
Abstract
Aims: We aimed to assess the impact of implant depth on hydrodynamic function following valve-in-valve (VIV) intervention using the ALLEGRA transcatheter heart valve (THV) in three different surgical valve designs.
Methods and results: Multiple implantation depths (+2 mm, –2 mm and –6 mm) were tested using a 23 mm ALLEGRA THV for VIV intervention in 19 mm, 21 mm, 23 mm, and 25 mm Epic, Mitroflow and Magna Ease bioprosthetic valves. Multimodality imaging and hydrodynamic evaluation was performed at each implantation depth. The 23 mm ALLEGRA valve had gradients <20 mmHg in the Mitroflow and Epic valves sized ≥21 mm, and in all sizes of the Magna Ease valve. Gradients did not increase significantly at lower implantation depths. The 19 mm Epic (+2 mm: 20.1±0.6 mmHg, –2 mm: 18.8±0.5 mmHg, –6 mm: 22.8±0.3 mmHg) and 19 mm Mitroflow (+2 mm: 24.1±0.2 mmHg, –2 mm: 31.5±0.3 mmHg, –6 mm: 25.6±0.2 mmHg) valves had elevated mean gradients. In larger sized surgical valves (≥23 mm) the regurgitant fraction was higher at low implantation depths. Pinwheeling was significantly worse in the smaller sized (≤21 mm) surgical valves and also at low (<–2 mm) implantation depth.
Conclusions: The 23 mm ALLEGRA valve had favourable (<20 mmHg) gradients in all surgical valves sized ≥21 mm, even when the THV was implanted low. In 19 mm sized Mitroflow and Epic valves, gradients were elevated (>20 mmHg). While there was no major difference in mean transvalvular gradients, leaflet pinwheeling was worse at lower implantation depths.
Introduction
Valve-in-valve (VIV) intervention is an alternative treatment to reoperation for patients with failed bioprosthetic surgical valves1,2. However, in small sized surgical valves, high gradients may persist after VIV, which may lead to poor clinical outcomes1. Different transcatheter heart valves (THV) can be utilised for VIV intervention; some designs may result in superior performance following VIV intervention3. The ALLEGRA valve (New Valve Technology, Hechingen, Germany) is a commercially available THV4,5. However, there is less experience using this THV for VIV intervention compared to other commercially available THVs. Early in vitro and clinical experience has shown favourable haemodynamic performance using the ALLEGRA THV for VIV interventions6,7. The optimum implantation depth and position of this THV relative to the surgical valve are poorly understood.
We assessed the impact of implant depth on hydrodynamic function using the ALLEGRA THV for VIV interventions in different surgical aortic bioprosthetic valves of different sizes.
Methods
Testing was performed at the Centre for Heart Valve Innovation bench testing laboratory (Vancouver, Canada), and ViVitro Laboratories (Victoria, Canada).
VIV intervention was assessed in 19 mm, 21 mm, 23 mm and 25 mm Mitroflow (Sorin Group Canada Inc., Burnaby, Canada), Epic™ (St. Jude Medical, St. Paul, MN, USA) and Magna Ease (Edwards Lifesciences, Irvine, CA, USA) aortic surgical bioprostheses, at three implantation depths (+2 mm, –2 mm, –6 mm).
VALVES
The ALLEGRA THV is a self-expanding TAVI prosthesis which is a trileaflet, bovine pericardial bioprosthetic aortic heart valve attached to a nitinol stent frame. The THV leaflets are positioned in a supra-annular position with the base of the leaflets inserting 12 mm above the inflow. In contrast, the base of the leaflets of an Evolut™ R (Medtronic, Minneapolis, MN, USA) and Edwards SAPIEN 3 (Edwards Lifesciences) inserts 13 mm and 3 mm above the inflow, respectively. The nitinol stent frame has a closed cell, diamond-shaped configuration with a variable cell size distribution. Six radiopaque gold markers are placed at the level of the valve plane to indicate the distal part of the semilunar valve. The ventricular inflow section of the prosthesis is covered by a bovine pericardial sealing skirt. The ALLEGRA THV is available in three sizes (23, 27 and 31 mm) to match aortic annulus dimensions ranging from 19 to 28 mm8. The 23 mm ALLEGRA valve was utilised for this study; it has an inflow and outflow diameter of 23.8 mm and 20.8 mm, respectively. The commissural outflow diameter of a 23 mm ALLEGRA is 24 mm. The frame height is 37.3 mm (Figure 1).
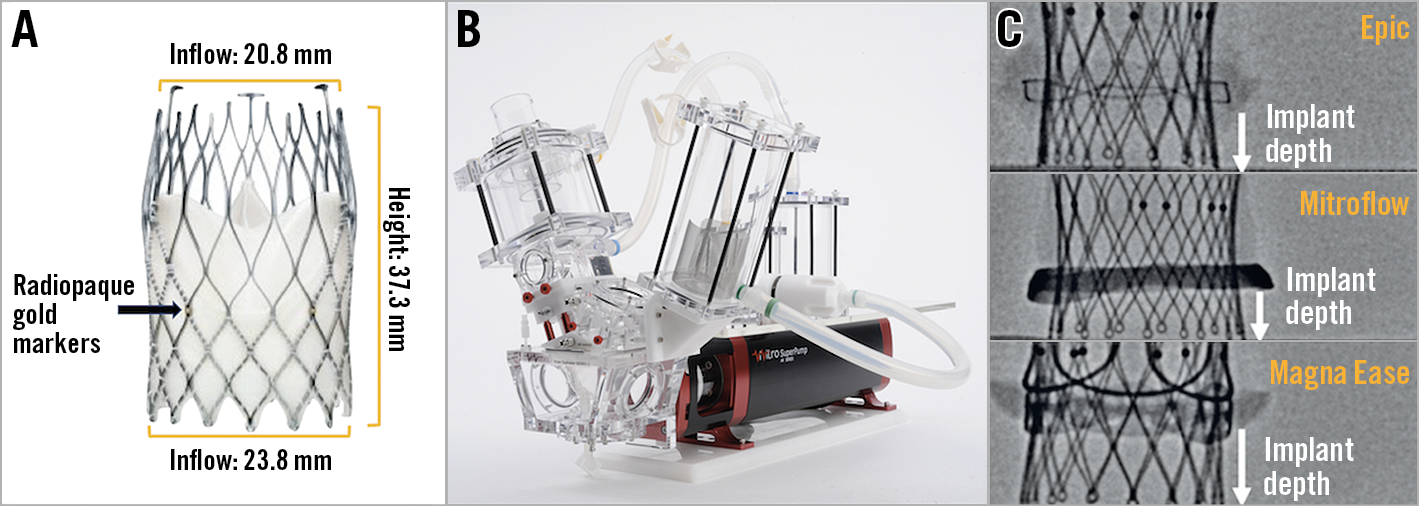
Figure 1. Bench testing methodology. A) Example of an ALLEGRA THV. B) Pulse duplicator used for hydrodynamic testing. C) Implantation depth was measured from the lower border of the radiopaque ring of the Mitroflow valve to the lowest point of the frame of the ALLEGRA THV. With the Epic and Magna Ease bioprostheses, implantation depth was measured from the lower border of the sewing ring to the lowest point of the THV.
The Mitroflow bioprosthesis consists of a polyester-covered acetyl homopolymer stent frame. Bovine pericardial sheets are sutured externally to form the leaflets9. The 19 mm, 21 mm, 23 mm and 25 mm Mitroflow valves have a true internal diameter of 15.4 mm, 17 mm, 19 mm and 21 mm, respectively10.
The Epic bioprosthesis consists of a Dacron covered acetyl copolymer stent. Porcine leaflets are sutured within the stent frame11. The 19 mm, 21 mm, 23 mm and 25 mm Epic valves have a true internal diameter of 17 mm, 19 mm, 21 mm and 23 mm, respectively10.
The Magna Ease valve is composed of a polytetrafluoroethylene cloth-covered cobalt-chromium stent. Bovine pericardial tissue is sutured within the stent frame. The 19 mm, 21 mm, 23 mm and 25 mm Magna Ease valves have a true internal diameter of 17 mm, 19 mm, 21 mm and 23 mm, respectively10.
VALVE-IN-VALVE PROCEDURE
Three implantation depths (+2 mm, –2 mm and –6 mm) were tested using a 23 mm ALLEGRA THV for VIV intervention in the four sizes of Epic, Mitroflow and Magna Ease tested. In the VIV configuration using the Mitroflow valve, implantation depth was measured from the lower border of the radiopaque ring of the valve to the lowest point of the frame of the ALLEGRA THV. With the Epic and Magna Ease bioprostheses, implantation depth was measured from the lower border of the sewing ring to the lowest point of the THV (Figure 1). Implantation depth was measured with both fluoroscopy and macroscopic measurements using digital scientific callipers.
IMAGING
Imaging was performed at each tested implant depth by the utilisation of high-resolution photography. The latter was performed at a pre-specified magnification and fixed camera height. Fluoroscopic images were acquired at a standard adult cardiac catheterisation laboratory (General Electric Healthcare, Chicago, IL, USA).
HYDRODYNAMIC ASSESSMENT
Hydrodynamic testing was performed at each implant depth tested, using a commercially available pulse duplicator (ViVitro Labs Inc., Victoria, Canada) (Figure 1). Valves were tested in accordance with International Standards Organization (ISO) 5840-3:2013 guidelines regarding in vitro pulsatile flow testing for heart valve substitutes implanted by transcatheter techniques12. Valves were placed in a holder fabricated from silicone with a durometer of scale Shore A hardness of 40±5. Justification for the selection of sample holder hardness was based on published data on acceptable tissue compliance matched with published data on the silicone material hardness scale13,14,15. The test fluid used was 0.9±0.2% sodium chloride test solution maintained at 37±2 °C (one drop of Cosmocil® [Lonza, Basel, Switzerland] preservative per 1 L).
Valves were tested on the aortic side of the pulse duplicator with a spring-loaded disc valve (ViVitro Labs) on the mitral side of the pulse duplicator. Measurements were based on average results taken from 10 consecutive cycles. High-speed video was captured at each step condition. Pulsatile forward flow performance was tested at a nominal beat rate of 70±1 beats per minute, systolic duration of 35±5%, mean aortic pressure of 100±2 mmHg, and simulated cardiac output of 5±0.1 litres per minute. Mean gradient (mmHg), regurgitant fraction (%) and effective orifice area (cm2) were assessed. The ISO standards for regurgitant fraction for a 23 mm THV stipulate a minimum performance requirement of <10%. The effective orifice area (EOA) was defined as the orifice area that has been derived from flow and pressure where qvRMS is the root mean square flow in ml/s, ∆P is the mean pressure difference (measured over the positive differential pressure period of the forward flow phase) in mmHg, and ρ is the density of the test fluid in g/cm3. This equation is derived from a simplified version of the Bernoulli equation.
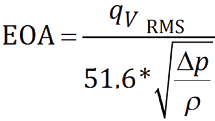
PINWHEELING
Pinwheeling, as defined by the ISO guideline for THV testing, refers to twisting of the leaflet free edges resulting from excessive leaflet redundancy12. The degree of pinwheeling was based on high-speed videos with backward pressure.
STATISTICS
Hydrodynamic variables are reported as mean±SD.
Results
VALVE HYDRODYNAMICS
TRANSVALVULAR GRADIENT
Mean transvalvular gradients for the tested surgical valves at different implantation depths are reported in Table 1 and Figure 2. There was no major difference in mean transvalvular gradients with lower implantation depths. The 23 mm ALLEGRA valve had <20 mmHg gradients in all surgical valves sized ≥21 mm at all implantation depths tested. The Magna Ease 19 mm valve also had favourable gradients with mean transvalvular gradients of 10.5±0.1 mmHg, 12.9±0.1 mmHg and 16.3±0.1 mmHg, at an implantation depth of +2 mm, –2 mm and –6 mm, respectively. In the 19 mm Epic valve, the mean transvalvular gradient was 20.1±0.6 mmHg, 18.8±0.5 mmHg and 22.8±0.3 mmHg, at an implantation depth of +2 mm, –2 mm and –6 mm, respectively. In the 19 mm Mitroflow valve, the mean transvalvular gradient was 24.1±0.2 mmHg, 31.5±0.3 mmHg and 25.6±0.2 mmHg, at an implantation depth of +2 mm, –2 mm and –6 mm, respectively.
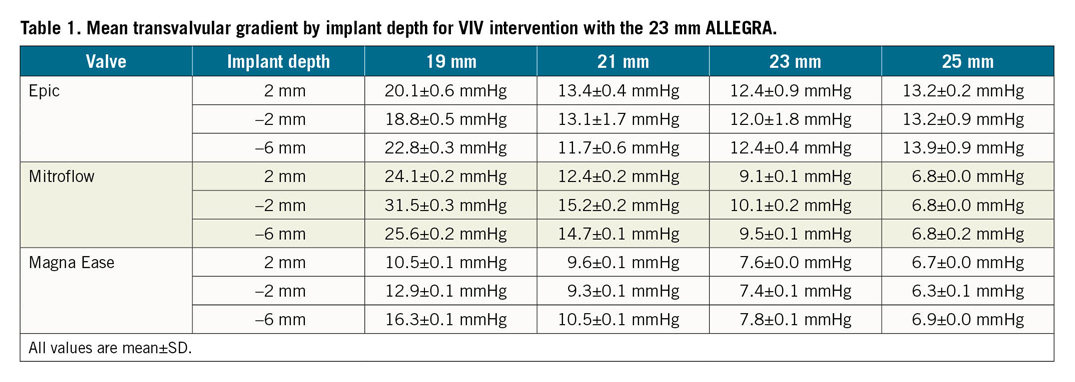
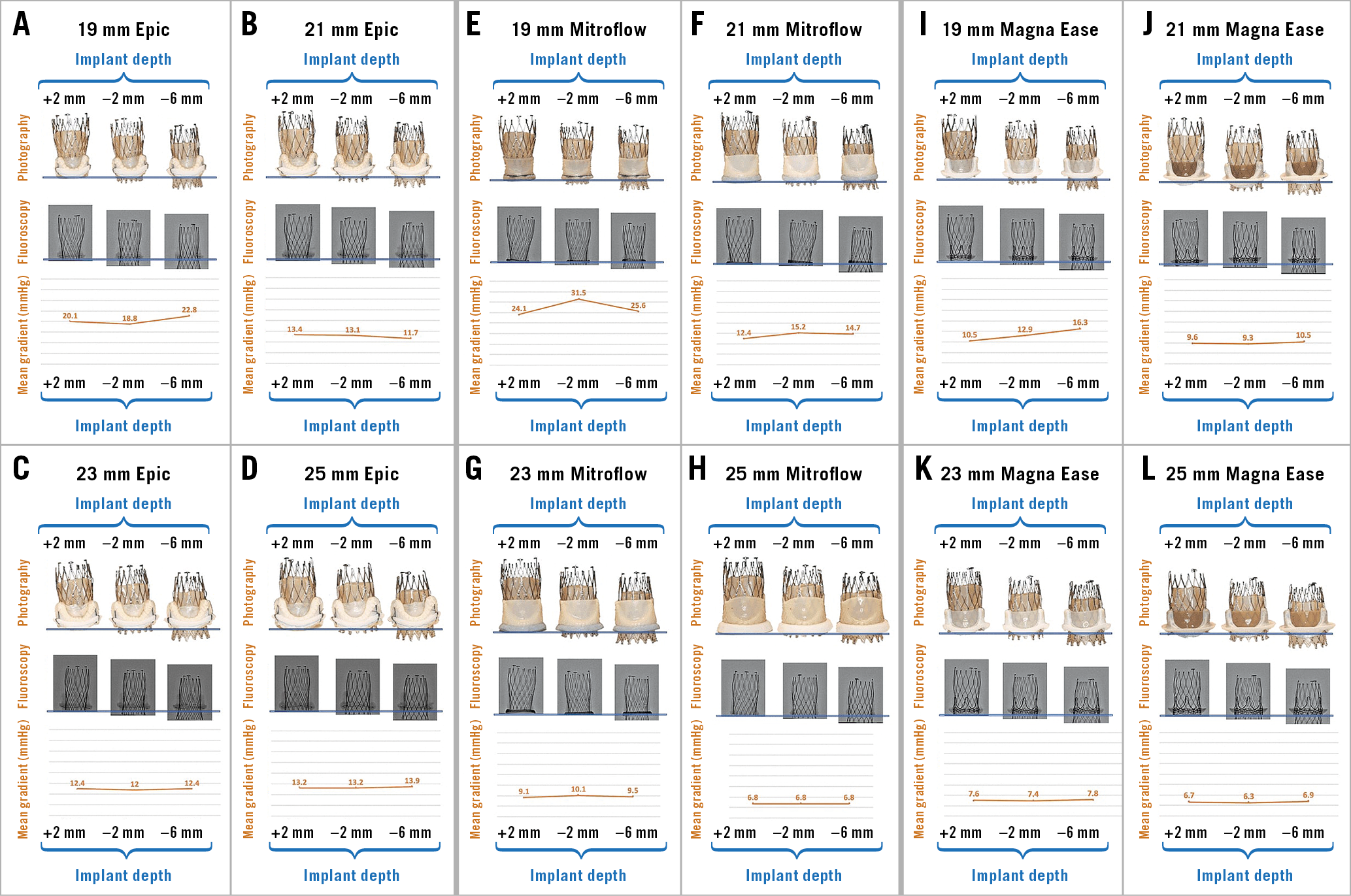
Figure 2. Photography, fluoroscopy and mean gradient by implant depth for VIV with the 23 mm ALLEGRA THV in Epic, Mitroflow and Magna Ease bioprosthetic valves. A) VIV with ALLEGRA THV in 19 mm Epic bioprosthetic valve. B) VIV with ALLEGRA THV in 21 mm Epic bioprosthetic valve. C) VIV with ALLEGRA THV in 23 mm Epic bioprosthetic valve. D) VIV with ALLEGRA THV in 25 mm Epic bioprosthetic valve. E) VIV with ALLEGRA THV in 19 mm Mitroflow bioprosthetic valve. F) VIV with ALLEGRA THV in 21 mm Mitroflow bioprosthetic valve. G) VIV with ALLEGRA THV in 23 mm Mitroflow bioprosthetic valve. H) VIV with ALLEGRA THV in 25 mm Mitroflow bioprosthetic valve. I) VIV with ALLEGRA THV in 19 mm Magna Ease bioprosthetic valve. J) VIV with ALLEGRA THV in 21 mm Magna Ease bioprosthetic valve. K) VIV with ALLEGRA THV in 23 mm Magna Ease bioprosthetic valve. L) VIV with ALLEGRA THV in 25 mm Magna Ease bioprosthetic valve.
EFFECTIVE ORIFICE AREA
Effective orifice areas for the tested surgical valves at different implantation depths are reported in Table 2. For surgical valves sized ≥21 mm, the EOAs remained similar irrespective of implantation depth of the 23 mm ALLEGRA valve. In the 19 mm Epic and Mitroflow surgical valves, EOAs remained similar irrespective of implantation depth. In the 19 mm Magna Ease, a lower implantation resulted in lower EOAs, with EOAs of 1.8±0.01 cm2, 1.6±0.01 cm2, and 1.4±0.01 cm2, at an implantation depth of +2 mm, –2 mm and –6 mm, respectively.
REGURGITANT FRACTION
Regurgitant fractions (RFs) for the tested surgical valves at different implantation depths are reported in Table 3. The ALLEGRA valve had a favourable RF (<10%) in the Epic valve in all sizes and at all implantation depths. The Mitroflow valve had a higher RF at an implant depth of +2 mm for valves sized 19 mm (+2 mm: 20.6±0.6%, –2 mm: 8.4±0.6%, –6 mm: 9.0±0.6%), 21 mm (+2 mm: 18.6±0.5%, –2 mm: 14.3±0.5%, –6 mm: 14.3±0.4%) and 23 mm (+2 mm: 16.9±0.7%, –2 mm: 13.7±0.5%, –6 mm: 13.9±0.5%). The 25 mm Mitroflow had a higher RF with lower implantation of the ALLEGRA THV (+2 mm: 11.0±0.4%, –2 mm: 21.3±0.4%, –6 mm: 23.0±0.5%). The Magna Ease had a higher RF in larger sized surgical valves and at lower implantation in the 23 mm (+2 mm: 16.5±0.5%, –2 mm: 16.9±0.9%, –6 mm: 20.7±0.6%) and 25 mm (+2 mm: 19.2±0.5%, –2 mm: 11.5±0.6%, –6 mm: 24.4±0.4%) sized valves.
PINWHEELING
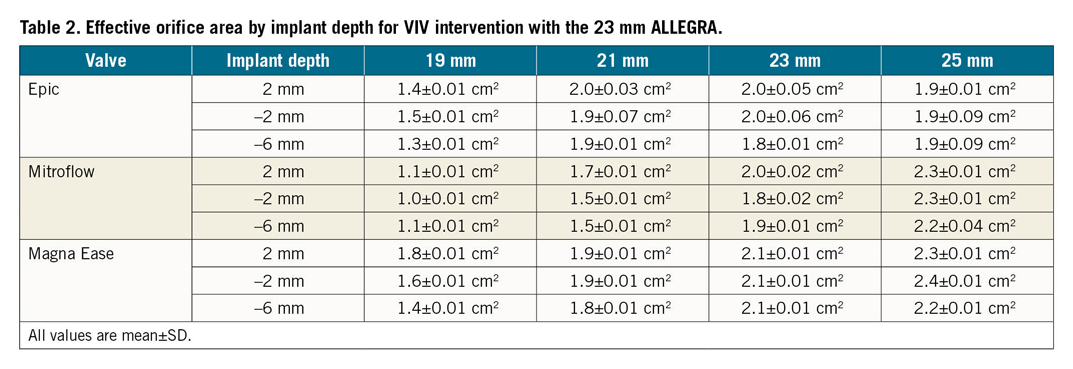
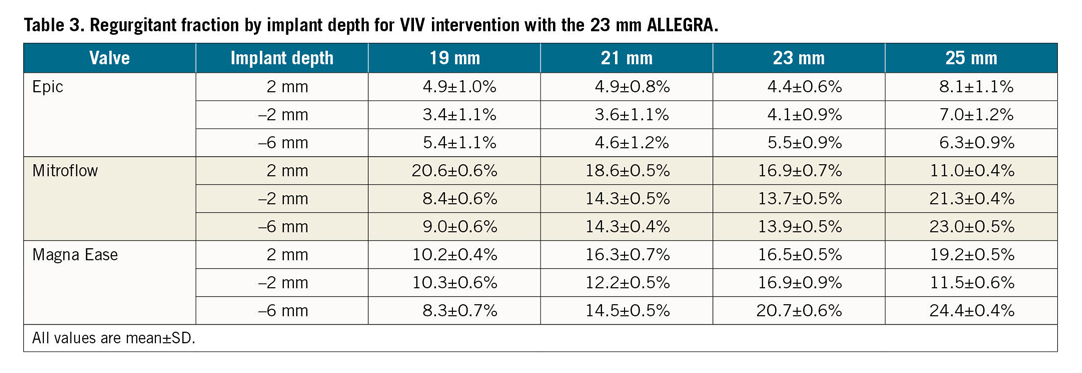
The degree of leaflet pinwheeling with maximum backward pressure is shown for the tested surgical valves at different implantation depths in Figure 3-Figure 5. Pinwheeling was significantly worse in the smaller sized surgical valves and also at lower implantation depths.
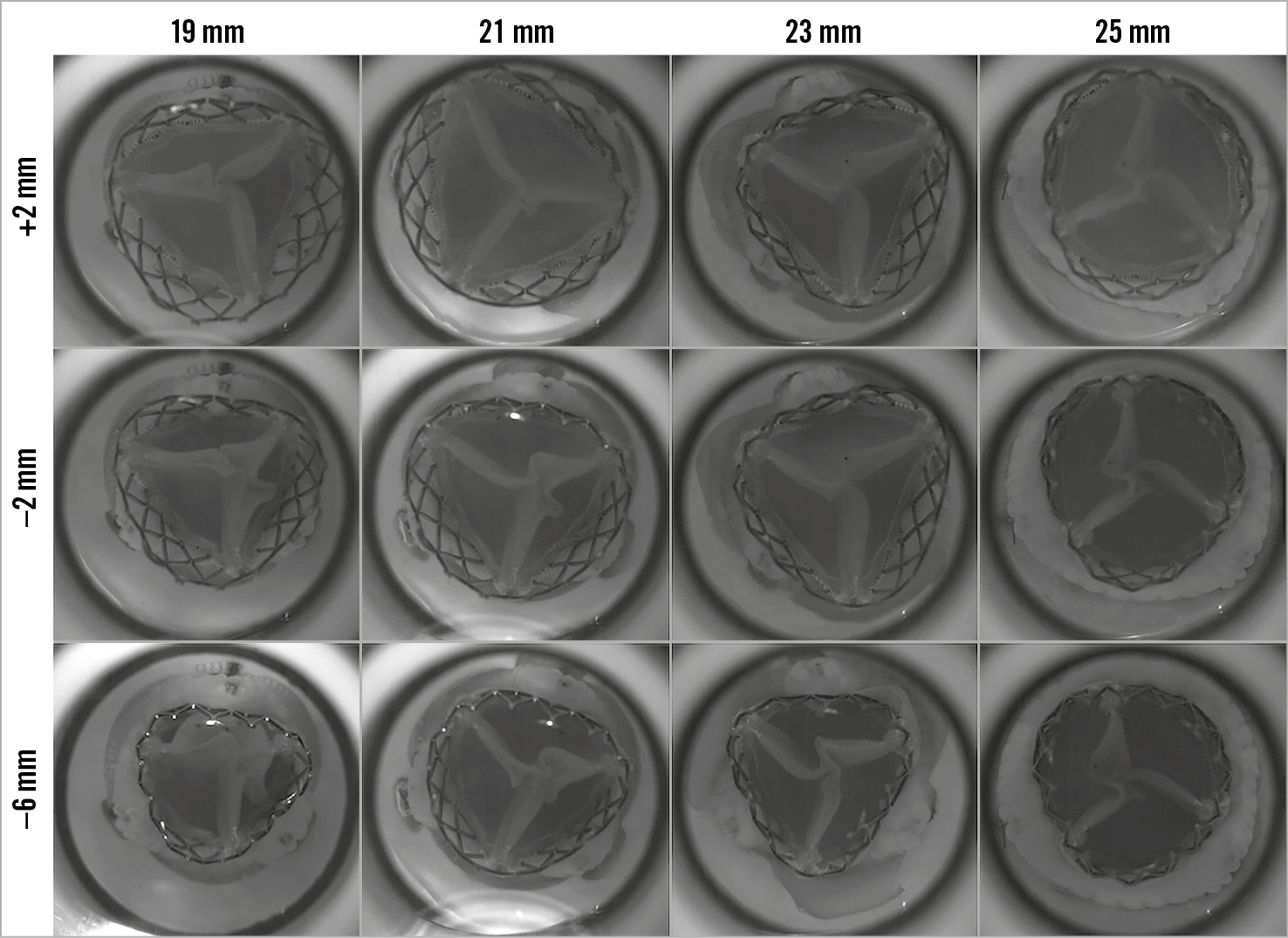
Figure 3. High-speed video images by implant depth for VIV intervention with the 23 mm ALLEGRA THV in Epic bioprosthetic valve with backward pressure.
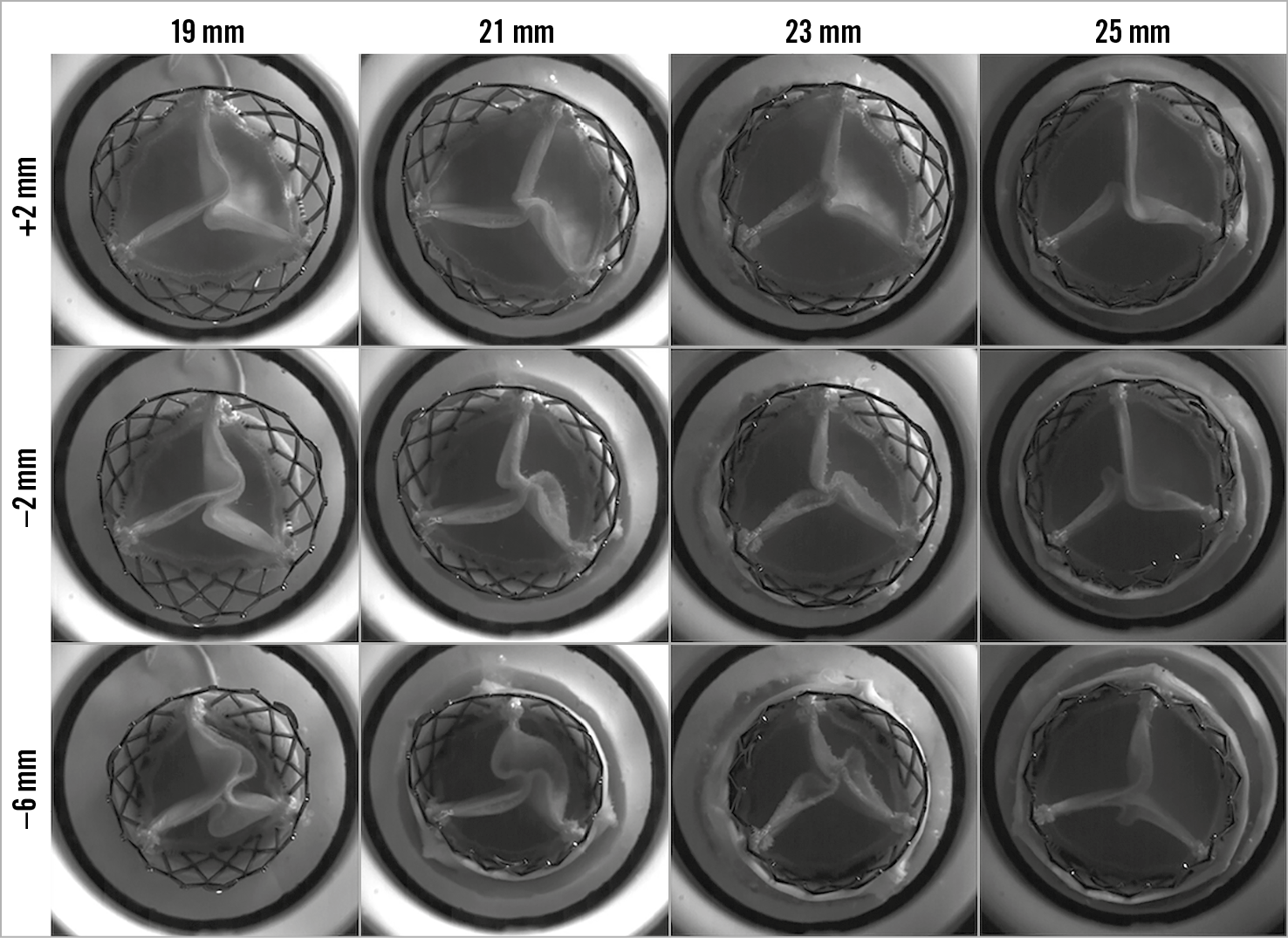
Figure 4. High-speed video images by implant depth for VIV intervention with the 23 mm ALLEGRA THV in Mitroflow bioprosthetic valve with backward pressure.
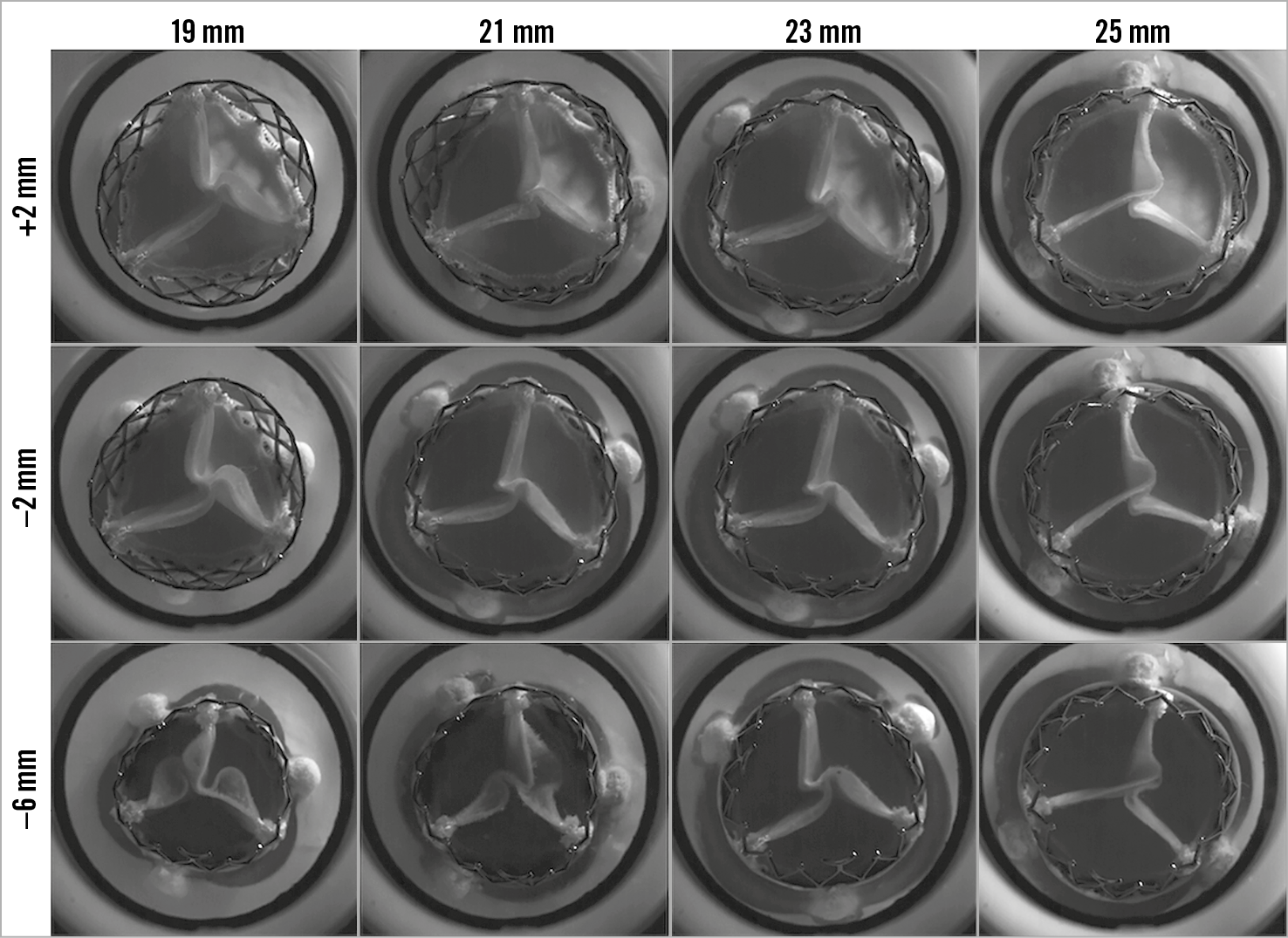
Figure 5. High-speed video images by implant depth for VIV intervention with the 23 mm ALLEGRA THV in Magna Ease bioprosthetic valve with backward pressure.
Discussion
In this study, a unique finding with the ALLEGRA THV was that implantation depth did not significantly affect transvalvular gradients in all three tested surgical valves. By contrast, bench studies with the SAPIEN XT (Edwards Lifesciences), CoreValve® Evolut™ (Medtronic), Portico™ (Abbott Vascular, Santa Clara, CA, USA), and ACURATE neo™ (Boston Scientific, Marlborough, MA, USA) THVs have all shown that lower implantation is associated with higher transvalvular gradients, compared to a high implant16,17. Prior bench studies have shown that other self-expanding valves, the Portico and CoreValve Evolut THVs, both had significantly lower mean gradients with a higher implantation. A lower implantation with these self-expanding valves has been shown to result in a higher mean gradient18.
In this study, the mean gradients remained generally consistent at all implantation depths, irrespective of surgical bioprosthetic valve type or size. Even with low implantation, the mean gradient did not vary significantly compared to a higher implant. Similarly, the EOAs also remained similar, even with lower implantation depths. This may potentially be related to the supra-annular position of the THV leaflets. Of note, even at an implant depth of –6 mm, the top of the THV leaflets still extended above the surgical valve posts in THVs with supra-annular leaflet position. Valve implantation at depths >6 mm, where the THV leaflets are at an intra-annular position, may potentially compromise EOAs and hydrodynamic function. Importantly, while mean gradients remained favourable, a low implantation with the ALLEGRA THV resulted in worse leaflet pinwheeling. Leaflet pinwheeling is a result of redundant leaflet tissue that can lead to increased strain and compromise THV durability19. Therefore, while gradients may be favourable, avoidance of a low implantation depth is still desirable to prevent leaflet pinwheeling. A low implantation was also associated with higher RF in the larger sized (>23 mm) valves tested in this study.
Based on prior bench studies, a higher implant is generally preferred to optimise hydrodynamic performance. However, this does not necessarily apply to all THVs or surgical valve designs. In this study, an implant depth of +2 mm was desirable for the ALLEGRA THV in both Epic and Magna Ease surgical valves. However, a +2 mm depth in the Mitroflow valve was associated with higher RF, while an implant depth of –2 mm resulted in the optimal hydrodynamic performance with the ALLEGRA THV. These differences are potentially related to design differences among the different surgical valves. At an implantation depth of +2 mm, the base of the ALLEGRA THV was at the upper border of the sewing ring, which may have led to higher RF. A potential lack of adequate sealing from the skirt of the ALLEGRA THV may have led to a high RF due to leak between the surgical valve and the ALLEGRA THV. Clinicians must be cognisant of the design features of each THV and their implications in different failed surgical valve types. In lieu of clinical evidence, bench testing can provide guidance on implantation depth to aid clinicians during clinical VIV interventions.
Limitations
Bench testing may not entirely reflect how the THV will expand in a patient’s native annulus, within a degenerated surgical bioprosthesis, or valve deployment under physiological conditions. The findings of this study require correlation with clinical series. It would also be important to ascertain the long-term implications of implant depth and the effect of pinwheeling in future studies.
Conclusions
The 23 mm ALLEGRA valve had favourable (<20 mmHg) gradients in all surgical valves sized ≥21 mm, even when the THV was implanted low. In 19 mm sized Mitroflow and Epic valves, gradients were elevated (>20 mmHg). While there was no major difference in mean transvalvular gradients, leaflet pinwheeling was worse at lower implantation depths.
|
Impact on daily practice Clinical experience with the ALLEGRA THV is currently limited for VIV interventions. This study has shown that the ALLEGRA THV has favourable transvalvular gradients in multiple surgical valve designs sized ≥21 mm. Importantly, the gradients remained similar at all implantation depths, with no significant increase in gradients with lower implantation. While transvalvular gradients remained favourable even at low implantation depths, there was worse leaflet pinwheeling with a low implantation. This may have important implications for leaflet wear and durability. Bench testing can provide guidance to aid clinicians during VIV interventions with newer THV designs, where clinical evidence may be limited. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
P. Blanke and J. Leipsic are consultants to Edwards Lifesciences and provide CT core lab services for Edwards Lifesciences, Medtronic, Neovasc, GDS, and Tendyne Holdings, for which no direct compensation is received. J. Leipsic has stock options in, is a consultant to, and receives institutional research support from HeartFlow and is supported by a Canadian Research Chair in Advanced CardioPulmonary Imaging. J. Sathananthan has received speaking fees from and is a consultant to Edwards Lifesciences. A. Sedaghat has received travel grants from Abbott, Boston Scientific, Edwards Lifesciences, New Valve Technologies and Medtronic. L. Søndergaard has received consultant fees and institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis. S. Toggweiler is a consultant and proctor for Boston Scientific and NVT GmbH. He has also received an institutional research grant from Boston Scientific. J.G. Webb is a consultant to, and has received research funding from Edwards Lifesciences, Abbott Vascular, and ViVitro Labs. D. Wood is a consultant to Edwards Lifesciences. M. Kütting is an employee of New Valve Technologies. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.

