
Abstract
Aims: Transcatheter heart valve (THV) implantation in failed bioprosthetic valves (valve-in-valve [ViV]) offers an alternative therapy for high-risk patients. Elevated post-procedural gradients are a significant limitation of aortic ViV. Our objective was to assess the relationship between depth of implantation and haemodynamics.
Methods and results: Commercially available THVs used for ViV were included in the analysis (CoreValve Evolut, SAPIEN XT and the Portico valve). THVs were implanted in small surgical valves (label size 19 mm) to simulate boundary conditions. Custom-mounted pulse duplicators registered relevant haemodynamic parameters. Twenty-eight experiments were performed (13 CVE, 5 SXT and 10 Portico). Ranges of depth of implantation were: CVE: –1.2 mm to 15.7 mm; SXT: –2.2 mm to 7.5 mm; Portico: 1.4 mm to 12.1 mm. Polynomial regression established a relationship between depth of implantation and valvular mean gradients (CVE: p<0.001; SXT: p=0.01; Portico: p=0.002), as well as with EOA (CVE: p<0.001; SXT: p=0.02; Portico valve: p=0.003). In addition, leaflet coaptation was better in the high implantation experiments for all valves.
Conclusions: The current comprehensive bench testing assessment demonstrates the importance of high device position for the attainment of optimal haemodynamics during aortic ViV procedures.
Abbreviations
EOA: effective orifice area
THV: transcatheter heart valve
ViV: valve-in-valve
VIVID: Valve-in-Valve International Data Registry
Introduction
Biological prostheses are increasingly preferred over mechanical valves, due to the lower rate of thrombotic complications1. Unfortunately, these tissue valves have limited durability and commonly fail within 10-20 years2-4, resulting in a high-risk patient population in need of repeat valve implantation5-8. Recent evidence suggests that transcatheter heart valve (THV) implantation in failed surgical valves (valve-in-valve [ViV]) is an appealing alternative to reoperation9,10. However, elevated post-procedural gradients related to THV underexpansion are unfortunately common following ViV, and possibly related to poor long-term device durability11.
Evidence suggests that THV devices with a supra-annular functional valve position are associated with lower gradients after ViV12,13. It seems that a THV that has a functional valve operating above the surgical valve annulus is unencumbered by the non-elastic portion of the original surgical bioprosthesis. Full expansion of the valve level of the THV can be expected in these cases, thus maximising the orifice area and lowering transvalvular gradients. Nevertheless, there are considerable design differences among the commercially available THVs, and each one functions in a different way. Some are supra-annular by design, while others tend to operate inside the surgical valve annulus (Figure 1). It is possible that a higher implantation of any THV would enable better expansion and lower gradients14. However, excessively high implantation could increase the risk of device malposition. Therefore, it is necessary to determine an optimal depth of implantation of THV devices in ViV procedures. The current in vitro study aimed to examine whether high implantation of three commonly used THV devices (i.e., aiming to achieve a supra-annular position of the THVs) would demonstrate improved haemodynamics after ViV.
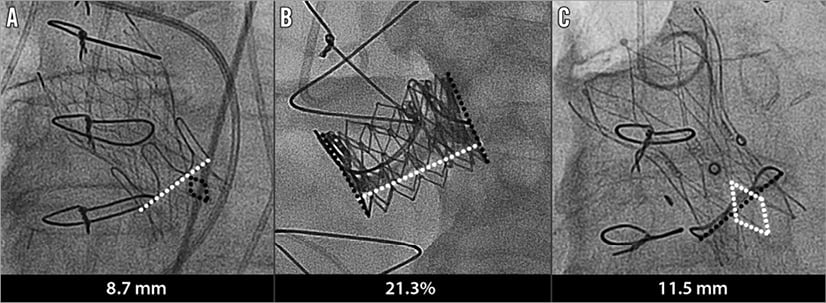
Figure 1. Examples of depth of implantation measurement for each of the transcatheter devices. A) CoreValve. B) SAPIEN XT. C) Portico valve.
Methods
THV MODELS AND VIV DEPLOYMENT
In vitro bench testing was performed with multiple implantation depths of three different THV devices implanted inside small surgical bioprostheses (label size 19 mm). Each position was assessed by X-ray imaging for its implantation depth prior to in vitro haemodynamic testing.
COREVALVE EVOLUT
CoreValve® Evolut® (Medtronic, Minneapolis, MN, USA) 23 mm devices were implanted in a 19 mm Epic™ Supra (St. Jude Medical, St. Paul, MN, USA) with a true internal diameter of 16.5 mm. Small and gradual changes in implantation depth were attempted between each experiment (13 positions). For the determination of implantation depth, the bottom diamond of the CoreValve Evolut was used as the reference for a scale. One straight line, starting at the surgical valve ring, was traced on each side of the device. The two values obtained were added together and their average was considered the final depth of implantation for each case.
SAPIEN XT
SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) 23 mm devices were implanted in a 19 mm Carpentier-Edwards PERIMOUNT valve (Edwards Lifesciences) with a true internal diameter of 17 mm. Small and gradual changes in implantation depth were attempted between each experiment (five positions). For the determination of implantation depth, one straight line was traced on each side of the SAPIEN XT. The values obtained were added and their average was considered the height of the device. Another line was traced at each side of the THV, starting from the surgical valve ring to the bottom of the THV. The values from both lines were added and their average was considered the depth numeric value. Finally, the depth was then defined as the percentage of the THV height below the stented surgical valve ring. Device height in millimetres was estimated by multiplying the depth value by the height of the expanded device (14.3 mm for the 23 mm device).
PORTICO VALVE
Portico™ (St. Jude Medical) 23 mm devices were implanted in a 19 mm Trifecta™ valve (St. Jude Medical) with a true internal diameter of 16 mm. Small and gradual changes in implantation depth were attempted between each experiment (10 positions). For Portico ViV procedures, the bottom diamond of the THV device was used as the reference for the creation of a scale. One straight line was traced on each side of the device. The two values obtained were added together and their average was considered as the final depth of implantation.
HAEMODYNAMIC MEASUREMENTS
THVs were tested at a temperature of 37°C in a custom-built pulse duplicator system. Heart rate, blood pressure, and cardiac output were used as control parameters for the waveform generator controlling a servo pump. Pressure was measured in the left atrium, left ventricle, left ventricular outflow tract, and ascending aorta with strain gauge pressure transducers (Deltran®; Utah Medical Products, Inc., Midvale, UT, USA). An electromagnetic flowmeter (CME 500 Series; Carolina Medical Electronics Inc., East Bend, NC, USA) was used to measure aortic valve flow rate. In addition, normal saline solution was used as recirculating fluid for the haemodynamic measurements. Pulse duplicator input parameters were used to match ISO 5840-1:2015 standard and Food and Drug Administration (FDA) specifications for testing heart valves: heart rate of 70 beats/min (bpm), 35% systolic duration of cycle period, mean atrial and aortic pressures of 10 and 100 mmHg, and cardiac output 5 litres/minute. These haemodynamic parameters were maintained constant throughout the study. Valvular mean pressure gradient, effective orifice area (EOA), and regurgitation volume were determined by the pulse duplicator software. EOA was calculated using the Gorlin equation. In addition, short-axis images of the opening and closing of the devices were recorded by a high-speed camera (FASTCAM SA4; Photron USA, Inc., San Diego, CA, USA) during the entire cardiac cycle at a rate of 1,000 frames per second. Relative haemodynamic values for pressure gradient, EOA, and regurgitation volume in comparison with the deepest THV position were reported.
Statistical analysis
Polynomial regression was performed for each THV device, with three correlations performed: implantation depth and final mean gradient, implantation depth and EOA, and implantation depth and regurgitation volume. F-tests were used to assess the fit of the data to linear, quadratic and cubic equations. F-tests were also used to test the significance of an increase in R2 between linear and quadratic equations, and also between quadratic and cubic equations. A two-tailed p<0.05 was considered statistically significant. Statistical analysis was performed using Microsoft Excel for Mac (Microsoft Corporation, Redmond, WA, USA).
Results
COREVALVE EVOLUT
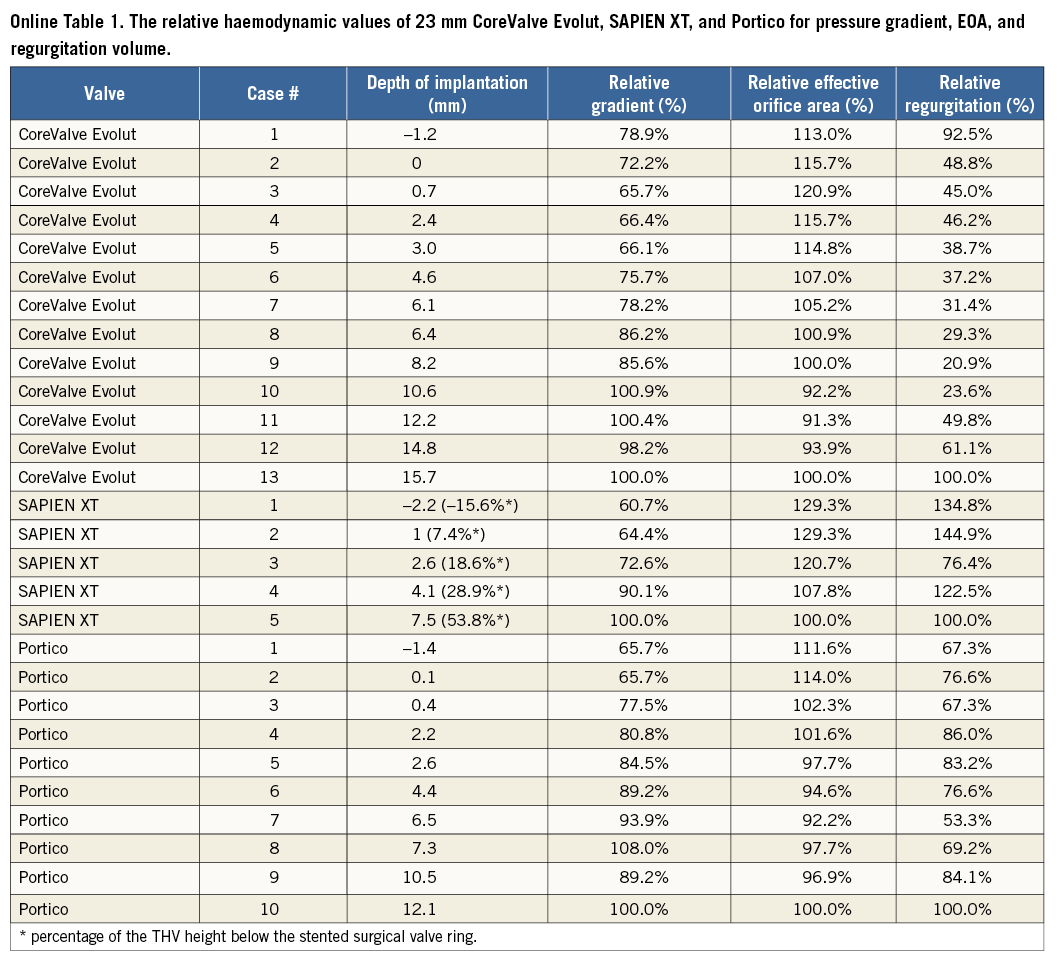
Thirteen in vitro experiments were performed at varied implantation depths from –1.2 mm to 15.7 mm (Figure 2). Online Table 1 shows the relative haemodynamic values for pressure gradient, EOA, and regurgitation volume. The relative values were calculated based on the case with the lowest implantation depth. Cubic regression demonstrated a correlation between higher implantation and lower mean gradients (p<0.001) (Figure 3). Supra-annular positioning of the CoreValve Evolut THV (0.7 to 3 mm depth of implantation) resulted in an average 17.2% lower mean transvalvular pressure gradient than in the common deeper position (6.1 to 8.2 mm depth of implantation). The reduction in mean transvalvular pressure gradient with increasingly supra-annular ViV deployment was due primarily to increases in valve orifice area. As shown in Figure 3, there was a correlation between higher implantation and larger EOAs (p<0.001). Furthermore, visual evaluation showed improved leaflet movement and closing morphology in high-implanted devices with lower transvalvular gradients (Moving image 1, Moving image 2). However, regurgitation volume increased considerably with higher implantation depth (Figure 3). Regurgitation volume was found to be lowest at the normal implantation depth, with a minimum value at 8.2 mm implantation depth. The largest regurgitation volume was found in the case with the lowest implantation depth (i.e., 15.7 mm implantation depth).
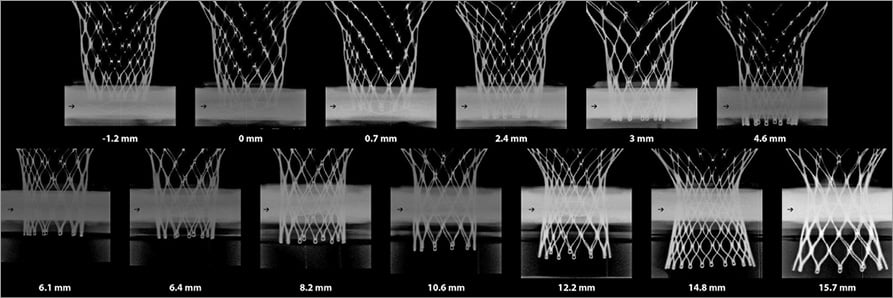
Figure 2. Thirteen CoreValve Evolut in vitro experiments with respective implantation depths.
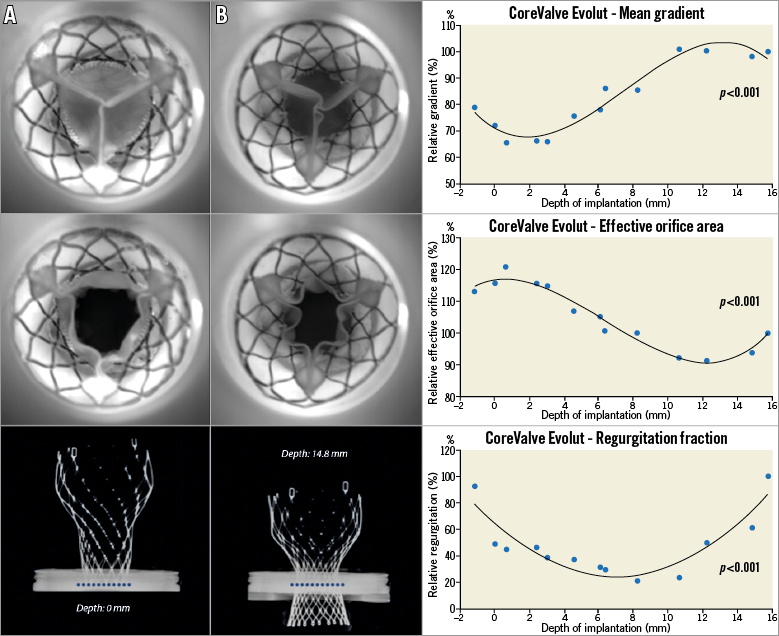
Figure 3. Thirteen 23 mm CoreValve Evolut in 19 mm St. Jude Epic experiments with respective depths. The first graph demonstrates the relationship between depth of implantation and EOA. The second graph demonstrates the correlation between depth and post-procedural gradients. Finally, the third graph shows the correlation between depth and relative regurgitation. Relative gradient is based on the gradient of the case with the lowest implantation. Experiment A demonstrates a high implantation of CoreValve Evolut. There was symmetric leaflet closure and larger orifice area, whereas experiment B demonstrates smaller orifice area. Notice the different implantation depths between the devices (the dotted line represents the surgical valve ring position).
SAPIEN XT
Five in vitro experiments were performed at varied implantation depths. The depth range was from –2.2 mm to 7.5 mm (Figure 4). Online Table 1 shows the relative haemodynamic values for pressure gradient, EOA, and regurgitation volume of SAPIEN XT THV at varied implantation depths. Similar to the CoreValve Evolut THV, the relative values were calculated based on the case with the lowest implantation depth. Cubic regression demonstrated a relationship between higher implantation depth and lower pressure gradients (p=0.01) (Figure 5). Supra-annular positioning of the SAPIEN XT THV (i.e., –2.2 mm depth of implantation) resulted in a 3.7% lower mean transvalvular pressure gradient than a deeper ViV position (i.e., 1 mm depth of implantation). The mean transvalvular pressure gradient increased as the depth of implantation increased, as the top portion of the THV could not be fully expanded to the manufactured size. In addition, as shown in Figure 5, a correlation between higher implantation depth and larger EOA was present (p=0.01). Visual evaluation showed that the oversized THV was constrained by the rigid bioprostheses, resulting in leaflet distortion at lower implantation depths (Figure 5). Better leaflet coaptation was visually evident in case of higher implantation (Figure 5, Moving image 3, Moving image 4). Moreover, excessively low implantation demonstrated leaflet overhang, resulting in two functional sets of leaflets, the original surgical leaflet and the THV leaflet (Figure 5, Moving image 5). Furthermore, regurgitation volume was found to be lowest at the 2.6 mm implantation depth.
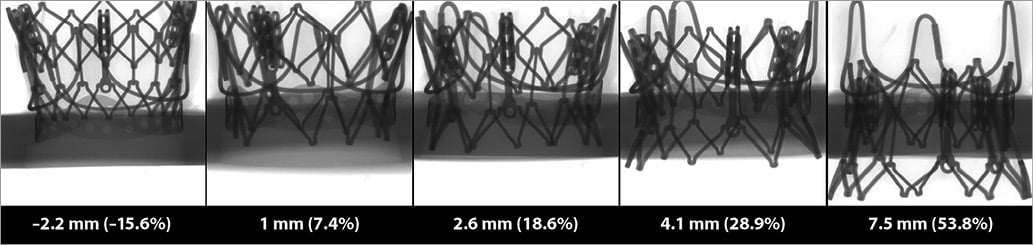
Figure 4. Five SAPIEN XT in vitro experiments with respective implantation depths.
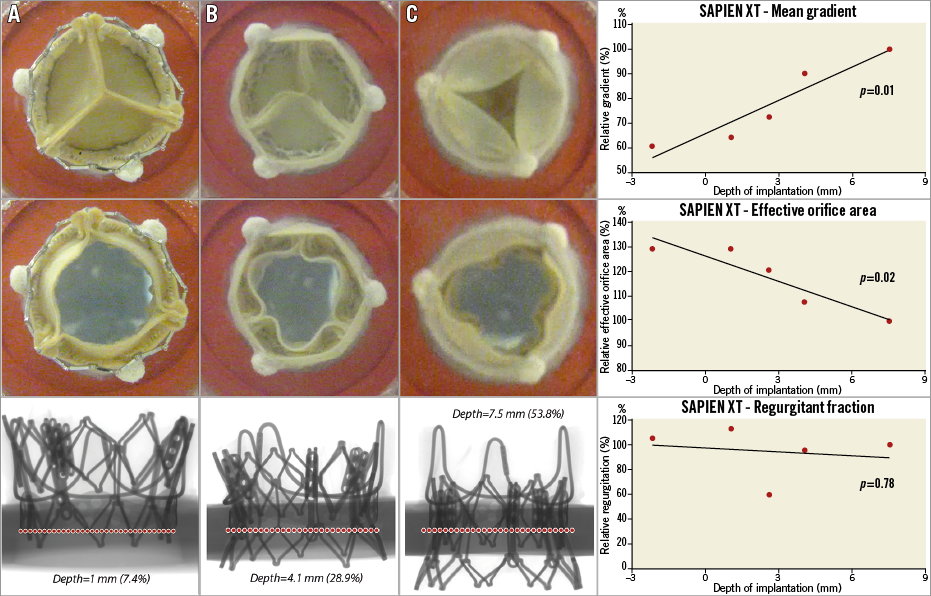
Figure 5. Five 23 mm SAPIEN XT in 19 mm PERIMOUNT with respective depths. The first graph demonstrates the relationship between depth of implantation and EOA. The second graph demonstrates the correlation between depth and post-procedural gradients. Finally, the third graph shows the correlation between depth and relative regurgitation. Relative gradient is based on the gradient of the case with the lowest implantation. Experiment A demonstrates a high implantation of SAPIEN XT. Notice the adequate closing of the leaflets and larger orifice area, whereas experiment B demonstrates smaller orifice area. Experiment C had a very deep implantation, resulting in leaflet overhang.
PORTICO VALVE
Ten experiments were performed in vitro at varied implantation depths (Figure 6). Online Table 1 shows the relative haemodynamic data for depth range of –1.4 mm to 12.1 mm. The relative values were calculated based on the case with the lowest implantation depth. A correlation was found between higher implantation and lower gradients (p<0.001) (Figure 7), as well as between higher implantation and larger EOA (p<0.001) (Figure 7). The lowest mean gradient and the largest EOA were observed at the highest THV implantation depth (i.e., –1.4 mm implantation depth). The reduction in mean transvalvular pressure gradient with increasingly supra-annular ViV deployment was due to increases in valve orifice area (Figure 7A, Figure 7B). Leaflet movement videos demonstrate better closing position in high-implanted devices (Moving image 6, Moving image 7). The largest regurgitation volume was found in the case with the lowest implantation position (i.e., 12.1 mm implantation depth). Regurgitation volume was found to be lowest at the 6.5 mm implantation depth.
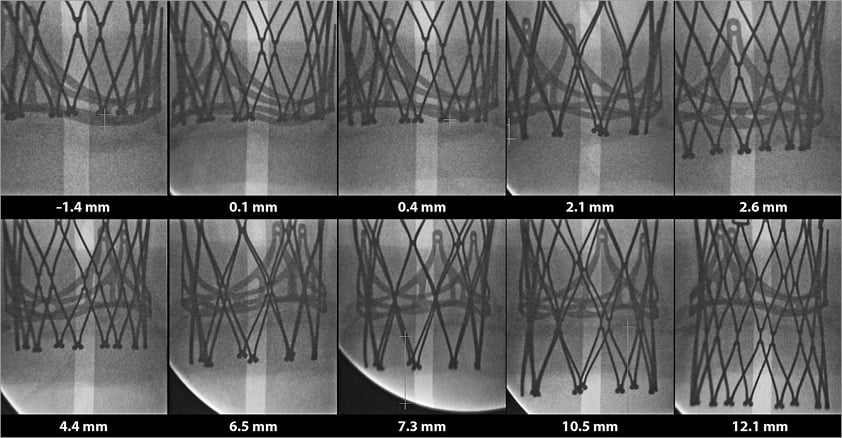
Figure 6. Ten Portico in vitro experiments with respective implantation depths.
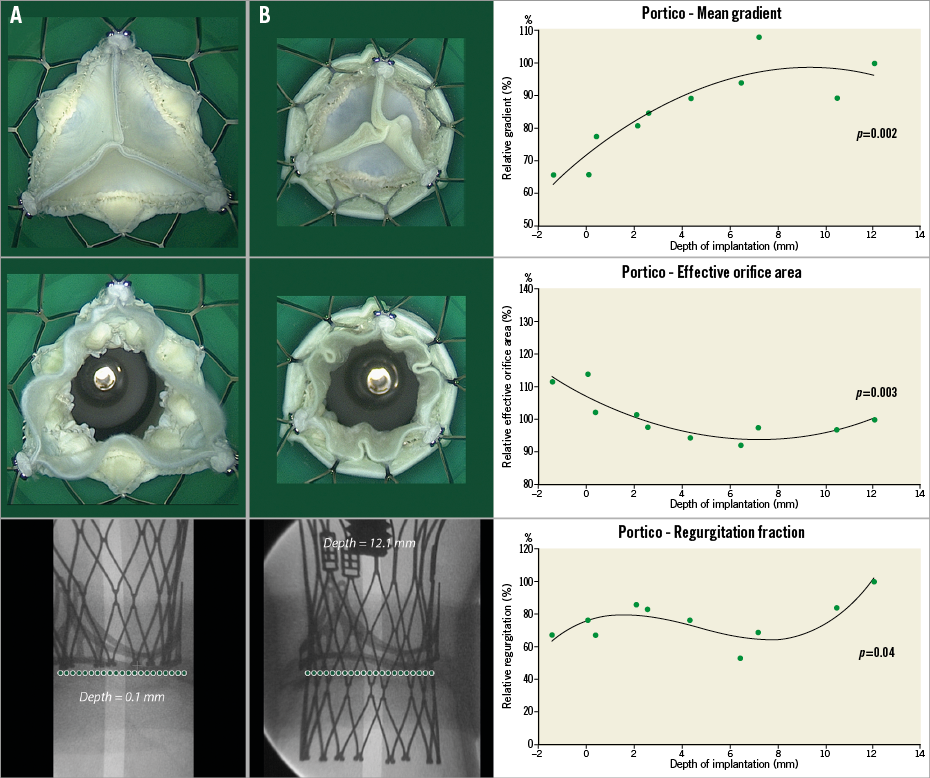
Figure 7. Ten 23 mm Portico in 19 mm St. Jude Trifecta with respective depths. The first graph demonstrates the relationship between depth of implantation and EOA. The second graph demonstrates the correlation between depth and post-procedural gradients. Finally, the third graph shows the correlation between depth and relative regurgitation. Relative gradient is based on the gradient of the case with the lowest implantation. Experiment A demonstrates a high implantation of the Portico valve. There was symmetric leaflet closure and larger orifice area, whereas experiment B demonstrates smaller orifice area.
Discussion
Transcatheter ViV is an alternative to conventional surgical valve replacement. Currently, the major limitation of ViV procedures is residual stenosis, which is common in this setting as surgical valve rings generally constrict THVs and do not allow full expansion. The current analysis is the largest comprehensive in vitro evaluation of the effect of positioning on ViV outcomes. Results suggest that supra-annular implantation of the three commonly used THVs within small bioprostheses can lead to lower gradient and larger EOA after ViV implantation. Visual evaluation of the three THVs showed improved leaflet movement and coaptation in high-implanted devices. The current analysis is in agreement with a previous in vivo analysis on the importance of depth of implantation in ViV procedures12.
Optimal THV function and leaflet coaptation require full device expansion to the manufactured size15. In addition to supra-annular positioning of THVs, ViV haemodynamics and leaflet coaptation in small degenerated bioprostheses may hypothetically be improved by using size-specific THVs that match bioprosthetic valve size15,16. Azadani et al16 evaluated the haemodynamic performance of 20 mm THVs created based on the Edwards SAPIEN valve design within 19 and 21 mm degenerated Carpentier-Edwards PERIMOUNT and Carpentier-Edwards porcine bioprostheses. ViV within the 19 mm PERIMOUNT and 21 mm porcine degenerated bioprostheses significantly reduced transvalvular pressure gradient and improved valve area. However, the 20 mm THVs migrated retrogradely into the left ventricle after ViV in 21 mm degenerated PERIMOUNT bioprostheses. Furthermore, ViV within the 19 mm porcine bioprosthesis was ineffective, with no reduction in pressure gradient.
The long-term durability and failure modes of THV devices in the ViV setting are unknown. The two distinct yet synergistic processes that contribute to the durability limitations of bioprosthetic valves are widely considered to be: 1) calcification, and 2) fatigue-induced structural deterioration17. Calcification is initiated primarily within residual connective tissue cells that have not been removed by fixation pretreatment procedure18-20. Furthermore, collagen and elastin fibres can serve as nucleation sites for calcification21,22. Alternatively, non-calcific tissue degeneration is due to fatigue-induced structural deterioration, induced by fibre debonding and shearing23.
Several studies have shown that high stress regions on bioprosthetic leaflets correlate with regions of calcification and mechanical damage due to fatigue17,19,23-27. A recent article by Abbasi et al11 demonstrated that incomplete deployment of a THV induced localised high stress regions within the belly of the THV during diastole. Generally, the stress is localised at the commissures during the diastolic phase of the cardiac cycle with complete THV expansion to the manufactured size. The computational simulations demonstrated that incomplete expansion of THVs would induce localised high stress regions within the leaflets, which over time may accelerate valve tissue degeneration. In addition, elevated gradients, more common in incompletely expanded devices, could be associated with a reduction in valve durability28. Therefore, long-term results of structural valve degeneration using the underexpansion strategy may possibly be related to poor device durability. In a recent analysis of a multicentre registry of 1,521 patients who underwent transcatheter aortic valve replacement, a ViV procedure was an independent predictor of valve haemodynamic deterioration, defined as a ≥10 mmHg increase in transprosthetic mean gradient during follow-up compared with discharge assessment29. However, as was shown in the current analysis, supra-annular position of THV leaflets potentially improves the haemodynamics of THVs constrained within the inexpansible bioprosthetic ring, with a reduction in leaflet coaptation distortion. It could be suggested that a high THV position during ViV may therefore improve long-term THV durability. Long-term assessment after ViV implantations when performed in the future may answer this question.
The VIVID Registry has recently evaluated the in vivo aspects of depth of implantation in ViV12. A total of 292 patients were included in that analysis, utilising core lab assessment of implantation depth. Optimal depth cut-offs were determined for the SAPIEN XT (0% to 10% of the device frame height, approximately 0 to ~2 mm) and for the CoreValve (0 to 5 mm). Low implantation was associated with a much higher rate of elevated gradients (mean ≥20 mmHg): 43.5% in the SAPIEN XT and 34.2% in the CoreValve vs. 18.5% and 15% in the high implantation group, respectively.
The present in vitro analysis further elucidates the pathophysiology of elevated mean gradients in low implantation ViV and validates the in vivo findings. Bench testing is especially important because it allows evaluation in controlled conditions. This permits a direct insight into valve functioning and the isolated effects of positioning on haemodynamics.
It is evident that high implantation could potentially benefit the vast majority of patients. Nevertheless, there are specific situations that could be considered as especially deserving of a more careful implantation. Most importantly, patients with small surgical valves have a more constricted valvular environment, in which high position may alleviate the risks of elevated gradients.
Limitations
The present study was limited to testing in in vitro conditions. The importance of THV depth of implantation on ViV haemodynamics has already been analysed in cases included in the VIVID Registry with similar findings that validate the importance of those revealed in the current analysis12. Nevertheless, the current in vitro analysis should be considered as similarly important for testing the significance of depth implantation, as these bench tests were performed in similar controlled conditions, something that could never be performed while assessing real-world in vivo clinical cases which differ in their left ventricular ejection fraction, pressures within the different cavities and in many other aspects. The SAPIEN 3 heart valve was not included in this analysis. Further studies in the future should address the effects of depth of implantation in novel THV devices. In addition, in the current study, we have only considered stented surgical bioprostheses for ViV. Therefore, extrapolating the current findings to stentless surgical bioprostheses would be inadequate. Our analysis was limited in testing one THV model per surgical valve. We also utilised different surgical valves for the different THV experiments. Distinct surgical valves could potentially affect haemodynamic outcomes. Future experiments could potentially test different THVs in each of the surgical valves, providing information about each specific model. Different surgical valve sizes could also provide more important information in future experiments. Furthermore, the bioprosthetic valves that were used in our experiments were not degenerated. Previous data suggest that an important predictor for having elevated gradients after ViV is significant stenosis of the failed surgical valve12. As a consequence, we needed to test the importance of implantation depth in very small surgical valves (label size 19 mm) that simulate conditions of valves with small effective orifice area.
Conclusion
The current analysis is the largest comprehensive in vitro evaluation of the effect of THV position on ViV outcomes. Our results demonstrate the importance of high device position for achieving optimal haemodynamics post procedure and support specific implantation targets for different THV devices during ViV procedures. Clinicians must carefully weigh the benefit of supra-annular THV implantation in reducing post-procedural gradients against the potential risk of malposition in potential ViV candidates.
| Impact on daily practice Elevated post-procedural gradients are relatively common in transcatheter valve-in-valve procedures. Comprehensive bench testing experiments show that high position of different types of transcatheter heart valve may improve haemodynamics after aortic valve-in-valve. In vitro results validate in vivo findings related to the importance of implantation depth. |
Acknowledgements
We would like to thank the St. Paul’s Foundation for their support. M. Simonato acknowledges the Science Without Borders Scholarship, provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Cientifíco e Tecnológico (CNPq), agencies of the Brazilian Federal Government.
Conflict of interest statement
D. Dvir reports consulting for Edwards Lifesciences and Medtronic. J. Webb reports consulting for Edwards Lifesciences. A. Petronio reports clinical proctoring for Medtronic and Boston Scientific, membership of the speaker’s bureau and consulting for Abbott Laboratories and Eli Lilly. A. Vahanian reports speaker’s fees and honoraria from Edwards Lifesciences, Abbott Laboratories and Valtech. The other authors have no conflicts of interest to declare.
Supplementary data
Online Table 1. The relative haemodynamic values of 23 mm CoreValve Evolut, SAPIEN XT, and Portico for pressure gradient, EOA, and regurgitation volume.
Moving image 1. Short-axis view of the CoreValve Evolut 23 mm implanted low (depth=14.8 mm). Leaflet closure is asymmetrical, with a whorled pattern in coaptation. This situation demonstrates incomplete device expansion.
Moving image 2. Short-axis view of the CoreValve Evolut 23 mm implanted high (depth=0 mm). Leaflet coaptation is fully symmetrical, without whorls or imperfections. The device is fully expanded, with a gradient approximately 30% lower and an EOA 15% greater than the reference low implantation experiment.
Moving image 3. Short-axis view of the SAPIEN XT 23 mm implanted low (depth=28.9%, 4.1 mm). A deep implantation is demonstrated, with improper leaflet contact and a whorled pattern, demonstrating incomplete expansion.
Moving image 4. Short-axis view of the SAPIEN XT 23 mm implanted high (depth=7.4%, 1 mm). This high implantation case has adequate and symmetrical leaflet coaptation, a consequence of full device expansion. This is reflected in the haemodynamic results, with a gradient 36% lower and an EOA almost 30% greater than the reference low implantation case.
Moving image 5. Short-axis view of the SAPIEN XT 23 mm implanted extremely low (depth=53.8%, 7.5 mm). This video exemplifies the leaflet overhang phenomenon, which consists in two functional sets of leaflets. In this situation, the XT frame did not compress the surgical valve leaflets against the stent posts.
Moving image 6. Short-axis view of the Portico 23 mm valve implanted low (depth=12.1 mm). The device in question has very poor leaflet coaptation, with an evident asymmetrical whorled pattern that shifts with each cycle.
Moving image 7. Short-axis view of the Portico 23 mm valve implanted high (depth=0.1 mm). The leaflets close in a much more symmetrical manner than the previous experiment, demonstrating the positive effects of high implantation. The gradient was 35% lower and the EOA 14% greater than the reference low implantation case.
Supplementary data
To read the full content of this article, please download the PDF.
Short-axis view of the CoreValve Evolut 23 mm implanted low (depth=14.8 mm). Leaflet closure is asymmetrical, with a whorled pattern in coaptation. This situation demonstrates incomplete device expansion.
Short-axis view of the CoreValve Evolut 23 mm implanted high (depth=0 mm). Leaflet coaptation is fully symmetrical, without whorls or imperfections. The device is fully expanded, with a gradient approximately 30% lower and an EOA 15% greater than the reference low implantation experiment.
Short-axis view of the SAPIEN XT 23 mm implanted low (depth=28.9%, 4.1 mm). A deep implantation is demonstrated, with improper leaflet contact and a whorled pattern, demonstrating incomplete expansion.
Short-axis view of the SAPIEN XT 23 mm implanted high (depth=7.4%, 1 mm). This high implantation case has adequate and symmetrical leaflet coaptation, a consequence of full device expansion. This is reflected in the haemodynamic results, with a gradient 36% lower and an EOA almost 30% greater than the reference low implantation case.
Short-axis view of the SAPIEN XT 23 mm implanted extremely low (depth=53.8%, 7.5 mm). This video exemplifies the leaflet overhang phenomenon, which consists in two functional sets of leaflets. In this situation, the XT frame did not compress the surgical valve leaflets against the stent posts.
Short-axis view of the Portico 23 mm valve implanted low (depth=12.1 mm). The device in question has very poor leaflet coaptation, with an evident asymmetrical whorled pattern that shifts with each cycle.
Short-axis view of the Portico 23 mm valve implanted high (depth=0.1 mm). The leaflets close in a much more symmetrical manner than the previous experiment, demonstrating the positive effects of high implantation. The gradient was 35% lower and the EOA 14% greater than the reference low implantation case.

