
Abstract
Aims: Residual gradients >20 mmHg after transcatheter valve-in-valve (ViV) implantation are associated with worse survival. This study aimed to evaluate the feasibility of high-pressure post-dilation with a non-compliant balloon after transcatheter ViV implantation in small surgical valves to optimise haemodynamics.
Methods and results: Thirty patients underwent ViV implantation in surgical valves with internal dimension ≤19 mm. High-pressure post-dilation to 16-20 atmospheres with a non-compliant balloon was performed in 12 patients and 18 patients underwent ViV without post-dilation. SAPIEN 3 and Evolut valves were used in 10 and two patients, respectively. The mean aortic valve (AV) gradient decreased by 11.3 mmHg following high-pressure post-dilation (18.7±7.9 mmHg immediately post ViV to 7.5±2.6 mmHg following high-pressure post-dilation, p<0.01). There were no cases of aortic root rupture. High-pressure post-dilation, compared to no post-dilation, was associated with lower invasive AV mean gradients at the end of the ViV procedure (8.2±3.5 mmHg vs. 17.3±7.9 mmHg, p=0.001) as well as lower day 1 (18.0±4.5 mmHg vs. 25.0±8.1 mmHg, p=0.016) and 30-day gradients (19.8±2.5 vs. 26.5±11.0, p=0.038) on transthoracic echocardiography.
Conclusions: High-pressure post-dilation of small surgical valves following transcatheter ViV implantation results in a significant improvement in post-procedure haemodynamics.
Abbreviations
CT : computed tomography
ID: internal dimension
SD : standard deviation
TAVR: transcatheter aortic valve replacement
ViV: valve-in-valve
Introduction
Up to 50% of patients undergoing surgical aortic valve replacement (SAVR) receive a small (size 19 or 21 mm) bioprosthesis1,2. Small surgical valves create a suboptimal platform for subsequent transcatheter valve-in-valve (ViV) implantation, resulting in higher post-ViV gradients and significantly impaired survival. In the Valve-in-Valve International Data Registry, severe prosthesis-patient mismatch following ViV implantation was noted in 31% of patients, with post-procedural gradient ≥20 mmHg being associated with worse outcomes3. Aortic ViV in patients with surgical valve size <21 mm was associated with worse one-year survival, compared to those with valve size >25 mm (74.8% versus vs 93.3%)3. With expansion of transcatheter aortic valve replacement (TAVR) technology, the number of transcatheter ViV implantation procedures for degenerative bioprosthetic surgical valves is expected to increase significantly; thus, optimisation of transcatheter valve haemodynamics following ViV is crucial. We sought to evaluate and describe a technique of fracturing the degenerative surgical bioprosthetic valves during transcatheter ViV implantation to optimise post-ViV haemodynamics. This technique expands the eligible cohort of patients who may benefit from a ViV procedure in the future.
Methods
POPULATION
Since the Food and Drug Administration approval of the Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) in July 2011, a total of 44 patients with small degenerative surgical bioprosthetic valves (as defined by valve internal dimension [ID] ≤19 mm) have undergone transcatheter ViV implantation at Cedars-Sinai Medical Center. High-pressure post-dilation of the ViV complex with a non-compliant balloon following transcatheter valve deployment was performed in 12 of these patients. The degenerative surgical valves in patients undergoing high-pressure post-dilation included Mitroflow (Sorin Group USA Inc., Arvada, CO, USA), Mosaic® and Hancock® (Medtronic, Minneapolis, MN, USA) and Edwards PERIMOUNT (Edwards Lifesciences) valves. Of the remaining 32 patients undergoing ViV implantation without high-pressure post-dilation, 18 patients undergoing transcatheter ViV in the same surgical valve types that underwent high-pressure post-dilation were included as the control group. The first case of high-pressure post-dilation was performed in November 2015; all cases prior to that date were performed without high-pressure post-dilation. Each patient was evaluated by our institutional Heart Team and determined to be high-risk for reoperation. Informed consent for the procedure was obtained from all patients.
PROCEDURE
The choice of the transcatheter heart valve size for the ViV procedure was determined by correlating the surgical valve manufacturer’s inner diameters, along with direct measurements by computed tomography (CT) when available. The transcatheter implantation technique was performed per our facility standard based on the evaluation of the patient’s vascular access and anatomy. All procedures were performed under general anaesthesia and transoesophageal echocardiographic guidance. The commercially available balloon-expandable Edwards valve (SAPIEN, SAPIEN XT or SAPIEN 3; Edwards Lifesciences) and self-expanding CoreValve®/Evolut™ valve (Medtronic) were used. Once the transcatheter valve was deployed, the aortic valve gradient was measured. Until November 2015, no high-pressure post-dilation with a non-compliant balloon was performed to optimise post-ViV haemodynamics. From November 2015 to October 2016, we performed high-pressure post-dilation in all consecutive patients (except one patient) undergoing ViV implantation in small surgical valves (true ID ≤19 mm). One patient undergoing ViV in a 21 mm Mitroflow valve experienced left main coronary occlusion immediately after valve deployment, requiring emergent initiation of cardiopulmonary bypass; high-pressure post-dilation was not performed in this patient despite high post-ViV gradients.
High-pressure post-dilation (16-20 atm) of the transcatheter heart valve was performed using a TRUE balloon (BARD Peripheral Vascular, Tempe, AZ, USA). The primary intent of the high-pressure post-dilation was to optimise post-ViV haemodynamics. This was achieved with either overexpansion or fracturing of the small surgical valve annular frame. The size of the TRUE balloon was determined based on the true ID of the surgical valve, the balloon size being 1 mm larger than the true ID. Post-dilation was performed during rapid ventricular pacing at 180 bpm utilising an indeflator to achieve the required high pressure. Post-dilation gradients were then recorded. If the operators determined that there was still a high residual gradient or incomplete expansion of the transcatheter valve with the first dilation, a second inflation was performed with the same or a larger balloon. Final gradients were then recorded. Post-dilation was considered successful if there was a >50% reduction in mean gradient following post-dilation, or if the final mean gradient was <10 mmHg. In patients at increased risk of coronary obstruction (especially Mitroflow valve with valve leaflets located on the outside of the stent frame), coronary protection was performed. All catheters were then removed, and haemostasis was achieved in standard fashion based on the access utilised in each case. Echocardiograms during follow-up were performed in a blinded manner by experienced sonographers and interpreted in a blinded manner by board-certified cardiologists.
IN VITRO BENCH TESTING
In vitro bench testing was performed by post-dilating a 19 mm Mitroflow valve with a 20 mm×4.5 cm TRUE balloon under fluoroscopic imaging (Figure 1, Moving image 1). Additional bench testing was performed with high-pressure inflation of a 21 mm Mitroflow valve under CT imaging, initially with a 22 mm×4 cm Z-MED II™ balloon (B. Braun Interventional Systems Inc., Bethlehem, PA, USA) and then with a 20 mm×4.5 cm TRUE balloon.
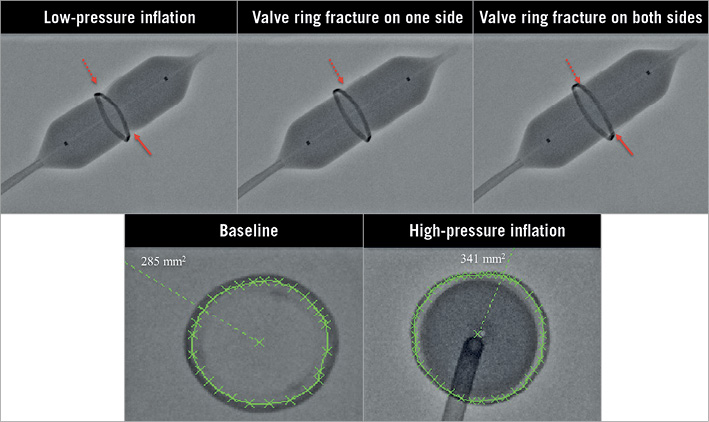
Figure 1. Bench testing model demonstrating fracturing of a 19 mm Mitroflow valve under fluoroscopy. A) Low-pressure inflation with an 18 mm× 4.5 cm TRUE balloon (arrows highlight the areas of underexpansion). B) High-pressure inflation resulted in fracture of the surgical valve on one side (dashed arrow). C) Continued high-pressure inflation resulted in a second fracture in the surgical valve (solid arrow). D) & E) The cross-sectional area of the surgical valve increased from 285 mm2 (D) to 341 mm2 (E) with fracture of the surgical valve.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean±standard deviation (SD) and categorical variables are expressed as n (%). The change in aortic valve gradients before and after post-dilation was compared using the paired t-test. Continuous variables were compared using a two-sided t-test and categorical variables were compared using the chi-square or Fisher’s test, as appropriate. The results were considered significant when the two-sided p-value was <0.05. All statistical analyses were conducted using SPSS, Version 24.0 (IBM Corp., Armonk, NY, USA) software.
Results
BASELINE DEMOGRAPHICS AND PROCEDURAL CHARACTERISTICS
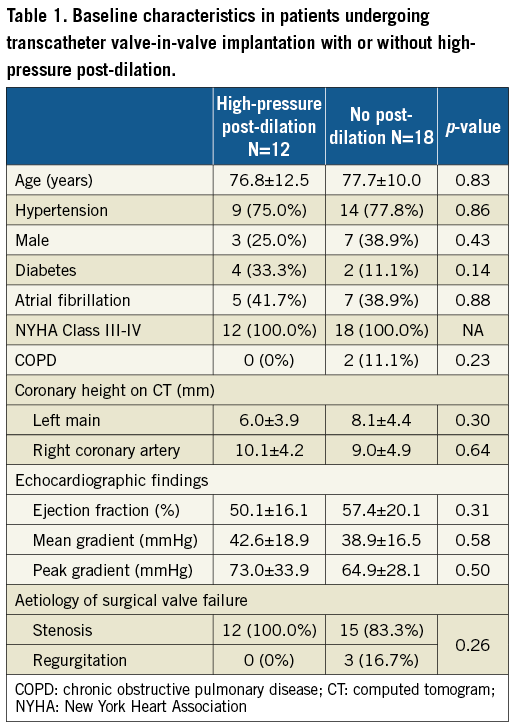
Baseline characteristics are summarised in Table 1. Of the 12 patients undergoing high-pressure post-dilation with a non-compliant balloon, the type of degenerative surgical valve was Mitroflow in eight patients, Mosaic in one patient, Hancock in one patient and Edwards PERIMOUNT in two patients. Mean age was 76.8±12.5 years. The time from index surgery to ViV was 6.5±5.0 years. All patients were symptomatic in New York Heart Association Class III-IV heart failure. One patient was in cardiogenic shock and actively receiving cardiopulmonary resuscitation at the time of the procedure. Ten patients underwent ViV by the transfemoral approach and two patients underwent ViV by the subclavian approach (one right and one left subclavian). Transcatheter ViV implantation was performed with a SAPIEN 3 valve in 10 patients (20 mm, n=6, and 23 mm, n=4) and an Evolut valve (23 mm) in two patients.
PROCEDURAL OUTCOMES
Procedural and haemodynamic outcomes are summarised in Table 2. The procedural details of patients undergoing high-pressure post-dilation are summarised in Table 3. Haemodynamic measurements were performed during the ViV procedure. The baseline mean aortic valve gradient was 42.0±18.8 mmHg. Immediately following transcatheter ViV deployment, the mean aortic valve gradient was 18.7±7.9 mmHg. High-pressure post-dilation of the surgical valve annular ring resulted in a mean reduction in the aortic valve gradient of 11.3 mmHg (18.7±7.9 mmHg immediately post-ViV deployment to 7.5±2.6 mmHg following high-pressure post-dilation, p<0.01) (Figure 2, Figure 3, Moving image 2-Moving image 7). There were no cases of aortic root rupture, aortic root haematoma or balloon rupture associated with high-pressure post-dilation. There was no central or paravalvular regurgitation noted in patients after high-pressure post-dilation. All patients survived to discharge, with a median length of stay of two days (range two to 13 days).
In one patient undergoing ViV by the right subclavian approach, there was significant difficulty encountered in advancing the valve delivery system into the ascending aorta due to significant calcification of the right brachiocephalic artery. Initially, a SAPIEN 3 delivery sheath was inserted but, due to significant calcification, the delivery sheath could not be advanced into the ascending aorta. Subsequently, it was decided to perform transcatheter ViV with an Evolut valve. The patient had excellent post-procedure haemodynamics; however, the patient was noted to have a frontal stroke on post-procedure day 1. There were no additional strokes noted up to 30 days in the patient cohort included in this study.
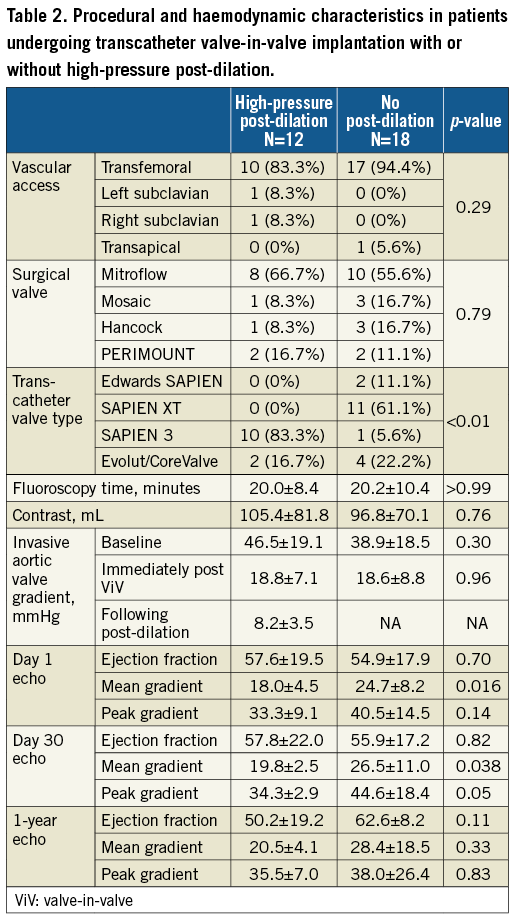
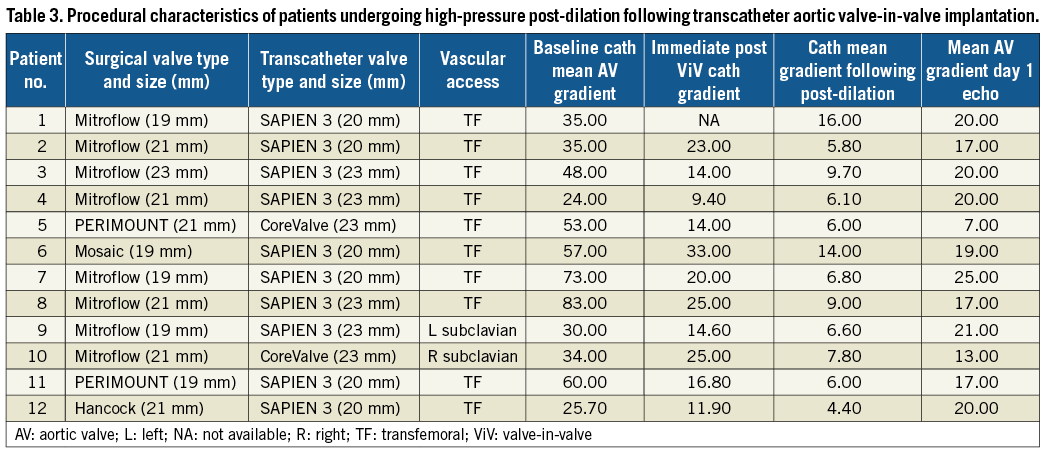
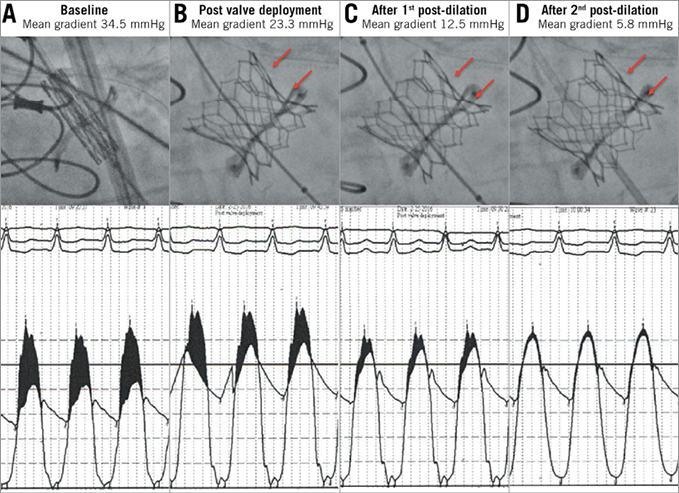
Figure 2. Fracturing of a 19 mm Mitroflow valve following transcatheter valve-in-valve implantation with a 20 mm SAPIEN 3 valve. A) High baseline aortic valve gradients due to degenerative 19 mm Mitroflow valve. B) High residual aortic valve gradient immediately following valve-in-valve implantation with a 20 mm SAPIEN 3. C) & D) Improved valve expansion and reduction in aortic valve gradients following post-dilation with an 18 mm×4.5 cm TRUE balloon (C) and 20 mm×4.5 cm TRUE balloon (D).
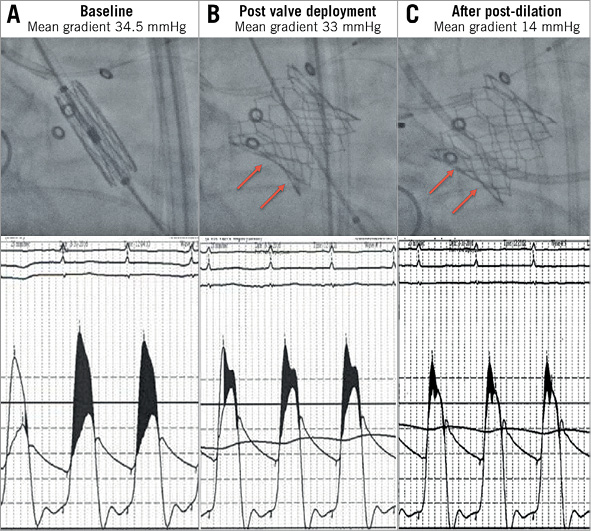
Figure 3. Fracturing of a 21 mm Mosaic valve with a 20 mm SAPIEN 3 valve following valve-in-valve implantation. A) High baseline aortic valve gradients due to degenerative 21 mm Mosaic valve. B) High residual aortic valve gradients immediately following valve-in-valve implantation with a 20 mm SAPIEN 3 valve. C) Fracture of the surgical valve with reduction in gradients following high-pressure post-dilation with a 20 mm×4.5 cm TRUE balloon.
POST-DILATION VERSUS NO POST-DILATION
We further compared haemodynamic and procedural outcomes among 12 patients with small surgical valves undergoing ViV with high-pressure post-dilation versus the 18 patients with the same surgical valve type and size undergoing ViV without post-dilation (Table 2-Table 4, Figure 4). Baseline mean aortic valve gradients were similar in the two groups (46.5±19.1 mmHg vs. 38.9±18.5 mmHg, p=0.29). The final invasive mean aortic valve gradients measured during the ViV procedure were significantly lower in patients undergoing high-pressure post-dilation, compared to those without post-dilation (8.2±3.5 mmHg vs. 17.3±7.9 mmHg, p=0.001). Mean aortic valve gradients assessed with transthoracic echocardiograms on day 1 post procedure (18.0±4.5 mmHg vs. 25.0±8.1 mmHg, p=0.016) and 30 days post procedure (19.8±2.5 mmHg vs. 26.5±11.0 mmHg, p=0.038) were lower in the high-pressure post-dilation cohort, compared to the patients undergoing ViV without high-pressure post-dilation. Procedural outcomes, per VARC-2 criteria, were not different between the two groups. Follow-up echocardiograms were available in seven out of 12 patients undergoing high-pressure post-dilation and nine out of 18 patients in the control group. The mean gradients continued to be lower in the post-dilation cohort, compared to the controls (20.6±4.6 mmHg vs. 28.4±18.5 mmHg, p=0.33). Among patients undergoing high-pressure post-dilation, there was no significant change in mean aortic valve gradients from 30 days to one year (19.8±2.5 mmHg vs. 20.6±4.6 mmHg, p=0.09).
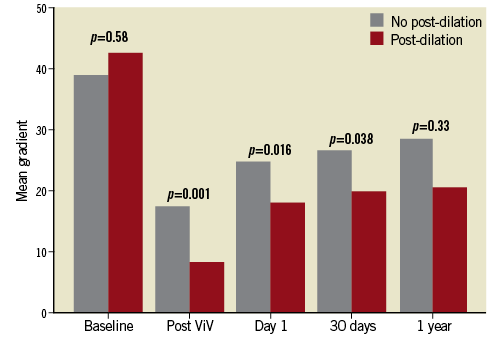
Figure 4. Comparison of mean aortic valve gradients between patients undergoing high-pressure post-dilation versus no post-dilation during transcatheter valve-in-valve implantation in small surgical bioprosthetic aortic valves.
BENCH TESTING
High-pressure inflation of a 20 mm×4.5 cm TRUE balloon in a 19 mm Mitroflow valve under fluoroscopic imaging demonstrated visible fracturing of one side of the Mitroflow valve frame at 14 atm, followed by fracturing of the other side of the stent frame at 18 atm, along with an audible click (Figure 1, Moving image 1). Fluoroscopic analysis of the valve in the short axis illustrated an increase in cross-sectional area from 285 mm2 to 341 mm2.
Additional bench testing was performed with a 21 mm Mitroflow valve under computed tomography (CT) imaging. A baseline CT of the Mitroflow valve was obtained. High-pressure dilation was initially attempted with a 22 mm×4 cm Z-MED II balloon. The balloon ruptured at 8 atm with no expansion of the surgical valve annulus. Subsequent successful fracturing of the ring was achieved with a 20 mm×4.5 cm TRUE balloon at 18 atm. A follow-up CT was then obtained and demonstrated an increase in the cross-sectional area of the Mitroflow valve from 283 mm2 to 299 mm2. The larger measured area with fluoroscopic vs. CT analysis is attributable to measuring the valve fluoroscopically while still on a high-pressure inflated balloon.
Discussion
We describe the technique of high-pressure post-dilation of small surgical bioprosthetic valves with a non-compliant balloon following transcatheter ViV implantation to optimise ViV haemodynamics. The haemodynamic improvement with high-pressure post-dilation results from further expansion of the surgical valve, and the extent of expansion of the valve frame, ranging from overstretching of the surgical valve frame (as noted in PERIMOUNT, Mosaic and Hancock valves) to fracturing of the valve frame (as noted in the Mitroflow valve).
A previous report described fracturing of a Mitroflow valve during ViV implantation with a SAPIEN XT valve4. However, unlike in our case series, the previous report described surgical valve fracture with predilation using a high-pressure non-compliant balloon prior to TAVR implant with the patient being fully haemodynamically supported on cardiopulmonary bypass. In our opinion, aggressive predilation of the surgical valve prior to ViV implantation can precipitate acute severe aortic insufficiency and haemodynamic instability. Moreover, the SAPIEN 3 valve, unlike the SAPIEN XT, can be expanded beyond its nominal size5. This permits further expansion of the valve once the surgical valve is fractured, potentially resulting in improved haemodynamics and leaflet coaptation.
The choice of transcatheter valve type between the balloon-expandable SAPIEN valve and self-expanding Evolut/CoreValve was determined by operator preference. It is plausible that high-pressure inflation of the self-expanding Evolut R may offer superior haemodynamics compared to the SAPIEN 3 valve in small surgical valves due to the supra-annular location of the leaflets3; however, there are also potential limitations. First, as seen in Table 1, the average coronary height in our series was low (LM 7.1±3 mm, RCA 9.5±2.5 mm). The relatively short SAPIEN 3 stent frame allows easier access to the coronaries for pre-emptive protection and percutaneous coronary intervention, if necessary. Second, the lower radial strength of the self-expanding valves in comparison to a balloon-expandable valve could potentially result in some recoil of the surgical valve annulus, despite aggressive post-dilation. The aortic valve gradients were noted to be higher on transthoracic echocardiograms performed on day 1 post TAVR, compared to intraprocedural invasive haemodynamics (Table 2). This pattern was noted in both post-dilation as well as no post-dilation groups. While this could be related to stent recoil following high-pressure post-dilation, it is more likely to be related to a change in haemodynamics (general anaesthesia at the time of TAVR, awake patient on day 1) since the gradients were noted to be higher in both post-dilation and no post-dilation groups on day 1 post TAVR. All cases during the study period were performed under general anaesthesia and transoesophageal echocardiographic guidance; thus, the type of anaesthesia did not influence the decision making in these cases.
There are several technical considerations with regard to this technique. First, instead of predilating the surgical valve to fracture the surgical valve annulus, we recommend post-dilating the transcatheter valve following ViV implantation to minimise the risk of haemodynamic compromise, as mentioned previously. Second, a non-compliant balloon must be used to achieve the pressure needed to fracture the surgical valve. As bench testing demonstrated, using a compliant balloon is inadequate to expand the surgical valve and can lead to balloon rupture with associated potential for aortic root injury. Third, an indeflator must be used to achieve the high pressures required to fracture the surgical prosthesis with the non-compliant balloon. Ring fracture, both on the bench and in vivo, was not obtained until at least 16-20 atm inflation pressure (approximately 264 psi of force) was reached. Reaching this pressure is not readily achievable with manual inflation. Fourth, given the high inherent risk of coronary occlusion with ViV procedures, particularly the Mitroflow valve, it is reasonable to have a low threshold for coronary protection when attempting this technique6,7. Percutaneous coronary intervention to the ostial left main coronary artery was performed exclusively in patients undergoing ViV in degenerative Mitroflow valves (four out of eight patients in the post-dilation versus three out of 10 patients in the no post-dilation group). The Mitroflow valve, due to its inherent design of the valve leaflets being located outside the stent frame, is associated with an increased risk of coronary compromise during ViV implantation. Only one of these cases (in the no predilation group) experienced haemodynamically significant coronary compromise; the remaining cases underwent left main stenting due to the proximity of the Mitroflow leaflets to the coronary ostium. Finally, while complete valve expansion is felt to optimise leaflet function and mobility, any adverse impact of high-pressure dilatation on valve durability or propensity for leaflet thrombosis is currently unknown. High-pressure post-dilation was safe in our reported series; however, we believe that the procedure does carry the usual risks of post-dilation, including aortic root rupture8 and stroke9. Thus, patients at increased risk of these complications, for instance, heavily calcified left ventricular outflow tract, aortic annulus or aortic arch, or aortopathy with a dilated ascending aorta, may not be the best candidates for high-pressure post-dilation. We had one adverse outcome (stroke) while utilising this technique. The stroke in the present case is more likely to be related to the access site, given the difficulty encountered in advancing the valve delivery system into the ascending aorta.
Limitations
Our study had the inherent limitations of a retrospective analysis. This was a single-centre, small study; thus, selection bias could have influenced the study results. The procedures were not performed as part of a structured protocol and different surgical and transcatheter valves were used in this proof-of-concept study. While the feasibility of this technique is demonstrated in Mitroflow, Mosaic, Hancock and PERIMOUNT valves, generalised applicability to all small surgical valves has not been shown and requires further investigation. Matched comparisons between individual transcatheter or surgical valve types were not performed due to the small number of patients in our study; however, it is unlikely that this influenced the results of our study.
Conclusions
This preliminary report of high-pressure post-dilation of the surgical valve ring with a non-compliant balloon following ViV implantation provides a treatment option to minimise the risk and/or severity of prosthesis-patient mismatch following ViV implantation in select small surgical valves. Future bench testing models and additional clinical investigation will determine the feasibility and applicability of this technique in other surgical valves and with other transcatheter valve systems. With further validation, this method may provide options for the large cohort of patients with untreated patient-prosthesis mismatch.
| Impact on daily practice High-pressure post-dilation with a non-compliant balloon following transcatheter valve-in-valve (ViV) implantation in small surgical valves results in expansion/fracturing of the surgical valve frame with consequent further expansion of the transcatheter heart valve. This is further associated with lower aortic valve mean gradients, sustained at least up to 30 days. The technique was safe and not associated with significant complications in our series. Optimisation of haemodynamics following ViV in small surgical valves with expansion/fracturing of the surgical valve frame will probably translate into improved clinical outcomes. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Bench testing model demonstrating fracturing of a 19 mm Mitroflow valve under fluoroscopy with an 18 mm×4.5 cm TRUE balloon.
Moving image 2. High-pressure post-dilation with fracturing of a 19 mm Mitroflow valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using an 18 mm×4.5 cm TRUE balloon.
Moving image 3. High-pressure post-dilation with fracturing of a 19 mm Mitroflow valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using a 20 mm×4.5 cm TRUE balloon.
Moving image 4. High-pressure post-dilation with overexpansion of a 21 mm Mosaic valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using a 20 mm×4.5 cm TRUE balloon.
Moving image 5. High-pressure post-dilation with fracturing of a 21 mm Mitroflow valve with high-pressure post-dilation of a 23 mm Evolut R valve using a 20 mm×4.5 cm TRUE balloon.
Moving image 6. High-pressure post-dilation with overexpansion of a 19 mm PERIMOUNT valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using a 20 mm×4.5 cm TRUE balloon.
Moving image 7. High-pressure post-dilation with overexpansion of a 21 mm Hancock valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using a 20 mm×4.5 cm TRUE balloon.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Bench testing model demonstrating fracturing of a 19 mm Mitroflow valve under fluoroscopy with an 18 mm×4.5 cm TRUE balloon.
Moving image 2. High-pressure post-dilation with fracturing of a 19 mm Mitroflow valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using an 18 mm×4.5 cm TRUE balloon.
Moving image 3. High-pressure post-dilation with fracturing of a 19 mm Mitroflow valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using a 20 mm×4.5 cm TRUE balloon.
Moving image 4. High-pressure post-dilation with overexpansion of a 21 mm Mosaic valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using a 20 mm×4.5 cm TRUE balloon.
Moving image 5. High-pressure post-dilation with fracturing of a 21 mm Mitroflow valve with high-pressure post-dilation of a 23 mm Evolut R valve using a 20 mm×4.5 cm TRUE balloon.
Moving image 6. High-pressure post-dilation with overexpansion of a 19 mm PERIMOUNT valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using a 20 mm×4.5 cm TRUE balloon.
Moving image 7. High-pressure post-dilation with overexpansion of a 21 mm Hancock valve with high-pressure post-dilation of a 20 mm SAPIEN 3 valve using a 20 mm×4.5 cm TRUE balloon.

