
Abstract
Aims: The valve-in-valve (ViV) procedure has become a valuable alternative for the treatment of failed surgical bioprostheses (BP) in high-risk patients. However, in small BP, the clinical outcomes have been suboptimal due to high post-procedural gradients. We aimed to examine the effect of size and position of the self-expanding transcatheter heart valve (THV) CoreValve on the haemodynamics of ViV within small BP.
Methods and results: Sizes 23 and 26 mm of the CoreValve were implanted in sizes 19 and 21 mm of three BP models: Trifecta, Mitroflow and Epic Supra. The THV was tested in three positions –normal (manufacturer recommendation), low (4 mm below normal) and high (4 mm above normal)– using a pulse duplicator. Haemodynamics were assessed by Doppler echocardiography and flowmeter, and GOA with a high-speed camera. Higher implantation was associated with lower residual gradients (normal position: –9%, high: –25% versus low). High position was, however, associated with increased risk of regurgitation in the Mitroflow and embolisation in the Epic Supra. Using a 26 mm THV instead of a 23 mm was associated with larger EOAs in the Trifecta, smaller in the Mitroflow, and increased risk of embolisation in the Epic Supra.
Conclusions: Supra-annular positioning of the CoreValve THV is associated with improved post-ViV haemodynamics in small surgical BP. The haemodynamic outcomes are highly dependent on the model and size of surgical BP.
Abbreviations
BP: bioprostheses
EOA: effective orifice area
GOA: geometric orifice area
mTPG: mean transvalvular pressure gradient
THV: transcatheter heart valve
ViV: valve-in-valve
Introduction
Nowadays, the vast majority of surgical aortic valve replacements are performed with bioprosthetic valves. However, the main limitation of these valve substitutes is that they have limited durability and commonly fail within 10-15 years11. Kaneko T, Vassileva CM, Englum B, Kim S, Yammine M, Brennan M, Suri RM, Thourani VH, Jacobs JP, Aranki S. Contemporary Outcomes of Repeat Aortic Valve Replacement: A Benchmark for Transcatheter Valve-in-Valve Procedures. Ann Thorac Surg. 2015;100:1298-304. ,22. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-89. . Patients with failed surgical bioprostheses (BP) are frequently at high surgical risk due to old age, comorbidities and the need for repeat surgery11. Kaneko T, Vassileva CM, Englum B, Kim S, Yammine M, Brennan M, Suri RM, Thourani VH, Jacobs JP, Aranki S. Contemporary Outcomes of Repeat Aortic Valve Replacement: A Benchmark for Transcatheter Valve-in-Valve Procedures. Ann Thorac Surg. 2015;100:1298-304. . Transcatheter heart valve (THV) implantation within the failed aortic surgical BP, i.e., valve-in-valve (ViV) procedure, provides a valuable, less invasive alternative to surgery for patients considered to be at high risk of reoperation22. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-89. .
Surgical BP often have a small and non-elastic stent, which limits the size of the THV that can be implanted for ViV33. Bapat VN, Attia R, Thomas M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: a concept of true internal diameter and its implications for the valve-in-valve procedure. JACC Cardiovasc Interv. 2014;7:115-27. . As a result, severe prosthesis-prosthesis mismatch and elevated post-procedural gradients are common following aortic ViV, especially when performed in small (≤21 mm) surgical BP44. Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, Barbanti M, Latib A, Schaefer U, Rodés-Cabau J, Treede H, Piazza N, Hildick-Smith D, Himbert D, Walther T, Hengstenberg C, Nissen H, Bekeredjian R, Presbitero P, Ferrari E, Segev A, De Weger A, Windecker S, Moat NE, Napodano M, Wilbring M, Cerillo AG, Brecker S, Tchetche D, Lefevre T, De Marco F, Fiorina C, Petronio AS, Teles RC, Testa L, Laborde JC, Leon MB, Kornowski R; Valve-in-Valve International Data Registry Investigators. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162-70. . These haemodynamic abnormalities have been associated with increased risk of mortality and worse functional capacity after ViV55. Webb JG, Mack MJ, White JM, Dvir D, Blanke P, Herrmann HC, Leipsic J, Kodali SK, Makkar R, Miller DC, Pibarot P, Pichard A, Satler LF, Svensson L, Alu MC, Suri RM, Leon MB. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Bioprostheses: PARTNER 2 Valve-in-Valve Registry. J Am Coll Cardiol. 2017;69:2253-62. . The position of the THV within the surgical BP may also influence haemodynamics and thus the clinical outcomes following ViV66. Simonato M, Azadani AN, Webb J, Leipsic J, Kornowski R, Vahanian A, Wood D, Piazza N, Kodali S, Ye J, Whisenant B, Gaia D, Aziz M, Pasala T, Mehilli J, Wijeysundera HC, Tchetche D, Moat N, Teles R, Petronio AS, Hildick-Smith D, Landes U, Windecker S, Arbel Y, Mendiz O, Makkar R, Tseng E, Dvir D. In vitro evaluation of implantation depth in valve-in-valve using different transcatheter heart valves. EuroIntervention. 2016;12:909-17. ,77. Simonato M, Webb J, Kornowski R, Vahanian A, Frerker C, Nissen H, Bleiziffer S, Duncan A, Rodés-Cabau J, Attizzani GF, Horlick E, Latib A, Bekeredjian R, Barbanti M, Lefevre T, Cerillo A, Hernandez JM, Bruschi G, Spargias K, Iadanza A, Brecker S, Palma JH, Finkelstein A, Abdel-Wahab M, Lemos P, Petronio AS, Champagnac D, Sinning JM, Salizzoni S, Napodano M, Fiorina C, Marzocchi A, Leon M, Dvir D. Transcatheter Replacement of Failed Bioprosthetic Valves: Large Multicenter Assessment of the Effect of Implantation Depth on Hemodynamics After Aortic Valve-in-Valve. Circ Cardiovasc Interv. 2016 Jun;9(6). . On the one hand, there is a large variety of designs, models and sizes of surgical BP; on the other hand, several models of THV (SAPIEN/SAPIEN XT/SAPIEN 3 [Edwards Lifesciences, Irvine, CA, USA] and CoreValve®/Evolut™ R/Evolut™ R Pro [Medtronic, Minneapolis, MN, USA]) are approved by the FDA for ViV and several other models of THV may soon become available for this procedure. Some studies have reported that THVs with a supra-annular design, such as the CoreValve, result in better haemodynamics following ViV compared to other types of THV44. Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, Barbanti M, Latib A, Schaefer U, Rodés-Cabau J, Treede H, Piazza N, Hildick-Smith D, Himbert D, Walther T, Hengstenberg C, Nissen H, Bekeredjian R, Presbitero P, Ferrari E, Segev A, De Weger A, Windecker S, Moat NE, Napodano M, Wilbring M, Cerillo AG, Brecker S, Tchetche D, Lefevre T, De Marco F, Fiorina C, Petronio AS, Teles RC, Testa L, Laborde JC, Leon MB, Kornowski R; Valve-in-Valve International Data Registry Investigators. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162-70. ,77. Simonato M, Webb J, Kornowski R, Vahanian A, Frerker C, Nissen H, Bleiziffer S, Duncan A, Rodés-Cabau J, Attizzani GF, Horlick E, Latib A, Bekeredjian R, Barbanti M, Lefevre T, Cerillo A, Hernandez JM, Bruschi G, Spargias K, Iadanza A, Brecker S, Palma JH, Finkelstein A, Abdel-Wahab M, Lemos P, Petronio AS, Champagnac D, Sinning JM, Salizzoni S, Napodano M, Fiorina C, Marzocchi A, Leon M, Dvir D. Transcatheter Replacement of Failed Bioprosthetic Valves: Large Multicenter Assessment of the Effect of Implantation Depth on Hemodynamics After Aortic Valve-in-Valve. Circ Cardiovasc Interv. 2016 Jun;9(6). . In vitro studies performed in pulse duplicators offer the opportunity of testing the multiple possible combinations of models, sizes, and positions of THVs within surgical BP using standardised haemodynamic conditions.
The objective of this in vitro study was to assess the effect of size and position of the self-expanding CoreValve THV on haemodynamic function of the ViV procedure within small surgical BP.
Methods
IN VITRO CARDIOVASCULAR SIMULATION
The in vitro experiments of bioprosthetic valves and ViV assemblies were performed using a cardiovascular pulse duplicator that has been previously described88. Tanné D, Bertrand E, Kadem L, Pibarot P, Rieu R. Assessment of left heart and pulmonary circulation flow dynamics by a new pulsed mock circulatory system. Exp Fluids. 2010;48:837-50. ,99. Evin M, Guivier-Curien C, Rieu R, Rodés-Cabau J, Pibarot P. Mitral valve-in-valve hemodynamic performance: An in vitro study. J Thorac Cardiovasc Surg. 2016;151:1051-9.e6. . More details are provided in Supplementary Appendix 1.
HAEMODYNAMIC CONDITIONS
For each BP and ViV assembly, eight physiological haemodynamic conditions were tested by varying the following cardiac parameters: heart rate (70 and 120 bpm), cardiac output (low flow: 3.1 L/min, normal flow: 4.3 L/min, high flow: 5.9 L/min), and mean aortic pressure (100 mmHg and 160 mmHg at normal flow).
TESTED BP AND ViV ASSEMBLIES
SURGICAL BP
We tested two sizes, i.e., 19 and 21 mm, of three different models of BP: Trifecta™ (St. Jude Medical, St. Paul, MN, USA), Mitroflow (Sorin [now LivaNova], Milan, Italy), and Epic™ Supra (St. Jude Medical) (Supplementary Table 1). All six tested BP had normal haemodynamic function and were tested alone on the pulse duplicator before ViV implantation in the previously described flow conditions (3 models×2 sizes×8 haemodynamic conditions=48 conditions).
ViV ASSEMBLIES
The THVs that were used for ViV in this study were the self-expanding nitinol-made 23 mm CoreValve Evolut and the 26 mm CoreValve (Supplementary Figure 1). The different ViV assemblies tested in this study are described in Supplementary Appendix 1. The degree of THV oversizing was defined as oversizing=100× (AreaTHV–IOABP)/IOABP, where AreaTHV is the area of the inflow portion of the CoreValve when fully deployed (4.12 cm22. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-89. for CoreValve Evolut 23 mm and 4.95 cm22. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-89. for CoreValve 26 mm) and IOABP is the internal orifice area of the BP based on the true internal diameters provided in the literature33. Bapat VN, Attia R, Thomas M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: a concept of true internal diameter and its implications for the valve-in-valve procedure. JACC Cardiovasc Interv. 2014;7:115-27. (ViV application, Supplementary Table 1).
THV DEPTHS OF IMPLANTATION
For each ViV combination described above, the THV was implanted in three different implant depth positions relative to BP annulus, i.e., low (–8 mm), normal (–4 mm) and high (0 mm) (Figure 1), resulting in (3 models×3 size assemblies×3 depth positions=27 ViV assemblies, total of 27×8 haemodynamic conditions=216 conditions) (Supplementary Appendix 1).
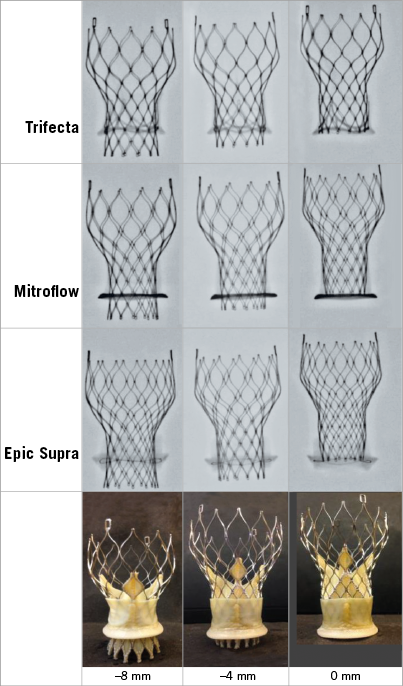
Figure 1. Implant depth positions of the transcatheter heart valve for ViV in each model of surgical bioprosthesis. The CoreValve was implanted in the Trifecta, the Mitroflow, and the Epic Supra. The low, normal, and high positions correspond to –8 mm, –4 mm, and 0 mm below the BP annulus.
DATA ACQUISITION
AORTIC VALVE GRADIENTS AND EOAs BY DOPPLER ECHOCARDIOGRAPHY AND FLOWMETER
Continuous wave Doppler was performed across the aortic valve assemblies with a Vivid 7 ultrasound system (GE Healthcare, Waukesha, WI, USA) and a 1.4 to 3.3 MHz phased-array probe. The transprosthetic flow velocities were recorded and averaged over four cardiac cycles in each implantation/haemodynamic condition. Mean transvalvular pressure gradients (mTPG) were calculated using the simplified Bernoulli formula and the valve effective orifice area (EOA) was calculated with the continuity equation by dividing the stroke volume measured with the flowmeter by the velocity-time integral of the transprosthetic velocities measured by Doppler. High residual gradient was defined as an mTPG >20 mmHg according to Valve Academic Research Consortium 2 criteria.
THV REGURGITATION AND STABILITY BY FLOWMETER AND VISUAL EXAMINATION
Aortic valve regurgitation fraction (regurgitant fraction=100× [regurgitant volume/stroke volume]) was measured by the electromagnetic flowmeter. THV migration was assessed by visual observation of the ViV assembly after withdrawal from the simulator. The position of the THV within the surgical BP was compared before and after ViV.
AORTIC VALVE GEOMETRIC ORIFICE AREA BY HIGH-SPEED CAMERA
A high-speed camera (SA3 Fastcam–120K N/B; Photron, Tokyo, Japan) was placed facing the outflow side of the valve. The geometric orifice area (GOA) was measured on images of the fully opened valve with a custom software detecting the contours of the minimal orifice area (Figure 2). The coefficient of flow contraction was computed as the ratio of EOA to GOA.
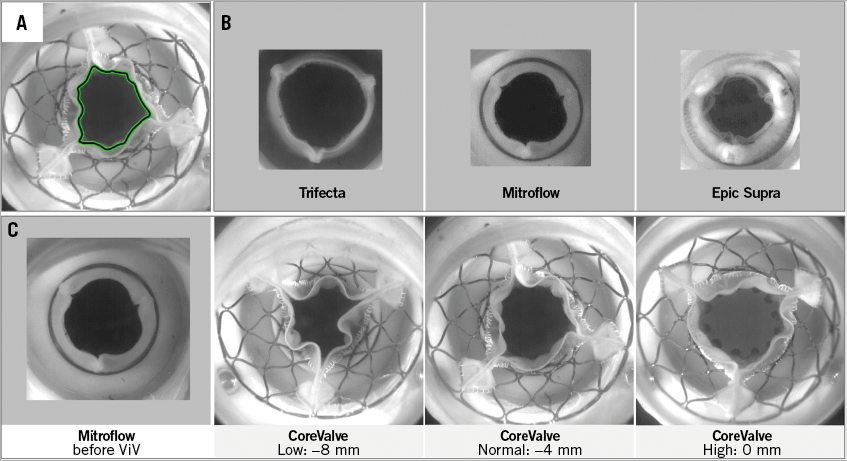
Figure 2. En face views of the aortic valves using a high-speed camera. A) Measurement of the geometric orifice area by drawing the contour of the valve orifice using a MATLAB programme. B) View of the three BP at maximum opening before valve-in-valve (ViV). C) View of the 19 mm Mitroflow before and after ViV implantation of the 23 mm CoreValve deployed at three different implantation depths.
STATISTICAL ANALYSIS
Data are presented as mean±SD averaged over all the tested assemblies. One-way repeated measurement ANOVA was performed to compare the pre- and post-ViV results according to the size and the position of the THV. The statistical analyses were performed using SigmaPlot 11 (Systat Software, Inc., San Jose, CA, USA) and a p-value <0.05 was considered statistically significant.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Results
EFFECT OF ViV ON HAEMODYNAMICS
The haemodynamic performance of the normal surgical BP prior to ViV is described in Figure 3, Moving image 1-Moving image 3, Supplementary Appendix 2, and Supplementary Table 2.
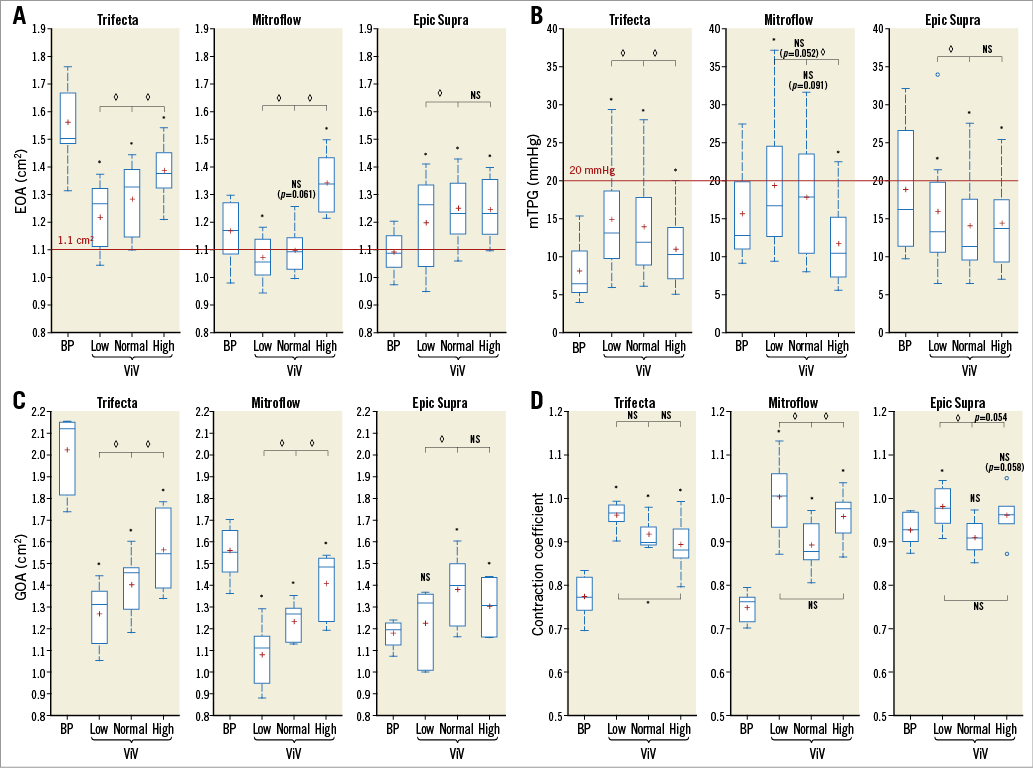
Figure 3. Valve haemodynamic performance according to the model of surgical bioprosthesis and the implant depth position of the transcatheter heart valve. A) Effective orifice area (EOA). B) Mean transprosthetic gradient (mTPG). C) Geometric orifice area (GOA). D) Flow contraction coefficient. Values are averaged over the three tested assemblies (BP19-CoreValve 23, BP21-CoreValve 23 and BP21-CoreValve 26) and over the three tested cardiac outputs at a heart rate of 70 bpm. Crosses represent the mean values. For the Epic Supra BP with CoreValve in the high position, only data of the two ViV assemblies with the 23 mm CoreValve are represented because the data for the 26 mm CoreValve are missing due to valve migration. The red lines represent the VARC-2 criteria for high residual gradient (mTPG >20 mmHg) and small EOA (<1.1 cm22. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-89. ). p<0.05 between CoreValve positions. *p<0.05 between CoreValve positions and BP alone groups. BP: bioprosthesis; NS: not statistically significant; ViV: valve-in-valve
Valve-in-valve implantation of the THV in the normal position was associated with deterioration in haemodynamics in the Trifecta (18% decrease in EOA, 72% increase in mTPG), no significant change in the Mitroflow (6% decrease in EOA, 12% increase in mTPG), and improvement in haemodynamics in the Epic Supra (+15% in EOA and –26% in mTPG) compared to the BP alone (Supplementary Table 2, Figure 3A, Figure 3B). The GOA decreased by 31% in the Trifecta and 21% in the Mitroflow, whereas it increased by 17% in the Epic Supra (Figure 3C). The flow contraction coefficient increased with ViV in the Trifecta and Mitroflow whereas it remained unchanged in the Epic Supra (Figure 3D). The number of cases with high residual gradients (mTPG >20 mmHg) after ViV in the normal position were: one (4.2% of total Trifecta conditions in normal position) for the Trifecta, three (12.5%) for the Mitroflow, and one (4.2%) for the Epic Supra (Supplementary Table 2).
EFFECT OF THV IMPLANTATION DEPTH ON ViV HAEMODYNAMICS
Regardless of the BP model, a higher implantation of the THV was associated with lower gradients (average –9% for the normal position and –25% for the high position compared to the low position) and larger EOAs (+4% and +14%, respectively) (Supplementary Table 2, Figure 3A, Figure 3B) and GOAs (+12% and +20%, respectively [Figure 3C]). High residual gradients after ViV in the low position occurred in two cases (8%) in the Trifecta, five cases (21%) in the Mitroflow, and three cases (13%) in the Epic Supra (Supplementary Table 2). Assessment of high-speed camera videos showed that leaflet opening became more regular, more extensive and with less plication of the free edges of the leaflets when the THV was implanted in a higher position (Figure 2, Moving image 4-Moving image 9).
EFFECT OF THV SIZING ON ViV HAEMODYNAMICS
Figure 4A and Figure 4B show the EOA and mTPG for 23 and 26 mm THV implanted in the three models of BP size 21 mm before and after ViV in the three tested THV positions. In the case of the Trifecta, implantation of a 26 mm THV was associated with an increase in EOA of 7% (+0.1 cm22. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-89. ) and a decrease in mTPG of 17% (–2 mmHg) compared to implantation with a 23 mm THV (Figure 4A, Figure 4B). In the case of the Epic Supra, implanting a 26 rather than a 23 mm THV was associated with better haemodynamics only in the low implant depth position (EOA: +7% and mTPG: –19.5%). In the high position the 26 mm THV migrated upwards (towards the aorta) which led to the development of moderate intervalvular regurgitation (Figure 5). In the Mitroflow, the implantation of a 26 rather than a 23 mm THV was not favourable and was associated with an increase in mTPG (+32%, +4.6 mmHg) and a decrease in EOA (–10%, –0.12 cm22. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-89. ) in the normal implant depth position.
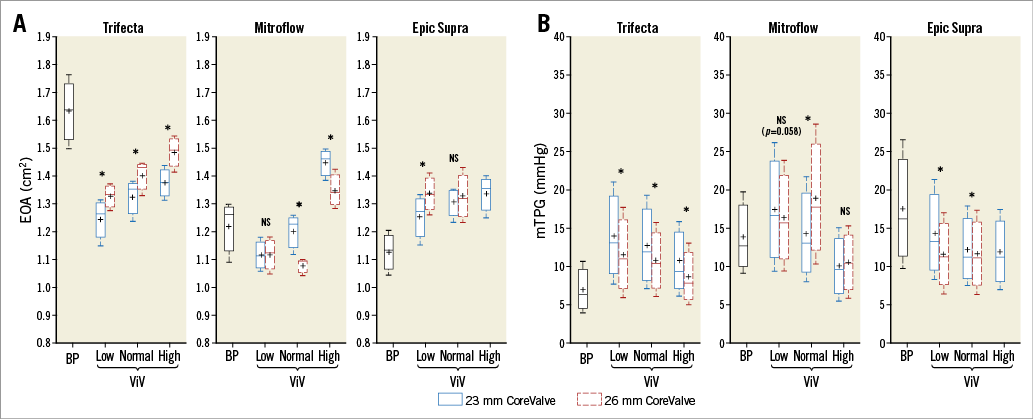
Figure 4. Valve haemodynamic performance according to the model of surgical bioprosthesis and the size and implant depth position of the transcatheter heart valve. A) EOA data. B) mTPG data. For the Epic Supra BP with CoreValve in the high position, only 23 mm CoreValve data are represented because the data for the 26 mm CoreValve are missing due to valve migration. BP: bioprosthesis; NS: not statistically significant; ViV: valve-in-valve
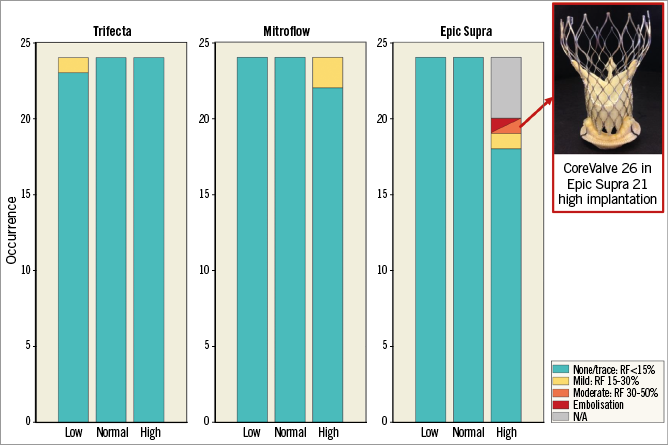
Figure 5. Effect of transcatheter heart valve implantation depth on intervalvular regurgitation and stability for each model of surgical bioprosthesis. One case of valve dislodgement and subsequent moderate intervalvular regurgitation was recorded with the 26 mm CoreValve implanted in the high position within the 21 mm Epic Supra. RF: regurgitant fraction
REGURGITATION AND STABILITY
Figure 5 presents the rates and severity of prosthetic valve regurgitation according to BP type and THV implant depth position. Before ViV (BP alone), no significant prosthetic valve regurgitation was observed. After ViV in the Trifecta, one (1.4%) case of mild regurgitation (regurgitant fraction >15% but <30%) was observed with the THV in the low position. After ViV in the Mitroflow, we observed two (2.8%) cases of mild regurgitation: these two cases occurred with the 26 mm THV in the high position.
With the Epic Supra, no case of regurgitation was observed in the normal and low THV positions. However, with the 26 mm THV implanted in the high position, there was a migration of the THV with moderate (i.e., regurgitant fraction >30% but <50%) intervalvular regurgitation (Figure 5). The effects of cardiac output and aortic pressure are described in Supplementary Appendix 2.
Discussion
The main findings of this in vitro study of ViV in small surgical BP are the following. 1) ViV with the CoreValve is associated with deterioration in valve haemodynamics (i.e., decrease in EOA and increase in gradient) in the Trifecta, haemodynamic improvement in the Epic Supra, and no significant change in the Mitroflow. 2) Overall, the high implantation depth position is associated with better haemodynamics compared to the low position; the normal position yields intermediate results between the low and high positions. However, the high position was associated with an increased risk of embolisation in the Epic Supra. 3) THV oversizing (i.e., using the 26 mm CoreValve instead of the 23 mm) was generally associated with larger EOAs in the Trifecta and Epic Supra but smaller EOAs in the Mitroflow.
EFFECT OF ViV ON HAEMODYNAMICS
ViV in the Trifecta resulted in significant reductions in GOA and EOA and ensuing increases in gradients. The Trifecta has a circular and rigid stent made of titanium with pericardial leaflets mounted outside the stent. Despite the radial forces exerted by the nitinol stent of the CoreValve, there is no possibility for expansion of the internal orifice diameter of the Trifecta. Consequently, the ViV resulted in a reduction of the GOA that is nevertheless attenuated when using a high implant depth position (Moving image 4, Moving image 5). The Mitroflow has a rigid polymeric circular stent and externally sewn leaflets. Despite a design similar to the Trifecta, the Mitroflow has less extensive opening of leaflets, which may allow some further expansion during ViV (Moving image 3).
To this effect, the ViV was associated with reduction in GOA with the Mitroflow but to a lesser extent than with the Trifecta. With both pericardial surgical BP, the flow contraction coefficient increased (i.e., less flow contraction) with the ViV, which, at least in part, attenuated the negative haemodynamic impact (i.e., decrease in EOA and increase in gradient) related to the reduction in GOA. The improvement in contraction coefficient is probably related to the fact that the implantation of the CoreValve within the pericardial BP provided a more progressive (funnel-like) valve inflow shape and so less flow contraction.
As opposed to the pericardial BP, Trifecta and Mitroflow, ViV resulted in an improvement and not a deterioration of the valve haemodynamics in the Epic Supra. The design of this porcine BP is very different compared to that of the pericardial BP. The stent made of an acetal copolymer has a scalloped shape and is partially flexible, and the porcine leaflets are mounted inside the stent. There is thus a potential for expansion of the internal orifice diameter with the radial forces generated by the stent of the THV during the ViV. As a matter of fact, the ViV was associated with an increase in GOA and EOA of the Epic Supra (Moving image 2, Moving image 8). With this BP, there was, however, minimal change in the flow contraction coefficient.
Given that the baseline haemodynamics of the Trifecta are substantially better than those of the Epic Supra or the Mitroflow, the haemodynamics post ViV were similar or better with the Trifecta than with the Epic Supra or the Mitroflow. These findings further emphasise the importance of implanting surgical BP with the largest possible internal orifice diameter, providing the largest GOA and EOA, and also ideally with stents (and thus internal orifice area) able to expand at the time of ViV.
EFFECT OF THV IMPLANTATION DEPTH
Regardless of the BP model and size, a higher implantation of the THV allowed reduction of the mTPG by increasing the GOA and thus the EOA (Moving image 4-Moving image 9). This is consistent with some in vitro studies66. Simonato M, Azadani AN, Webb J, Leipsic J, Kornowski R, Vahanian A, Wood D, Piazza N, Kodali S, Ye J, Whisenant B, Gaia D, Aziz M, Pasala T, Mehilli J, Wijeysundera HC, Tchetche D, Moat N, Teles R, Petronio AS, Hildick-Smith D, Landes U, Windecker S, Arbel Y, Mendiz O, Makkar R, Tseng E, Dvir D. In vitro evaluation of implantation depth in valve-in-valve using different transcatheter heart valves. EuroIntervention. 2016;12:909-17. ,1010. Azadani AN, Reardon M, Simonato M, Aldea G, Nickenig G, Kornowski R, Dvir D. Effect of transcatheter aortic valve size and position on valve-in-valve hemodynamics: An in vitro study. J Thorac Cardiovasc Surg. 2017;153:1303-15.e1. ,1111. Midha PA, Raghav V, Condado JF, Arjunon S, Uceda DE, Lerakis S, Thourani VH, Babaliaros V, Yoganathan AP. How Can We Help a Patient With a Small Failing Bioprosthesis?: An In Vitro Case Study. JACC Cardiovasc Interv. 2015;8:2026-33. and with the in vivo study from the VIVID registry77. Simonato M, Webb J, Kornowski R, Vahanian A, Frerker C, Nissen H, Bleiziffer S, Duncan A, Rodés-Cabau J, Attizzani GF, Horlick E, Latib A, Bekeredjian R, Barbanti M, Lefevre T, Cerillo A, Hernandez JM, Bruschi G, Spargias K, Iadanza A, Brecker S, Palma JH, Finkelstein A, Abdel-Wahab M, Lemos P, Petronio AS, Champagnac D, Sinning JM, Salizzoni S, Napodano M, Fiorina C, Marzocchi A, Leon M, Dvir D. Transcatheter Replacement of Failed Bioprosthetic Valves: Large Multicenter Assessment of the Effect of Implantation Depth on Hemodynamics After Aortic Valve-in-Valve. Circ Cardiovasc Interv. 2016 Jun;9(6). , which reported that a higher THV implantation is independently associated with lower post-procedural gradients with both self-expanding and balloon-expandable THVs.
The supra-annular design of the CoreValve allows the THV leaflets to operate with more space and less restriction compared to THV leaflets that would be located at the level of the BP internal orifice. The stent of the THV has an hourglass shape and is thus less constrained and has a larger internal orifice above the annulus of the BP than at the level of the annulus. With the low implant depth position of the THV, this advantage of the supra-annular position and operation of the THV leaflets is partially lost, whereas it is maximised with the highest position. The haemodynamic benefit of the high vs. normal vs. low positions is essentially determined by an increase in the GOA and thus the EOA. On the other hand, a higher position is associated with some slight decrease in the flow contraction coefficient probably because of the attenuation of the funnel effect (at the valve inflow) created by the portion of the THV stent below the internal orifice of the surgical BP.
The high position of the THV may however be associated with an increased risk of THV migration and embolisation, and intervalvular regurgitation following ViV in small BP, particularly in the case of some specific models such as the Epic Supra. This risk appears to be increased with important THV oversizing. Indeed, the Epic Supra valve has relatively shorter stent posts and leaflets compared to the Trifecta or Mitroflow BP, which may explain the greater susceptibility to THV migration with the high position (Figure 5, inset). This instability of the THV may also be related to the effacement of the hourglass shape of the THV stent at its base (due to restriction of the apical border of the stent within the BP annulus), especially when the ViV is performed within small BP and with a large degree of THV oversizing. These conditions may increase the upward forces applied at the base of the stent, thus leading to “pop-off” of the THV in the direction of the aorta.
In the Mitroflow, mild intervalvular regurgitation was observed in two cases with high THV position. One possible explanation may be the indentations at the apical border of the stent, which leaves some open space not covered by porcine tissue. With the high position, these open spaces at the base of the THV stent are above the BP annulus, thus increasing the risk of intervalvular leak. The newer generations of CoreValve, i.e., the Evolut R and Evolut R Pro have extended sheets of porcine tissue, which fill, at least in part, these apical indentations, and a layer of pericardium wrapped around the base of the stent (Evolut R Pro only). These features may help to reduce the risk of intervalvular regurgitation with the high THV position.
EFFECT OF THV OVERSIZING
In small surgical BP, the implantation of a 23 mm CoreValve is already associated with important THV oversizing. The results of this study show that even more important oversizing, i.e., using a 26 rather than a 23 mm CoreValve, is associated with somewhat larger EOAs and lower gradients with the Trifecta and the Epic Supra but not with the Mitroflow. The only case of THV dislodgement that we observed in this study occurred with a 26 mm THV implanted in the high position within the 21 mm Epic Supra. Furthermore, although this was not assessed in the present study, important THV oversizing may increase the mechanical stress on the leaflets and may thus impair the long-term durability of the THV1212. Martin C, Sun W. Transcatheter Valve Underexpansion Limits Leaflet Durability: Implications for Valve-in-Valve Procedures. Ann Biomed Eng. 2017;45:394-404. . Hence, the small haemodynamic benefit achieved by major THV oversizing may not offset the risk of THV migration and of potentially impaired durability.
Study limitations
We tested only three models of surgical BP and one model of THV. Further studies are necessary to expand these analyses to other models of BP and THVs used in the clinical setting. We used BP with normal morphology and function. Further in vitro studies are necessary to test different models, sizes, and positions of THVs within failed surgical BP explanted from patients. It is possible that in such studies the gradients may be higher than those observed in the present study with normal BP. We tested only three THV positions. Positions higher than the high position (0 mm) or lower than the low position (-8 mm) are not clinically relevant. We could have tested an intermediate position between normal and high (i.e., 2 mm below the BP annulus). However, it is likely that the results would have been between those of the normal and high positions. We were not able to assess the effect of THV position and oversizing on the risk of coronary artery obstruction, which is a potential complication of ViV. Further studies including measurements of pull-out forces are required to validate the THV stability and risk of migration, especially in the highest position.
Conclusions
Supra-annular positioning of the CoreValve is associated with good overall haemodynamics following ViV in small surgical BP. The results of this study allow tailoring the sizing and positioning of the THV for ViV according to the model and size of the surgical BP. For the 19 and 21 mm Epic Supra, a 23 mm CoreValve implanted in the normal (4 mm below BP annulus) position appears to provide the best conditions to optimise the haemodynamics and stability. For the 19 and 21 mm Mitroflow, a 23 mm CoreValve implanted in the normal or high (0 mm) position provides optimal results. For the 19 and 21 mm Trifecta, the best haemodynamic results are obtained, respectively, with the 23 and 26 mm THV implanted in a high position. However, further studies are needed for better assessment of the stability of the THV position and its durability in these BP as well as in other ViV combinations of BP-THV models.
|
Impact on daily practice To optimise the haemodynamics and stability of the ViV, the selection of the THV size and position should be tailored to the model and size of the surgical BP. In the Trifecta, the high position (0 mm relative to BP annulus) of the THV provides the best haemodynamic performance and large oversizing provides further incremental haemodynamic benefit with no obvious side effects. In the Mitroflow, the high position is associated with lower gradients but slightly more (mild) intervalvular regurgitation; large oversizing is not recommended. For the Epic Supra, the high THV position and the large oversizing are not recommended as these conditions are not associated with any further haemodynamic benefit (i.e., decrease in gradients) compared to the normal position and, furthermore, they may be associated with increased risk of THV migration and intervalvular regurgitation. |
Acknowledgements
We would like to thank Julien Diperi for his help in machining the simulated aortic annuli.
Funding
This study was funded by a Foundation Scheme Grant (#FDN-143225) from Canadian Institutes of Health Research (Ottawa, Ontario, Canada). P. Pibarot holds the Canada Research Chair in Valvular Heart Disease. J. Rodés-Cabau holds the Research Chair on the Development of Interventional Therapies for Structural Heart Diseases - Family Jacques Larivière Foundation.
Conflict of interest statement
P. Pibarot and J. Rodés-Cabau report receiving research grants from Edwards Lifesciences and Medtronic. J.F. Obadia reports receiving a research grant from Abbott and is a consultant for St. Jude Medical, Landanger, Delacroix-Chevalier, Edwards and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Appendix 2. Results.
Supplementary Table 1. Design features of each surgical bioprosthesis model including stent characteristics and internal diameters.
Supplementary Table 2. Valve-in-valve haemodynamic performance according to surgical bioprosthesis model and transcatheter heart valve implant depth position.
Supplementary Figure 1. Transcatheter heart valves used in the study.
Moving image 1. En face view of the 19 mm Trifecta valve.
Moving image 2. En face view of the 19 mm Epic Supra valve.
Moving image 3. En face view of the 19 mm Mitroflow valve.
Moving image 4. En face view of the 23 mm CoreValve implanted in the 19 mm Trifecta valve in the low implant depth position.
Moving image 5. En face view of the 23 mm CoreValve implanted in the 19 mm Trifecta valve in the high implant depth position.
Moving image 6. En face view of the 23 mm CoreValve implanted in the 19 mm Mitroflow valve in the low implant depth position.
Moving image 7. En face view of the 23 mm CoreValve implanted in the 19 mm Mitroflow valve in the high implant depth position.
Moving image 8. En face view of the 23 mm CoreValve implanted in the 19 mm Epic Supra valve in the low implant depth position.
Moving image 9. En face view of the 23 mm CoreValve implanted in the 19 mm Epic Supra valve in the high implant depth position.
To read the full content of this article, please download the PDF.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. En face view of the 19 mm Trifecta valve.
Moving image 2. En face view of the 19 mm Epic Supra valve.
Moving image 3. En face view of the 19 mm Mitroflow valve.
Moving image 4. En face view of the 23 mm CoreValve implanted in the 19 mm Trifecta valve in the low implant depth position.
Moving image 5. En face view of the 23 mm CoreValve implanted in the 19 mm Trifecta valve in the high implant depth position.
Moving image 6. En face view of the 23 mm CoreValve implanted in the 19 mm Mitroflow valve in the low implant depth position.
Moving image 7. En face view of the 23 mm CoreValve implanted in the 19 mm Mitroflow valve in the high implant depth position.
Moving image 8. En face view of the 23 mm CoreValve implanted in the 19 mm Epic Supra valve in the low implant depth position.
Moving image 9. En face view of the 23 mm CoreValve implanted in the 19 mm Epic Supra valve in the high implant depth position.

