Access was femoral in 80% and apical in 20% of the cases. All patients had CPS back-up. An Atlas GOLD high-pressure balloon 20 mm was used to fracture Mitroflow size 19 (30% of the cases), and the mean threshold for fracture was 15±1 atm. In Mitroflow size 21 (70% of the cases), an Atlas GOLD balloon 22 mm was used, and the mean threshold for fracture was 13±3 atm. An Edwards S3, 23 mm (86%) or an Edwards SAPIEN XT, 23 mm (14%) was implanted after fracturing Mitroflow size 21 and a SAPIEN XT 20 mm was implanted after fracturing Mitroflow size 19. Mean duration of hospital stay after the procedure was 5±1 days. One patient had visual impairment after the procedure: MRI of the brain revealed a minor stroke. The patient recovered fully within a few days. One patient developed advanced AV block after the procedure and had a permanent pacemaker implanted.
VALVE AND CLINICAL CHARACTERISTICS BEFORE AND AFTER THE PROCEDURE (Table 3, Figure 2)
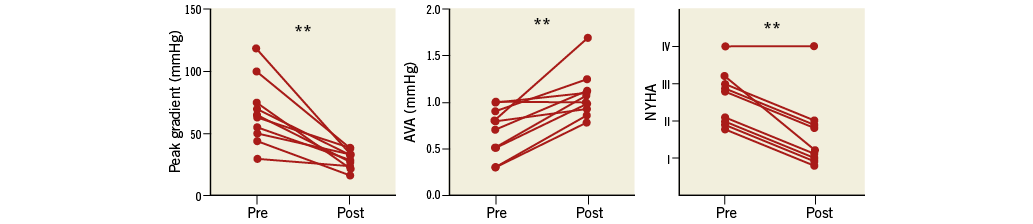
Figure 2. Aortic valve haemodynamics and patients’ functional class before and 30 days after BF-VIV. ** p<0.01. AVA: aortic valve area calculated by transthoracic echocardiography; NYHA: New York Heart Association (functional class)
All patients were still alive at the end of follow-up (mean follow-up 438±255 days). The procedure significantly improved NYHA functional class (p=0.0054) with 10% of patients in NYHA Class IV, 40% in NYHA Class III and 50% in NYHA Class II before the procedure and 10% in NYHA Class IV, 50% in NYHA Class II and 40% in NYHA Class I after the procedure. Mean aortic valve area (AVA) and peak transvalvular peak gradients changed, respectively, from 0.7±0.3 cm² and 66 mmHg before the procedure to 1.1±0.3 cm2 (p=0.0011) and 29 mmHg (p=0.0020) after the procedure. Severe (10%), moderate (40%) and minor aortic regurgitation was present before the procedure and resolved after BF-VIV. One patient had a minor paravalvular aortic regurgitation after the procedure. The internal diameter and valve area of an unused Mitroflow valve are 15.5 mm and 1.9 cm2 for the size 19 and 17 mm and 2.3 cm2 for the size 21, respectively. Cardiac CT after VIV revealed a mean stent valve internal diameter and area for the size 20 valves of 19 mm and 2.9 cm2 and for the size 23 valves of 20 mm and 3.2 cm2. The CT measurements were taken at the level of the narrowest area of the implanted transcatheter valve. Eccentricity index of the implanted stent valves ranged from 0.92 to 1.02 (mean 0.98±0.03).
Discussion and limitations
The feasibility to fracture a surgical bioprosthetic valve before VIV was reported for the first time with a 19 mm Perimount valve (Edwards Lifesciences) in the pulmonic position in a patient with congenital heart disease9. We recently reported the two first-in-man cases of fracturing the ring of small Mitroflow bioprostheses by high-pressure balloon predilatation in transcatheter aortic VIV implantation8. Our experience with this novel treatment as an alternative to high-risk repeat surgery has now been extended: in this paper we describe the results of the first ten patients treated by this novel technique (BF-VIV) with a mean follow-up of more than one year. Our report of the first-in-man aortic cases was preceded by in vitro testing of the fracture mechanics of the Mitroflow valves8. We also extended this experimental testing of high-pressure balloon fracturing to a number of other frequently used surgical biological heart valves, such that we currently have established data on fracture threshold, mechanism, number and zones of fractures and valve structure alterations after fracture for a number of bioprostheses10.
The BF-VIV treatment in this patient cohort of failing small Mitroflow aortic valves resulted in a marked improvement in parameters of aortic valve function and haemodynamics (Figure 2). All patients demonstrated a reduction in the transaortic pressure gradient and an increase in the aortic valve area as estimated by echocardiography at follow-up after one month. Aortic bioprosthetic regurgitation was eliminated by VIV and only one patient had trace of paravalvular leakage after transcatheter valve implantation. Cardiac CT documented a larger internal area of the implanted transcatheter valves than the internal area of the Mitroflow valves into which they were implanted (areas of new non-degraded valves). This was an additional proof that ring fracture had actually occurred. The improvement in aortic valve function achieved by BF-VIV was translated into an improvement in NYHA functional class. Only one patient did not improve clinically. This patient was in NYHA Class IV before treatment and had a very poor left ventricular function and multiple comorbidities. All patients in this cohort were alive at the end of follow-up (mean 438 days) despite advanced age, high EuroSCORE II and a heavy burden of comorbidities.
The inserted surgical valve prosthesis will normally protect against aortic root rupture in VIV therapy. However, this may not apply when the integrity of the surgical valve ring is broken by high-pressure balloon predilatation. In theory, there could also be sharp and irregular elements from the fractured bioprosthesis, thereby increasing the risk of damaging the aortic root. Finally, expansion of the surgical valve ring to a diameter larger than the de novo diameter could possibly induce aortic tissue damage by stretching. However, in the present study we did not see any signs of aortic root damage as evaluated by clinical manifestation, echocardiography or cardiac CT. Moreover, our in vitro series of experiments did not demonstrate any sharp or protruding elements after fracturing the Mitroflow valves8. When the inner acrylic valve ring of the Mitroflow prosthesis is fractured and expanded by the high-pressure balloon and subsequently by the inserted stent valve, it is probably the space between the inner ring and the outer sewing ring of the prosthesis that is reduced, ensuring a minimal distension of the aortic annulus itself.
Another possible complication from BF-VIV with the Mitroflow could by coronary ostial compression. In fact, this risk may theoretically be increased with the Mitroflow compared with other bioprostheses because this valve is inserted in a supra-annular position and the bovine pericardial sheet that forms the valve leaflets is sutured to the outside of the inner acrylic stent frame. It is therefore essential to do preprocedural evaluation by cardiac CT in order to assess the distance from the bioprosthesis to the coronary ostia. If there is a borderline distance to the coronary ostia, a coronary guidewire may be advanced into the relevant coronary artery before BF-VIV. We did this in two of the patients in this cohort; however, coronary ostial compression did not occur in any of the ten BF-VIV-treated patients.
When the ring or frame of a bioprosthetic valve is broken by high-pressure ballooning, the subsequent expansion of a stent valve inside the prosthesis will not necessarily be even and circular. That could be influenced for instance by the number and distribution of fracture zones and structural integrity of the valve. However, with the Mitroflow in vitro experiments, we observed a single and regular fracture line in the acrylic valve ring and, in the current series of BF-VIV Mitroflow patients, cardiac CT demonstrated an eccentricity index of one.
Conclusions
We found that this novel method of BF-VIV is a valid alternative to surgical valve replacement in high-risk patients with failing small Mitroflow bioprosthetic valves. Given the previously high number of implanted small Mitroflow valves7, BF-VIV has the potential to benefit a high number of heart valve patients. The concept may possibly be extended to other small surgical tissue valves as indicated by additional in vitro experiments performed by our group10. The future design of surgical tissue valves should take into consideration the ability to carry out later implantation of a transcatheter valve of sufficient size.
| Impact on daily practice Transcatheter valve-in-valve (VIV) treatment cannot usually be offered to patients with small degraded surgical bioprostheses due to further narrowing of the small valve orifice. We report the first ten cases of fracturing small dysfunctional Mitroflow bioprostheses by high-pressure balloon dilatation to increase the internal diameter of the surgical valve before VIV (BF-VIV). This novel treatment concept is a promising alternative to surgical valve replacement in patients with small failing Mitroflow bioprostheses. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Pre-procedure contrast injection reveals severe aortic valve regurgitation.
Moving image 2. Inflating the Atlas GOLD 20 mm high-pressure balloon reveals a stenotic Mitroflow valve. With higher pressure, the balloon breaks the stenosis and a waist is then seen from the intact Mitroflow valve ring. With increasing pressure, the Mitroflow fractures at 14 atm with an audible click, a drop in balloon pressure and resolution of the waist in the high-pressure balloon.
Moving image 3. A SAPIEN XT size 20 is implanted in the fractured Mitroflow size 19.
Moving image 4. Post-procedure contrast injection to confirm valve position and function.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Pre-procedure contrast injection reveals severe aortic valve regurgitation.
Moving image 2. Inflating the Atlas GOLD 20 mm high-pressure balloon reveals a stenotic Mitroflow valve. With higher pressure, the balloon breaks the stenosis and a waist is then seen from the intact Mitroflow valve ring. With increasing pressure, the Mitroflow fractures at 14 atm with an audible click, a drop in balloon pressure and resolution of the waist in the high-pressure balloon.
Moving image 3. A SAPIEN XT size 20 is implanted in the fractured Mitroflow size 19.
Moving image 4. Post-procedure contrast injection to confirm valve position and function.

